Abstract
Stem cells are a promising tool for treatment of a variety of degenerative diseases. Human amniotic epithelial stem cells (hAECs) have desirable and unique characteristics that make them a proper candidate for cell therapy. In this study, we have investigated the effects of BMP-4 (bone morphogenetic protein-4) and its inhibition on differentiation of AECs into ectodermal lineages. Analysis of AEC-derived ectodermal lineages (neurons and keratinocytes) was performed by using flow cytometry technique for Map2 and β-tubulin (as neuron markers), Olig2 and MBP (as oligodendrocyte markers), and K14 and K10 (as keratinocyte markers). The results of this study illustrated that noggin (as BMP antagonist), BMP4, and both BMP4 and heparin (together or separately) increased neural and keratinocyte marker expression, respectively. The expression of markers MAP2, olig2, and K14 in hAECs has been significantly decreased 21 days after exposure to differentiation medium (without growth factors) compared with isolation day, which supports the hypothesis that AECs can be dedifferentiated into pluripotent cells. Moreover, activation and inhibition of BMP signaling have no effects on viability of hAECs. The results of this study showed that BMP signaling and its inhibition are the key factors for ectodermal lineage differentiation of amnion-derived stem cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, cell therapy holds a great promise for the treatment of various diseases such as neurodegenerative diseases (e.g., spinal cord injury, multiple sclerosis, and Parkinson disease) and wound healing. Using proper cell source for this purpose is very important. Human amniotic epithelial cells (hAECs), which are isolated from human placenta, have favorable and unique characteristics that make them highlighted and attractive for cell therapy. Remarkable properties of hAECs are low immunogenicity, non-tumorigenicity, secretion of several growth factors such as epithelial growth factor (EGF), keratinocyte growth factor (KGF), transforming growth factors (TGFβ) (Niknejad et al. 2008; Si et al. 2015; Zhang et al. 2017), as well as immunomodulatory and anti-inflammatory features. Moreover, these cells can be achieved in extensive amounts without ethical concerns in comparison with the other fetal stem cells (Liu et al. 2012). Furthermore, AECs express stem cell surface markers and the pluripotency transcription factors such as octamer-binding protein 4 (Oct-4), Nanog, Lefty A, sex determining region Y-box 2 (SOX-2), and teratocarcinoma-derived growth factor 1 (TDGF1) (Maymó et al. 2018). hAECs are able to differentiate to all three germ layers of endodermal (pancreatic and hepatic cells), mesodermal (cardiac cells), and ectodermal (nervous system and skin) lineages (Wang et al. 2018).
It has been revealed that hAECs express ectodermal lineage markers for both neural and glial cells, such as neurofilament, neuron-specific enolase, microtubule-associated protein 2, GFAP and myelin basic protein, 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase), and A2B5 (Miki 2011; Miki et al. 2005; Parolini et al. 2008). Moreover, AECs have capability to differentiate to functional neural cells that synthesize and release acetylcholine, norepinephrine, and dopamine (Ilancheran et al. 2009). We also reported that AECs can be differentiated to midbrain dopaminergic neurons (Niknejad et al. 2012). The other ectodermal lineage that AECs can be differentiated is keratinocytes which express markers such as K19, K14, β1 integrin, K5, K8, K18, K19, K16, K18, K19, and E-cadherin (Mahmood et al. 2015; Yu et al. 2015). Differentiation of cultured hAECs toward skin keratinocytes has been demonstrated in the previous studies (Fatimah et al. 2013).
There are several pathways involved in ectodermal lineage differentiation of hAECs. One of the main pathways that affect both neural and keratinocyte differentiation is BMP signaling. From reproductive point of view, BMP signaling is a building block in ectodermal fate in embryo. For instance, BMP activity stimulates epidermal fate and its inhibition causes CNS development (Patthey and Gunhaga 2014). Among 20 known BMP family members, BMP4 is a key regulator in stem cell commitment which directly induces epidermal fate and regulates neural differentiation (Qu et al. 2016). Noggin is a BMP signaling antagonist which directly binds to BMP-4 and blocks its activities. Several in vitro studies on embryonic and bone marrow-derived stem cells have reported that activation or inhibition of BMP4 (by Noggin) involves in ectodermal lineage differentiation (Fazeli et al. 2015; Mira et al. 2010; Morell et al. 2015). However, there are limited data on the effects of BMP signaling on ectodermal differentiation of AECs. Therefore, the aim of this study was to investigate the effect of BMP-4 on differentiation of AECs to three early ectodermal lineages keratinocyte, neuron, and glia cells.
Materials and methods
Isolation of hAECs
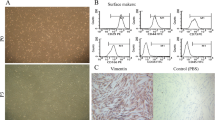
Human placenta was obtained after elective cesarean delivery from healthy mothers after obtaining informed consent and transferred to the lab under sterile conditions. All experimental procedures were done after approving by ethics committee of Shahid Beheshti University of Medical Sciences. The amnion layer was mechanically peeled off of the chorion and washed several times with cold phosphate-buffered saline (PBS). To isolate AECs, amniotic membrane (AM) was incubated at 37 °C with 0.15% trypsin-EDTA (Merck, Germany). The cells from the first 10 min of digestion were discarded to exclude debris. The supernatants from the second and third 40-min digests were pooled. Trypsin was inactivated with fetal bovine serum (Gibco, UK) and cell suspension was centrifuged at 1200 rpm for 12 min. The cells were suspended in Dulbecco modified Eagle’s medium (DMED) (Gibco, UK) containing 100 U/mL penicillin/streptomycin (Thermo Fisher, USA) and 10% FBS. Trypan blue (Sigma-Aldrich, USA) dye exclusion was used to determine viability of the isolated cells (Fig. 1a, b).
a Process of human amniotic epithelial cell isolation from amniotic membrane. b The figure shows the phenotype of isolated human amniotic epithelial cells. c The characterization of isolated epithelial cells with pancytokeratin. The inset shows DAPI nuclear staining of the same field. Scale bar: 50 μm. d A schematic diagram which shows differentiation process of human amniotic epithelial cells
Immunocytochemistry
Characteristic analysis of freshly isolated AECs was performed at the protein expression level by immunocytochemistry. The cells were fixed at room temperature with 4% paraformaldehyde for 10 min, washed with PBS, and incubated with 10% goat serum and 0.1% Triton X-100 for 1 h. The cells were incubated with pancytokeratin primary antibody (Sigma, 1:200) overnight at 4 °C. The secondary antibody conjugated with rhodamine (Chemicon, 1:100) was applied for 30 min after washing with PBS. The total number of cells was counted by staining nuclei with 4′,6-diamidino-2-phenylindole (DAPI) (Sigma).
Differentiation protocol
After reaching 90% confluency, 2 × 104 cells were plated in 12-well plates in aforementioned culture medium. After 3 days, differentiation process was initiated by replacing culture medium with differentiation medium including DMEM supplemented with 10% FBS, 100 IU/mL penicillin, 100 mg/mL streptomycin, 5 ng/mL epidermal growth factor (EGF) (Sigma-Aldrich, USA), 1 mM sodium pyruvate (Sigma-Aldrich, USA), 1% insulin/transferrin/selenium (ITS) (Gibco, UK), 2 mM ascorbic acid (Sigma-Aldrich, USA), 10 ng/mL bFGF (Sigma-Aldrich, USA), and 1 μM RA (Sigma-Aldrich, USA). Differentiation medium was changed twice a week for 7 days. After that, we added 50 or 100 ng/mL noggin for neuronal differentiation and 25 or 50 ng/mL BMP-4 for oligodendrocyte differentiation. Moreover, for keratinocyte differentiation, the differentiation medium was supplemented with 10 and 20 μg/mL of heparin. Additionally, 25 ng/mL BMP-4 was added to differentiation medium (with or without heparin) for keratinocyte differentiation (the differentiation protocol is shown in Fig. 1d.
Cell fixation and flow cytometry
After 21 days, the differentiated cells were fixed by adding 4% formaldehyde and characteristic analysis of AEC-derived ectodermal cells (neurons and keratinocytes) was performed by flow cytometer. Stained cells were analyzed using a BD FACS Calibur system (BD Biosciences) and analyzed with FlowJo software. To detect Map2, β-tubulin, Olig2, MBP, K14, and K10 markers, the following primary antibodies were applied: mouse monoclonal anti-MAP-2 antibody (Santa Cruz, USA, 1:100), rabbit polyclonal anti-beta tubulin antibody (Abcam, USA, 1:100), rabbit polyclonal anti-olig2 antibody (Abcam, USA, 1:100), mouse monoclonal anti-MBP antibody (Abcam, USA, 1:100), mouse monoclonal anti-cytokeratin 14 antibody (Abcam, USA, 1:100), mouse monoclonal anti-cytokeratin 10 antibody (Abcam, USA, 1:100). Additionally, the following secondary antibodies were used: anti-mouse AlexaFluor® antibody (Abcam, USA, 1:100) and anti-rabbit AlexaFluor® antibody (Abcam, USA, 1:100), goat anti-mouse antibody (Abcam, USA, 1/500), and goat anti-rabbit (Abcam, USA, 1/500).
Cell viability assay
To evaluate the effects of differentiation protocols on the viability of differentiated cells, the viability of cells was examined after 21 days of differentiation using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (Sigma-Aldrich, USA) as we described recently (Azizian et al. 2018). In summary, 20-μL MTT prepared solution (5 mg of MTT per 1 mL PBS) was added to each well at differentiation day 21 and incubated for 4 h. Subsequently, the formazan crystals were dissolved in dimethyl sulfoxide (DMSO) (Merck, Germany) and absorbance of dissolved formazan was measured at 570 nm using ELISA reader (BioTek, USA). Untreated cells were taken as control with 100% viability. The cell viability (%) was estimated by the following formula:
Statistical analysis
The data were obtained from three independent experiments and each one was triplicate (n = 3). The results were expressed as mean ± SD. Analysis of variance (ANOVA) was used with Turkey’s post-test to determine statistical significance. P value less than 0.05 was considered to be statistically significant.
Results
The viability of isolated and differentiated hAECs
hAECs were isolated from full-term amnion of healthy women after two-step enzyme digestions. The viability of isolated cells was more than 95%, as detected with trypan blue. The purity of hAECs was more than 98% as evaluated by pan cytokeratin expression (Fig. 1c). Differentiation protocols did not decrease the viability of hAECs (Fig. 2). Moreover, the concentrations of BMP4 and Noggin have no effects on the differentiated cells’ viability. The result showed that the percentage of viability in the treatment groups was 93.8–100%.
The effect of BMP-4 on expression of MAP-2 and β-tubulin markers in hAECs
The effect of noggin (BMP antagonist) on MAP-2 and β-tubulin expression was confirmed before and after differentiation for 21 days by flow cytometry analysis. The results indicated that noggin increases MAP2 and β-tubulin neuronal markers as shown in Fig. 3. There is no significant difference between 50 and 100 ng/mL noggin concentrations. The percentage of MAP2 expression was 74.07 ± 13.33% for 50 ng/mL noggin-treated group and 66.82 ± 4.275% for the 100 ng/mL noggin group. The percentage of β-tubulin expression was 71.15 ± % for 50 ng/mL noggin-treated group and 63.3 ± 1.13% for the 100 ng/mL noggin group.
a–e Flow cytometer analysis of MAP-2 and β-tubulin expression of hAECs treated with Noggin after 21 days. Flow cytometry diagrams for detection of MAP2 and β-tubulin expression: a, a´ Map2 control (untreated cells), b, b´ Map2 marker expression in noggin-treated group of AECs. c, c´ β-tubulin control (untreated cells), d, d´ β-tubulin marker expression in noggin-treated group of AECs. e Analysis of MAP2 and β-tubulin marker expression of the hAECs treated with noggin. *p < 0.05
The effect of BMP-4 on expression of Olig2 and MBP markers in hAECs
To evaluate the effect of BMP-4 on oligodendrocyte differentiation of hAECs, the expression of olig2 and MBP was measured before and after 21 days of differentiation. The results indicated that BMP-4 increases olig2 and MBP glial markers as shown in Fig. 4. There is no significant difference between 25 and 50 ng/mL BMP-4 concentrations. The percentage of Olig2 expression was 60.8 ± 0.5% for 25 ng/mL BMP-4-treated group and 57.9 ± 4.2% for the 50 ng/mL BMP-4 group. The percentage of MBP expression was 41.85 ± 0.75% for 25 ng/mL BMP-4-treated group and 44.4 ± 0.6% for the 50 ng/mL BMP-4 group.
a–e Flow cytometer analysis of olig2 and MBP marker expression of the hAECs treated with BMP-4. hAECs were treated with BMP4 after 21 days. Flow cytometry diagrams for detection of Olig2 and MBP markers: a, a´ olig2 control (untreated cells), b, b´ olig2 marker expression in BMP-4-treated group of AECs. c, c´ MBP control (untreated cells), d, d´ MBP marker expression in BMP-4-treated group of AECs, e analysis of olig2 and MBP marker expression of the hAECs treated with BMP-4. *p < 0.05, **p < 0.001, and ***p < 0.0001
Differentiation of hAECs into keratinocytes in the presence and absence of BMP-4
We evaluated the effect of heparin and BMP-4 on keratinocyte differentiation of hAECs. At the 21th day of differentiation, we detected the k14 and k10 expression levels. Both BMP4 and heparin increase keratinocyte marker K14 and k10 after 21 days. The results showed that there is no significant difference between 10 and 20 μg/mL heparin concentrations on keratinocyte differentiation (Fig. 5). The combination of BMP4 and heparin showed the same results on the k14 and k10 expression levels as BMP4 or heparin alone. The results of k14 expression for BMP4-treated, heparin-supplemented differentiation medium, and heparin + BMP4 -reated group were 76.65 ± 1.7%, 67.45 ± 0.35%, and 67 ± 1.4%, respectively. The results of k10 expression for BMP4-treated, heparin-supplemented differentiation medium, and heparin + BMP4-treated group were 74.70 ± 1.2%, 51.05 ± 1.05%, and 72.02 ± 0.95%, respectively (Fig. 6).
a–d Flow cytometer analysis of K10 marker expression of the hAECs treated with heparin after 21 days. Flow cytometry diagram for detection of K10: a, a´) control (untreated cells), b, b´ 10 μg/mL heparin-treated group, c, c´ 20 μg/mL heparin-treated group, d analysis of K10 marker expression of the hAECs treated with heparin. *p < 0.05
a–i Flow cytometer analysis of keratinocyte markers K14 and K10 in the hAECs treated with BMP4 and heparin after 21 days. Flow cytometry diagrams for detection of K14 and K10 markers: a, a´ K14 control (untreated cells), b, b´ K14 marker expression in heparin-treated group of AECs, c, c´ K14 marker expression in heparin+ BMP4-treated group of AECs, d, d´ K14 marker expression in BMP4-treated group of AECs. e, e´ K10 control (untreated cells), f, f´ K10 marker expression in heparin-treated group of AECs, g, g´ K10 marker expression in heparin+ BMP4-treated group of AECs, h, h´ K10 marker expression in BMP4-treated group of AECs, i analysis of K14 and K10 marker expression of the hAECs treated with BMP4 and heparin after 21 days. *p < 0.05, **p < 0.001
Simultaneous expression of Map2, Olig2, and K14 markers in hAECs
The effect of 25 ng/mL BMP-4 on simultaneous expression of Map2, Olig2, and K14 markers was detected with the three color flow cytometry after 21 days of differentiation. The results indicated that 29.77% of differentiated AECs co-expressed markers Map2, Olig2, and K14 in the same cell population (Fig. 7).
Dedifferentiation of hAECs after culture
The results showed that the fresh isolated hAECs endogenously express the neural and keratinocyte markers. On the day of isolation, expressions of MAP-2, Olig-2, and K14 were 14.3%, 9.92%, 27%, respectively. Based on the results, the culture protocol (without growth factors) decreased the number of mentioned markers in hAECs. At day 21 of culture, less than 1% of the cultured cells expressed markers MAP-2, Olig-2, and K14, as shown in Fig. 8.
Discussion
In this study, we investigated the effect of BMP signaling and its inhibition on neuronal, glial, and keratinocyte differentiation of human AECs. Noggin (as an antagonist of BMP4) was used to induce neuronal differentiation of hAECs through inhibition of BMP signaling.
The results demonstrated that treatment of cultured hAECs with two concentrations of noggin effectively promoted MAP2 and β-tubulin neuronal marker expression. This result is consistent with previous studies which showed the induced neural differentiation of stem cells by Noggin (Fazeli et al. 2015; Gerrard et al. 2005; Morell et al. 2015). The data of the current study demonstrate that the differentiation is not depending on the concentrations of Noggin. However, it was reported that different concentrations of Noggin had different effects on neural differentiation of PB-MSCs (Fazeli et al. 2016). It seems that the concentration-dependent effect of noggin may be related to the type of the cells. The selection of dose for noggin was based on previous studies; in one of them, we evaluate 100 ng/mL of noggin for neural differentiation (Niknejad et al. 2010). In the current study, the concentration 50 ng/mL was also included. Therefore, in this study, we compared three concentrations of 0, 50, and 100 ng to differentiate human amniotic epithelial cells into neuronal lineage. Moreover, the same concentrations of noggin were used by the other researchers (Fazeli et al. 2015; Seifi et al. 2014). The same applied to BMP and the effects of concentrations 0, 25, and 50 were compared in this study which was consistent with previous studies (Armenteros et al. 2018; Zhu et al. 2019). However, the optimum concentrations should be obtained based on the cell type which would be evaluated in the future studies.
In this research, we investigated olig-2 (as an oligodendrocyte marker) expression with two BMP-4 concentrations (25 and 50 ng/mL). We observed that BMP-4 increased the level of olig-2 and MBP expression, but the difference between two concentrations was not significant. Our data is consistent with the previous studies that have shown that BMP-2 and -4 stimulate neurogenesis in cultured neural crest stem cells (Kléber et al. 2005; Le Douarin and Dupin 2003; Kessaris et al. 2007).
Since the previous studies have shown that activation of BMP signaling caused epidermal differentiation (van Eijl et al. 2018), we examined the effects of BMP4 on differentiation of AECs to keratinocytes. The results demonstrated that treatment of cultured hAECs with BMP4 effectively promoted K14 and K10 expression. As majority of keratinocyte differentiation factors such as EGF act through heparin binding protein and heparin has shown keratinocyte differentiation (Hasegawa et al. 2016; Shirakata et al. 2005), it was also used in order to investigate its effect on keratinocyte differentiation of AECs. Our results demonstrated that the cultured AECs with heparin-supplemented differentiation medium showed K14 and K10 expression but what was interesting here was that combination of BMP4 and heparin does not possess additive or synergistic effects on keratinocyte differentiation and there is no significant difference in K14 and K10 expression levels in either presence or absence of BMP4. This result can be explained through affecting both BMP and heparin on the same intracellular target. It has frequently been shown that treatment of stem cells with BMP-4 increased Smad1/5/8 phosphorylation. Moreover, the time-dependent effects of heparin have been shown on SMAD1/5/8 phosphorylation (Chang et al. 2009; Kanzaki et al. 2011; Paulsen et al. 2011; Zhao et al. 2006; Poli et al. 2011; Zhu et al. 2014). Therefore, it seems that this result is due to the fact that both heparin and the BMP4 affect the similar target SMAD1/5/8 pathway in keratinocyte differentiation of hAECs. However, further research will be required to determine the exact intracellular target of heparin and BMP4 in differentiation process of amnion-derived cells.
The effect of BMP-4 on the simultaneous expression of Map2, Olig2, and K14 markers was investigated after 21 days of differentiation. Data revealed that about 27% of the mentioned markers co-expressed after differentiation in the same cell population of AECs. This result was consistent with the previous studies which showed that during embryonic development, the stem cells can transit between the two cell states and co-expressed embryonic developmental markers (Acloque et al. 2009; Adamzyk et al. 2016).
The results showed that differentiated cells with mentioned growth factors were viable after 21 days. It seems that activation and inhibition of BMP signaling involved in hAECs neuro-ectodermal differentiation process though they have no effects on viability of these cells.
Another result of this study showed that MAP2, olig2, and K14 marker expression was significantly decreased in hAECs after 21 days of culture (without BMP4, noggin, heparin). Since cell viability did not decrease, the reduction of the markers can be explained due to reversion of the cells which supports the hypothesis that AECs can be dedifferentiated to pluripotent stem cells. Consistently, we observed increased expression of Oct-4 and Nanog after 7 days of culturing of hAECs, as shown in our previous study (Niknejad et al. 2012). hAECs endogenously express OCT4, SOX2, KLF4, and c-MYC, four key factors used in cellular reprogramming (Easley IV et al. 2012). Therefore, we concluded that hAECs became dedifferentiated and they have the ability to be suitable source of iPSCs (induced pluripotent stem cells).
In conclusion, the results of the current study showed that BMP signaling and its inhibition play a critical role in differentiation of hAECs into ectodermal lineage and hAECs is a proper candidate for cell therapy in skin and neural degenerative diseases. However, further studies would be required to investigate more specific ectodermal markers on gene and protein expression levels.
References
Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA (2009) Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest 119:1438–1449
Adamzyk C, Labude N, Schneider R, Jaekel J, Hoffmann V (2016) Human umbilical cord-derived mesenchymal stem cells spontaneously form 3D aggregates and differentiate in an embryoid body-like manner. J Stem Cell Res Ther 1:00037
Armenteros T, Andreu Z, Hortigüela R, Lie DC, Mira H (2018) BMP and WNT signalling cooperate through LEF1 in the neuronal specification of adult hippocampal neural stem and progenitor cells. Sci Rep 8:9241
Azizian S, Hadjizadeh A, Niknejad H (2018) Chitosan-gelatin porous scaffold incorporated with chitosan nanoparticles for growth factor delivery in tissue engineering. Carbohydr Polym 202:315–322
Chang S-F, Chang T-K, Peng H-H, Yeh Y-T, Lee D-Y, Yeh C-R, Zhou J, Cheng C-K, Chang CA, Chiu J-J (2009) BMP-4 induction of arrest and differentiation of osteoblast-like cells via p21CIP1 and p27KIP1 regulation. Mol Endocrinol 23:1827–1838
Easley CA IV, Miki T, Castro CA, Ozolek JA, Minervini CF, Ben-Yehudah A, Schatten GP (2012) Human amniotic epithelial cells are reprogrammed more efficiently by induced pluripotency than adult fibroblasts. Cell Rep 14:193–203
Fatimah SS, Tan GC, Chua K, Tan AE, Azurah AGN, Hayati AR (2013) Effects of keratinocyte growth factor on skin epithelial differentiation of human amnion epithelial cells. Burns 39:905–915
Fazeli Z, Ghaderian SMH, Rajabibazl M, Salami S, Shiran NV, Omrani MD (2015) Expression pattern of neuronal markers in PB-MSCs treated by growth factors noggin, bFGF and EGF. Int J Mol Cell Med 4:209
Fazeli Z, Omrani MD, Ghaderian SMH (2016) CD29/CD184 expression analysis provides a signature for identification of neuronal like cells differentiated from PBMSCs. Neurosci Lett 630:189–193
Gerrard L, Rodgers L, Cui W (2005) Differentiation of human embryonic stem cells to neural lineages in adherent culture by blocking bone morphogenetic protein signaling. Stem Cells 23:1234–1241
Hasegawa T, Kadono N, Kuriyama K, Yano H, Nakashima A, Hirai Y (2016) Inductive influence of heparin-like polysaccharide on the keratinocyte differentiation. J Dermatol Sci 84:e42
Ilancheran S, Moodley Y, Manuelpillai U (2009) Human fetal membranes: a source of stem cells for tissue regeneration and repair? Placenta 30:2–10
Kanzaki S, Ariyoshi W, Takahashi T, Okinaga T, Kaneuji T, Mitsugi S, Nakashima K, Tsujisawa T, Nishihara T (2011) Dual effects of heparin on BMP-2-induced osteogenic activity in MC3T3-E1 cells. Pharmacol Rep 63:1222–1230
Kessaris N, Pringle N, Richardson WD (2007) Specification of CNS glia from neural stem cells in the embryonic neuroepithelium. Philos Trans R Soc B Biol Sci 363:71–85
Kléber M, Lee H-Y, Wurdak H, Buchstaller J, Riccomagno MM, Ittner LM, Suter U, Epstein DJ, Sommer L (2005) Neural crest stem cell maintenance by combinatorial Wnt and BMP signaling. J Cell Biol 169:309–320
Le Douarin NM, Dupin E (2003) Multipotentiality of the neural crest. Curr Opin Genet Dev 13:529–536
Liu YH, Vaghjiani V, Tee JY, To K, Cui P, Oh DY, Manuelpillai U, Toh B-H, Chan J (2012) Amniotic epithelial cells from the human placenta potently suppress a mouse model of multiple sclerosis. PLoS One 7:e35758
Mahmood R, Choudhery MS, Mehmood A, Khan SN, Riazuddin S (2015) In vitro differentiation potential of human placenta derived cells into skin cells. Stem Cells Int
Maymó JL, Riedel R, Pérez-Pérez A, Magatti M, Maskin B, Dueñas JL, Parolini O, Sánchez-Margalet V, Varone CL (2018) Proliferation and survival of human amniotic epithelial cells during their hepatic differentiation. PLoS One 13:e0191489
Miki T (2011) Amnion-derived stem cells: in quest of clinical applications. Stem Cell Res Ther 2:25
Miki T, Lehmann T, Cai H, Stolz DB, Strom SC (2005) Stem cell characteristics of amniotic epithelial cells. Stem Cells 23:1549–1559
Mira H, Andreu Z, Suh H, Lie DC, Jessberger S, Consiglio A, San Emeterio J, Hortigüela R, Marqués-Torrejón MÁ, Nakashima K (2010) Signaling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampus. Cell Stem Cell 7:78–89
Morell M, Tsan Y-c, O'Shea KS (2015) Inducible expression of noggin selectively expands neural progenitors in the adult SVZ. Stem Cell Res 14:79–94
Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM (2008) Properties of the amniotic membrane for potential use in tissue engineering. Eur Cells Mater 15:88–99
Niknejad H, Peirovi H, Ahmadiani A, Ghanavi J, Jorjani M (2010) Differentiation factors that influence neuronal markers expression in vitro from human amniotic epithelial cells. Eur Cell Mater 19:22–29
Niknejad H, Deihim T, Ahmadiani A, Jorjani M, Peirovi H (2012) Permanent expression of midbrain dopaminergic neurons traits in differentiated amniotic epithelial cells. Neurosci Lett 506:22–27
Parolini O, Alviano F, Bagnara GP, Bilic G, Bühring HJ, Evangelista M, Hennerbichler S, Liu B, Magatti M, Mao N (2008) Concise review: isolation and characterization of cells from human term placenta: outcome of the first international workshop on placenta derived stem cells. Stem Cells 26:300–311
Patthey C, Gunhaga L (2014) Signaling pathways regulating ectodermal cell fate choices. Exp Cell Res 321:11–16
Paulsen M, Legewie S, Eils R, Karaulanov E, Niehrs C (2011) Negative feedback in the bone morphogenetic protein 4 (BMP4) synexpression group governs its dynamic signaling range and canalizes development. Proc Natl Acad Sci 108:10202–10207
Poli M, Girelli D, Campostrini N, Maccarinelli F, Finazzi D, Luscieti S, Nai A, Arosio P (2011) Heparin: a potent inhibitor of hepcidin expression in vitro and in vivo. Blood 117:997–1004
Qu Y, Zhou B, Yang W, Han B, Yu-Rice Y, Gao B, Johnson J, Svendsen CN, Freeman MR, Giuliano AE (2016) Transcriptome and proteome characterization of surface ectoderm cells differentiated from human iPSCs. Sci Rep 6:32007
Seifi T, Ghaedi K, Tanhaei S, Karamali F, Kiani-Esfahani A, Peymani M, Baharvand H, Nasr-Esfahani M-H (2014) Identification, cloning, and functional analysis of the TATA-less mouse FNDC5 promoter during neural differentiation. Cell Mol Neurobiol 34:715–725
Shirakata Y, Kimura R, Nanba D, Iwamoto R, Tokumaru S, Morimoto C, Yokota K, Nakamura M, Sayama K, Mekada E (2005) Heparin-binding EGF-like growth factor accelerates keratinocyte migration and skin wound healing. J Cell Sci 118:2363–2370
Si J, Dai J, Zhang J, Liu S, Gu J, Shi J, Shen SG, Guo L (2015) Comparative investigation of human amniotic epithelial cells and mesenchymal stem cells for application in bone tissue engineering. Stem Cells Int
van Eijl RE, van Buggenum JA, Tanis SE, Hendriks J, Mulder KW (2018) Single-cell ID-seq identifies BMP signaling as a driver of a late stage epidermal differentiation program. bioRxiv:350850
Wang G, Zhao F, Yang D, Wang J, Qiu L, Pang X (2018) Human amniotic epithelial cells regulate osteoblast differentiation through the secretion of TGFβ1 and microRNA-34a-5p. Int J Mol Med 41:791–799
Yu S, Xu Y, Li Y, Xu B, Sun Q, Li F, Zhang X (2015) Construction of tissue engineered skin with human amniotic mesenchymal stem cells and human amniotic epithelial cells. Eur Rev Med Pharmacol Sci 19:4627–4635
Zhang Q, Bu S, Sun J, Xu M, Yao X, He K, Lai D (2017) Paracrine effects of human amniotic epithelial cells protect against chemotherapy-induced ovarian damage. Stem Cell Res Ther 8:270
Zhao B, Katagiri T, Toyoda H, Takada T, Yanai T, Fukuda T, Chung U-i, Koike T, Takaoka K, Kamijo R (2006) Heparin potentiates the in vivo ectopic bone formation induced by bone morphogenetic protein-2. J Biol Chem 281:23246–23253
Zhu X-J, Liu Y, Dai Z-M, Zhang X, Yang X, Li Y, Qiu M, Fu J, Hsu W, Chen Y (2014) BMP-FGF signaling axis mediates Wnt-induced epidermal stratification in developing mammalian skin. PLoS Genet 10:e1004687
Zhu S, Liu W, Ding HF, Cui H, Yang L (2019) BMP4 and Neuregulin regulate the direction of mouse neural crest cell differentiation. Experimental and therapeutic medicine 17:3883–3890
Acknowledgments
The authors would like to thank the operation room personnel of Erfan hospital for their assistance in this research. The results of this paper were extracted from a part of PhD thesis of F. Biniazan, A. Manzari, and F. Safaeinejad.
Funding
Research reported in this publication was supported by Research Grant Committee from the National Institutes for Medical Research Development (NIMAD), Tehran, Iran, under award number 963951. This work was supported by a grant from the Iran National Science Foundation (INSF).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
In this research, human placenta were obtained from healthy mothers after obtaining informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Felor Biniazan, Asma Manzari-Tavakoli, and Fahimeh Safaeinejad are alphabetically ordered co-first authors
Rights and permissions
About this article
Cite this article
Biniazan, F., Manzari-Tavakoli, A., Safaeinejad, F. et al. The differentiation effect of bone morphogenetic protein (BMP) on human amniotic epithelial stem cells to express ectodermal lineage markers. Cell Tissue Res 383, 751–763 (2021). https://doi.org/10.1007/s00441-020-03280-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-020-03280-z