Abstract
The focus of immunotherapeutics has been placed firmly on anti-tumour T cell responses. Significant progress has been made in the treatment of both local and systemic malignancies, but low response rates and rising toxicities are limiting this approach. Advancements in the understanding of tumour immunology are opening up a new range of therapeutic targets, including immunosuppressive factors in the tumour microenvironment. Macrophages are a heterogeneous group of cells that have roles in innate and adaptive immunity and tissue repair, but become co-opted by tumours to support tumour growth, survival, metastasis and immunosuppression. Macrophages also support tumour resistance to conventional therapy. In preclinical models, interference with macrophage migration, macrophage depletion and macrophage re-education have all been shown to reduce tumour growth and support anti-tumour immune responses. Here we discuss the role of macrophages in prognosis and sensitivity to therapy, while examining the significant progress which has been made in modulating the behaviour of these cells in cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The potential of utilizing the host immune system to eradicate cancers has been hotly debated over the course of the last century. Many doubted the ability to prime the host immune system to a tumour which has already successfully evaded detection and generated a profoundly immunosuppressive tumour microenvironment (TME). Over the last two decades a range of immunotherapies have made it to the clinic, clearly proving the point of principle, but the ability of immunotherapy to target more aggressive and less immunogenic tumours is still in doubt [1].
To comprehend the limitations of current T cell immunotherapeutics, namely T cell checkpoint inhibitors (CPIs), which skew the balance of stimulatory and inhibitory signals, it can be useful to imagine the tumours as exerting an immunosuppressive force, and the immune system as having a finite immune potential (Fig. 1). Immunosuppression will rise with cancer progression and possibly plateau, but at a level both beyond the limit of the normal immune potential and even further from the immune potential of an immunocompromised cancer patient, thus even with a plentiful supply of neoantigens the immune system is rendered ineffective. CPIs function to boost the immune potential of the host to a point at which it can feasibly compete and overcome the immunosuppression generated by the tumour. While this is desirable in an anti-cancer context, the effect of such an untethered immune response in the host can have serious deleterious effects beyond the tumour [2, 3].
The major successes in immunotherapies for cancer patients have relied upon the direct modulation of T cell activation, either by targeting T cell costimulatory proteins such as CTLA-4 and PD-1, or by adoptive T cell transfer using ex vivo T cell activation. However, the side effects associated with these drugs appear to be dose-dependant, cumulative with previous cycles of therapy and additive with other similar regimes. This apparent limit has led to a shift in research to identify suitable complimentary therapies that kill tumour cells in a way which primes the TME for T cell activation by inducing immunogenic forms of cell death [4, 5].
Developments in the field of immunology, and the elucidation of the myriad of components interacting in the TME, are leading to the development of a new range of immunotherapeutics that focus on an expanding set of targets with therapeutic and diagnostic potential.
In contrast to many approved immunotherapeutics that boost the immune potential, one interest has been in trying to actively reverse the immunosuppression generated by the tumour by disrupting immunosuppressive factors in the TME or by disrupting cells normally co-opted by tumours.
One specific vein of research has focused on a subset of the myeloid cell compartment comprising the monocyte–macrophage lineage which can be subverted and recruited to the tumour as tumour associated macrophages (TAMs). While TAMs can comprise up to 50% of the tumour mass, they have been less intensively studied than other immune subsets [6]. There is a growing body of literature showing their prognostic value, and they are emerging as promising therapeutic targets in oncology.
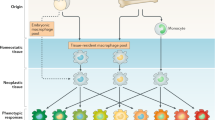
Synopsis of macrophage origin and classification
TAMs are predominantly derived from circulating populations of monocytes. As a simplified paradigm, macrophages have been categorized as classically activated M1 (inflammatory) which are anti-tumour, or alternatively activated M2 (wound repair) which are pro-tumour. The M1 M2 dichotomy was developed by in vitro observations but recent advances have led to a more complex spectrum of activation states. Both monocyte and macrophage populations frequently display hybrid M1/M2 phenotypes, or phenotypes that cannot be adequately defined using the M1:M2 system [7]. It has been identified that the M1/M2 system is leading to confusion and inconsistency between researchers and ultimately impeding progress [8].
Others advocate the use of in vivo function to classify M1 M2 macrophages, focusing on the iNOS (M1): arginase (M2) ratio. With cells being defined as inhibitors of cell growth and killers or as promoters of cell proliferation and wound repair (Fig. 2) [9]. Flow cytometry has, however, led to the distinction of a range of macrophage and monocyte types based on their relative expression of various cell surface markers.
Synopsis of M1:M2 macrophage dichotomy. CD11b monocytes (MO) can mature with a heterogeneity of phenotypes which together represent a spectrum with M1 and M2 macrophages representing the two extremes of that spectrum. In vitro, IFNγ, LPS and TNFα drive M1 polarization whereas IL-4, IL-10 and IL-13 drive M2 polarization. M1 macrophages express CD68, CD11b, CD38. CD16/32, MHC II and CD80/86, their primary function is dependent on the expression and function of inducible nitric oxide synthase (iNOS) which results in the extracellular accumulation of nitric oxide (NO) and citrulline which, along with other cytokines, can drive cytotoxic anti-tumour Th1 responses. M2 macrophages express CD68, CD11b, CD163, CD206, Galectin 3 and Egr2, their primary function is dependent on the expression and function of arginase which results in the extracellular depletion of arginine and the accumulation of ornithine and urea which are key to wound repair mechanisms but can also promote immune suppression and tumour progression
From a clinical perspective, the study of macrophages faces a unique challenge, in that we find it more amenable to study discretely defined subsets of cells, but it is becoming increasingly evident that this is not possible with such a heterogeneous set of cells. While many continue to report based on two distinct subtypes, it is important to remember that the activation states of macrophages incorporate discrete populations and spectrums or continuums where cells can adopt hybrid states [10].
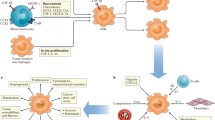
Contribution to tumourigenicity
Macrophages have been implicated in all aspects of tumour growth and spread, but they are also known to be critical mobilizers of the adaptive immune system (Fig. 3). As such they play an enigmatic role in tumour development and the generation of anti-tumour responses.
Synopsis of pro- and anti- tumoural effects exerted by macrophages. Key enzymes and cytokines produced by M1 and M2 macrophages that have the effect of driving or inhibiting cancer progression. M1 cells can drive inflammation and cytotoxic Th1 responses while M2 cells can produce factors such as vascular endothelial growth factor (VEGF) and Prostaglandin E2 (PGE2), and are involved in the depletion of activated T cells, recruitment of regulatory T cells, tissue remodelling, angiogenesis and tumour progression
In line with their roles in immune stimulation and antigen presentation, there is evidence high macrophage infiltration in the early stages of tumour growth can result in tumour destruction while low levels of infiltration support tumour growth [11, 12]. Macrophages can promote anti-tumour responses but advanced tumours have been shown to polarize TAMs into an M2-like phenotype [13].
Tumours can secrete a range of chemoattractants that promote recruitment of monocyte and macrophage populations [14]. TAMs become co-opted to promote tumour cell proliferation and survival, tumour vascularization and immunosuppression along with supporting extravasation and growth of tumour cells at distal sites [15].
The importance of TAMs is evident across the literature, they can affect patient prognosis and determine sensitivity to a range of therapies. Preclinical studies and early stage clinical trials have implicated them as prime therapeutic targets [16, 17].
Effect of macrophage infiltration and polarization on patient prognosis
Prognostic significance of circulating and infiltrating macrophages
A high density of macrophage infiltration into the tumour has been cited as a negative prognostic indicator in a range of solid and haematological malignancies (Tables 1, 2). Colorectal cancer displays a contrasting trend whereby high macrophage infiltration can result in increased patient survival.
Arguably the most robust prognostic evidence is available for breast cancer and Hodgkin’s Lymphoma. A distinct gene signature in breast cancer has shown high macrophage density is prognostic if combined with a high CD4+ helper T cells to cytotoxic T cell ratio. The signature closely correlated to the development of secondary tumours that could accurately predict survival in women after complete resection [18]. A macrophage gene signature has been developed for Hodgkin’s Lymphoma that can accurately predict survival and response to therapy, indicating that the pro-tumour effect of macrophages is not restricted to solid tumours [19].
Prognostic significance of histologic localization
Histologic examination of colorectal cancer, for which TAM infiltration is a positive prognostic indicator, revealed infiltration at the tumour front in colon cancer leads to enhanced survival and reduced liver metastasis, irrespective of CD8 T cell infiltration [20,21,22,23]. The proximity of the TME to the intestinal microbiome has been hypothesized as a potential explanation for the differential behaviour of macrophages in colorectal cancer. It is possible that the continuous supply of pathogen-associated molecular patterns (PAMPs) available to macrophages may outweigh the ability of the tumour to polarize the cells to an M2-like phenotype. This hypothesis may also explain why similar results have been seen in gastric cancer, in which tumours may have varying access to the intestinal microbiome depending on the localization of the tumour. Thus, high infiltration of macrophages in the tumour nests in gastric cancer is associated with enhanced antigen presentation and T cell activation, and a positive prognosis [24].
The histological localization of macrophages in breast cancer has shown no correlation with prognosis, while in endometrial cancer high TAM infiltration into the tumour hotspot (tumour core of necrotic cells) is associated with advanced clinical staging, myometrial invasion and histological differentiation [25,26,27]. Characterization in other tumour types is warranted.
Prognostic significance of polarized macrophages
It is possible the results of many studies were adversely affected by failure to distinguish pro- and anti-tumour populations. When differentiated in non-small cell lung cancer (NSCLC), it was found that high M1-like macrophage infiltration was associated with prolonged survival, while the level of M2-like infiltration had no impact on survival [28, 29]. This is in contrast to an earlier meta-study examining the prognostic relevance of overall CD68+ infiltration in NSCLC that found no link with OS [30].
Similarly, in patients with hepatocellular carcinoma after curative resection, high numbers of CD11c+ dendritic cells and low numbers of CD206+ macrophages correlated with extended OS, whereas CD68+ TAM infiltration displayed no prognostic significance [31]. In ovarian cancer there is inconsistent evidence on the prognostic effect of CD68+ cell infiltration, however, differentiation of the populations revealed that a high M1-like:M2-like ratio is prognostically favourable [32,33,34,35]. Together these data indicate whole macrophage counts used to explore the prognostic effects in other cancers may not accurately reflect the true trend or scale of the effect imposed by pro-tumourigenic macrophage populations. The ability to draw robust prognostic indications from TAM frequency emphasizes their central role in disease progression.
Role of macrophages in therapeutic response
Effect of chemotherapy on macrophages
Conventional chemotherapies are considered immunosuppressive due to toxic systemic effects on rapidly proliferating leukocytes and bone marrow progenitors resulting in leukocyte depletion. Chemotherapy has also been shown to stimulate the secretion of colony stimulating factor 1 (CSF1) by tumour cells, which is a potent chemoattractant for macrophages, and results in an accumulation of TAMs in the TME which contribute to chemoresistance [36].
Prognostic significance of macrophages in response to chemotherapy
High levels of infiltrating CD68+ and CD163+ cells are a negative prognostic marker for patients with esophageal cancer undergoing pre-operative neoadjuvant chemotherapy and indicates patients are less likely to respond to chemotherapy [37]. The CD8:CD68 cell ratio is a predictive biomarker for response to neoadjuvant chemotherapy in breast cancer patients [18, 38]. These effects were found to be at least in part due to the upregulation of CSF1 by tumour cells in response to CT. A high density of CD163+ cells at the invasive front in oral squamous cell carcinoma was found to correlate to a poorer outcome after surgery following 5-FU based chemoradiotherapy [39].
On examination of the histologic localization of macrophages, CD68+ in the parenchyma negatively correlated to lymphatic metastasis after neoadjuvant chemotherapy, in contrast to the number in the dense fibrous stroma which directly correlated to the number of positive lymph nodes, indicating the role of macrophages depends on intratumoural localization in breast cancer [38].
The role of macrophages in chemoresistance
Macrophages are central coordinators of immune responses during chemotherapy [40]. Blockade of macrophage recruitment increased the efficacy of paclitaxel in breast cancer, resulting in diminished growth of both primary and metastatic tumours [18]. Suppression of CD8+ effector T cells by the production of IL-10 has been shown to reduce anti-cancer cytotoxicity [41]. IL-10 production by macrophages also limits the efficacy of chemotherapy in breast cancer and was subsequently shown to indirectly enhance tumour growth by down regulating IL-12 production by DCs which is required for cytotoxic CD8+ T cell responses.
Macrophages are critical mediators of wound and tissue repair and it is possible that these functions can be naturally adapted by the tumour to generate chemotherapeutic resistance. M2-like macrophages derived from THP-1 cells, were shown to reduce apoptosis in addition to enhancing tissue repair and angiogenesis in response to etoposide, a topoisomerase inhibitor [42, 43].
Both macrophage depletion and re-education to an M1 state have been shown to increase the efficacy of chemotherapy [44,45,46]. The induction of M1 polarization using host-produced histidine-rich glycoprotein to reduce signalling by the M2 driver PIGF has been shown to restore sensitivity to chemotherapy, reduce tumour growth and reduce metastasis, indicating that M1 polarization can combat all major aspects of disease [44].
Macrophage modulating therapies have an advantage over many other immunotherapeutics because they can be used to synergistically improve outcome with chemotherapy, whereas the results of combining CPIs with chemotherapy have shown very little or no effect on OS or quality of life [18].
Effect of radiotherapy on macrophages
Conventional fractionated radiotherapy is considered immunosuppressive, as radiation primarily leads to apoptotic cell death, but it can also lead to necrotic cell death and mitotic catastrophe [47, 48].
The accumulation of macrophages in the TME after radiotherapy is due to the ability of MOs to survive clinically relevant doses of radiotherapy coupled with an influx of monocytes after radiotherapy [49, 50]. While this may seem attractive in the generation of an abscopal effect, there is much research showing that the influx of monocytes and macrophages is responsible for therapy failure due to their role in vasculogenesis and angiogenesis [51, 52].
Role of macrophages in radioresistance
Murine models of oral and brain cancer have shown macrophages infiltrating the tumour after radiotherapy were primarily M2-like and supported vasculogenesis and tumour growth [52,53,54,55].
Curiously, ionizing radiation skews macrophages from an M2-like to an M1-like phenotype, suggesting an enigmatic role of macrophages in radiotherapy [56]. Characterization of the TME post irradiation reveals decreased levels of the anti-inflammatory markers CD163, IL-10, VCAM-1, and MRC1 while significantly increasing the inflammatory markers iNOS, CD80, CD86 and HLA-DR [57]. However, irradiated macrophages were still able to enhance tumour cell invasion and supported the angiogenic process of tumour cells indicating the retention of M2-like traits. Blocking macrophage influx into the TME after RT has been shown to enhance response in murine models [49].
Prognostic significance of macrophages in response to radiotherapy
Prognostically, there is limited evidence on the effect of macrophages in patients undergoing radiotherapy. Macrophages have been shown to predict response to short course pre-operative radiotherapy for colon cancer, with data suggesting a high infiltration of M1-like macrophages is likely to result in a reduced response, no effect was seen by M2-like macrophages [58].
Role of macrophages in response to checkpoint inhibitors
CPIs have been the most notable achievement in the development of immunotherapy for cancer patients, but there has been limited interest in the role of myeloid cells in their clinical application to date.
Macrophages are key coordinators of adaptive immune responses, and express a range of T cell costimulatory and co-inhibitory molecules, known as the B7 family [59]. Crosstalk between tumour cells and macrophages can regulate the expression of B7 family molecules on both tumour cells and macrophages [60]. The TME is abundant in IL-10 and TNF-α, which can both upregulate PD-L1 expression on macrophages, via STAT3 signalling, which is responsible for the inactivation and depletion of activated T cells [61,62,63]. PD-L1 has been implicated as a major signalling molecule associated with immune escape by tumours [64].
In addition to their role in facilitating T cell responses, macrophages are critical mediators of many therapeutics that employ antibodies with fully humanized Fc domains. While the primary function of antibodies is the activation or neutralization of their targets, the choice of antibody Fc domains are known to influence their efficacy. CD16, the receptor for IgG1 is expressed primarily by macrophages and NK cells and is responsible for the neutralization of antibody targets via antibody-dependent cellular cytotoxicity (ADCC) or phagocytosis [65, 66]. The capacity to generate ADCC responses is dependent on two variables. Firstly, the ability of the Ab used to bind FC receptors, and secondly on the activation state of the FcR expressing cell.
Anti-CTLA4
Ipilimumab is a fully human IgG1 mAb that interacts with FcγRIIIA (CD16) expressing cells. Ipilimumab efficacy relies on two mechanisms. Firstly, interference with CTLA4 binding on effector T cells, and secondly, FcγR mediated depletion of Tregs by ADCC [67, 68].
In a small study of 29 patients receiving Ipilimumab for the treatment of melanoma, responders had a higher number of CD68+CD163+ macrophages in the TME before treatment and decreased Treg infiltration after therapy. Responders had the highest level of circulating non-classical CD16+CD14low macrophages at baseline [69]. In a study of 209 melanoma patients receiving Ipilimumab, low absolute monocyte counts and low circulating Lin−CD14+HLA-DR−/low MDSCs were significantly associated with improved survival [70]. These studies indicate macrophages play an active role in response.
Anti-PD-1/PD-L1
Both PD-1 and PD-L1 are expressed by macrophages, and as such the effect of these neutralizing antibodies may have a depletory effect on macrophage numbers. PD-1 is expressed on infiltrating macrophages and lymphocytes of melanoma patients responding to anti-PD-1 therapy [71]. Response was primarily correlated to the proliferation of intratumoural CD8+ T cells and the role of PD-1+ macrophages was not examined. A reduction of the proinflammatory cytokine CCL3 is associated with prolonged survival in metastatic renal cell carcinoma patients receiving Atezolizumab [71, 72].
Evaluation of immunologic correlates during CPI administration is required to improve our understanding of the biology of response and development of resistance. Due to the very limited number of patients receiving CPIs, our understanding of the global effect of CPIs on non-T cell immune subsets is still in its infancy.
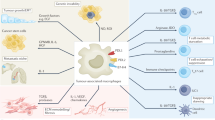
Macrophage modulation in cancer
A wide range of efforts have been made to enhance anti-tumour responses by modulating the behaviour of macrophages. These can be distinguished into three groups:
-
(1)
Skewing of monocyte/macrophage polarization.
-
(2)
Inhibition of macrophage migration to the TME.
-
(3)
Depletion of monocyte/macrophage populations.
Interest has been shown in a wide range of modulatory mechanisms with varying degrees of success. The most promising include granulocyte–macrophage colony-stimulating factor (GM-CSF), the CCL2/CCR2 axis and the CSF1/CSF1 receptor (CSF1R) axis.
Treatment with GM-CSF
GM-CSF promotes the expansion of granulocytes and monocytes, polarizes macrophages to an M1-like anti-tumour phenotype and can skew cells towards a type one phenotype capable of driving anti-tumour Th1 responses [73,74,75,76,77]. GM-CSF has been approved for the second line treatment of paediatric high-risk neuroblastoma in combination with IL-2 and 13-cis-retinoic acid, and has been recommended for the amelioration of febrile neutropenia in solid and haematological malignancies by The American Society of Clinical Oncology [78].
There is currently a phase 2/3 trial in the recruitment phase examining the administration of recombinant GM-CSF, BCG and 4 lethally irradiated melanoma cell lines for the treatment of pre-malignant melanoma (NCT01729663).
Sipuleucel-T is a therapeutic vaccine approved for castration-resistant prostate cancer, composed of autologous PBMCs cultured ex vivo with PAP-GM-CSF. Despite gaining approval, it only modestly enhanced OS (25.8 vs. 21.7 months) with no improvement in time to progression [79]. GVAX is a vaccine comprised of a patient’s own cancer cells stimulated to secrete GM-CSF and then irradiated to prevent further proliferation. GVAX has recently been given breakthrough designation for pancreatic cancer in combination with CRS-207, a listeria-based therapeutic vaccine, after positive phase 2 results. Interestingly, GVAX has been shown to induce PD-L1 positive ‘post-immunotherapy lymphoid aggregates’ in murine models of pancreatic adenocarcinoma that may prime the tumour into an immunogenic state [80, 81]. Building on that work the authors performed an early stage clinical trial with GVAX and Ipilimumab which showed clinical benefit [82]. These studies were performed before the approval of anti-PD-1 antibodies, and it is likely this combination will offer enhanced outcomes. A clinical trial is now recruiting (NCT02648282).
There have been fears surrounding the administration of GM-CSF due to observations of constitutive GM-CSF expression by advanced cancers [83]. GM-CSF can induce pleiotropic effects depending on its concentration and receptor, including differing effects on survival and proliferation. Tumour cells can utilize GM-CSF in an autocrine or paracrine mechanism to stimulate growth and proliferation [84, 85].
Rationale for the modulation of the CCL2/CCR2 axis
CCR2 is a chemokine receptor present on inflammatory monocytes that it is required for mobilization from the bone marrow and recruitment to the TME. Tumours can upregulate CCL2 expression, its cognate ligand, from both tumour cells and stromal cells resulting in an upregulation of CCR2+ inflammatory monocytes and matrix metallopeptidase 9+ (MMP-9) neutrophil infiltration [86,87,88,89,90,91,92,93].
CCL2 has been shown to increase the survival of PBMCs and clearance of apoptotic cells which may be beneficial in an anti-tumour context,; however, CCL2 also drives M2 polarization suggesting it is more likely to play a negative role in cancer patients [94, 95]. Inhibition of the CCL2/CCR2 pathway has been shown to potently inhibit the development of metastasis in murine models of hepatocellular carcinoma, breast and prostate cancer [96,97,98,99]. Murine models of pancreatic ductal adenocarcinoma (PDAC) have shown that CCR2 inhibitors can induce a 3-fold reduction in tumour burden [100].
Both chemotherapies and radiotherapy have been shown to upregulate CCL2 production by tumour cells and stromal cells [101, 102]. Addition of anti-CCL2 antibodies is additive with chemotherapy in models of ovarian and prostate cancer, and with radiotherapy in models of PDAC [98, 103,104,105].
Prognostic significance of CCL2 and CCR2
CCL2 expression has been linked to cancer progression in hepatocellular carcinoma, prostate cancer, colorectal cancer, breast cancer and gastric cancer and has been shown to promote the induction of tumour growth, tumour cell migration, neovascularization and metastasis [88, 92, 97, 106,107,108,109,110,111,112,113,114,115,116,117]. Prognostically, high CCL2 in combination with VEGF in tumour conditioned media has been shown to increase the chance of early relapse in breast cancer [118]. High intratumoural CCL2 expression is related to a lower 5-year survival (5YS) in gastric cancer [119]. Intratumoural expression of both CCL2 and CCR2 are associated with a lower OS and increased risk of recurrence in non-metastatic clear-cell renal cell carcinoma [120].
Clinical modulation of the CCL2/CCR2 axis
Clinical inhibition of CCL2 initially failed to generate significant effects. Carlumab—a mAb against CCL2—was found to be safe and tolerable in patients but reduction in free CCL2 was short lived and failed to achieve an objective response in solid tumours (NCT01204996) [121, 122]. MLN1202, a similar antibody, was trialled in patients with bone metastasis from solid tumours, and resulted in reduced urinary N-telopeptide (uNTX) levels but with minimal therapeutic success [123]. Further to the poor therapeutic responses; in murine models a bounce back effect in CCL2 levels was observed in which levels quickly returned to baseline or higher than pre-treatment levels resulting in accelerated death [124].
An orally active CCR2 antagonist PF-04136309, has been shown to reduce growth of PDAC and enhance survival. Phase 1b trials with FOLFIRINOX have shown that it is safe, tolerable, and enhances survival [125]. Levels of peripheral circulating monocytes are inversely related to survival in pancreatic cancer [100]. Systemic CCR2 inhibition inhibits the mobilization of inflammatory monocytes from the bone marrow, consequently lowering monocyte infiltration to the TME. Preclinical models suggest the results in PDAC may translate into other tumour types, however, the unique TME of PDAC, with high innate immune cell infiltration and T cell immune privilege, must be considered unique so recapitulation of the results in other tumour types is uncertain [126, 127].
CCR2+ macrophages suppress the infiltration of MMP-9+ neutrophils to the TME. In murine models of cervical cancer, when macrophages are depleted in the TME, pro-tumourigenic neutrophils are recruited. Consequently, no major difference in tumour incidence or tumour burden is seen between CCR2 null and wild type mice, with only a small delay from dysplasia to carcinoma being noted [128]. It is possible that this compensatory influx of neutrophils may be inhibited by the dense desmoplastic in pancreatic cancer, indicating the therapeutic benefit of PF-04136309 may be restricted to pancreatic cancer.
Rationale for modulation of the CSF1/CSF1R axis
CSF1 is a secreted cytokine that binds CSF1R on cells and which can control the production, migration, function and differentiation of macrophages. CSF1R is predominantly expressed on myeloid cells of the monocyte–macrophage lineage and its inhibition has been used in various preclinical models for local macrophage/monocyte depletion. CSF1R mediated depletion has been shown to increase the efficacy of chemotherapy, radiotherapy, angiogenic inhibitors, and CPIs [36, 55, 129,130,131]. In addition to enhancing monocyte migration, CSF1 binding has been shown to promote the development of M2-like macrophages [132, 133].
Targeting CSF1R has the added advantage of being highly expressed on potently immunosuppressive MDSCs and can inhibit the migration of both macrophages and monocytic MDSCs to the TME [119, 120]. Along with M2-like macrophages, MDSCs secrete high levels of indolamine 2,3-dioxygenase (IDO) and have been implicated in resistance to CPIs and rapid outgrowth of B16 cell line tumours [119].
Unlike GM-CSF which results in upregulation of PD-L1 expression on immune infiltrates, inhibition of CSF1 signalling appears to upregulate CTLA-4 on tumour infiltrating CD8+ CTLs in addition to enhancing PD-L1 expression on macrophages and tumour cells, but with a concomitant decrease in PD-1 expression by monocytes and macrophages [131]. Inhibition of signalling by CSF1R on macrophages has been shown to enhance antigen presentation and T cell effector functions. Combination with CPIs was shown to induce tumour regression in murine models of PDAC [131].
While CCL2:CCR2 inhibitors can inhibit the mobilization of monocytes from the bone marrow and may result in a build-up of potentially pro-tumour cells elsewhere, anti-CSF1R antibodies deplete macrophages. There has been evidence that CSF1/CSF1R inhibition can increase metastasis in breast cancer via a compensatory increase in expression of G-CSF, however, this has not been seen in other tumour models [134].
Prognostic significance of CSF1 and CSF1R
CSF1R overexpression is associated with a negative prognosis in breast cancer patients [135]. In murine models, CSF1R overexpression is associated with reduced survival in endometrial, hepatocellular and colorectal cancer and targeting of both CSF1 and CSF1R have been shown to increase survival [136].
Clinical modulation of the CSF1/CSF1R axis
There are a range of anti-CSF1R antibodies currently in clinical trials designed to generate ADCC of tumour cells over expressing CSF1R and TAM depletion (Table 3).
CSF1R is a member of the KIT family of tyrosine kinases. Imatinib Mesylate can act as a tyrosine kinase inhibitor to these kinases. A trial using Imatinib in KIT+ patients showed clear clinical efficacy with 20/27 achieving stable disease, 1 complete response and 4 partial responses. Because of the promiscuity of Imatinib, toxicities due to off target effects were significant with 1 in 4 discontinuing treatment due to intolerable AEs [137,138,139,140].
There have been efforts to design tyrosine kinase inhibitors that target CSF1R, but they have lacked specificity to CSF1R and induced intolerable side effects unrelated to macrophage behavior. A novel compound, DCC-3014, displays remarkable specificity and was due to be used in a First-In-Human trial by the end of 2016 but is yet to commence [141].
While the efficacy of CSF1R inhibitors has not yet led to their clinical approval, effective depletion of TAM numbers has been a positive development which may effectively compliment other therapies.
Combination of macrophage modulation and T cell checkpoint inhibitors
Progress has not been aided by a relative under characterization of macrophage behaviour during the administration of current immunotherapeutics and analysis of how they may impact response. This is more striking when considering the central role monocytes and macrophages play in shaping the immune response. There has been limited publication of the relationship between response to CPIs and myeloid cells, but the level of immunological interrogation of patients focusing on myeloid subsets is not clear.
Ipilimumab (10 mg/kg) has been successfully trialled with subcutaneous recombinant GM-SCF in metastatic melanoma with an enhanced OS of 17.5 vs 12.7 months, and was better tolerated than Ipilimumab alone [142]. The mechanism resulting in reduced toxicities is not known; however, there was no difference in the objective response rate and no significant change in PFS. There is currently a phase 2/3 clinical trial examining the combination of Nivolumab and Ipilimumab with or without GM-CSF in unresectable melanoma (NCT02339571).
Positive results of clinical trials examining macrophage modulation will intuitively result in future trials combining them with CPIs. Some of these combinational approaches are entering early stage clinical trials, but there have also been a number of trials which have indirectly combined CPIs with macrophage modulation and seen positive results.
Trabectedin is a drug approved for soft tissue sarcoma that binds the minor groove on DNA resulting in a poorly characterized DNA damage in all cells, but critical to its anti-tumour efficacy is its ability to selectively induce apoptosis in monocytes and macrophages, reduce recruitment of CD68+ monocytes to the TME and reduce CCL2 and CXCL8 levels [143,144,145]. Trabectedin has been shown to be synergistic with anti-PD-1 antibodies in murine models of ovarian cancer with the generation of systemic anti-tumour immunity [146]. It has been approved for the treatment of soft tissue sarcoma under the trade name Yondelis, and is currently in clinical trials for use in breast, prostate and paediatric sarcomas. The prolonged period of treatment required to see an effect on macrophage populations makes it unlikely to exert an observable effect in fast growing or late stage tumours.
MGN1703, a DNA-based TLR agonist is being trialled in advanced solid malignancies with Ipilimumab (NCT02668770). Similarly, IMO-2125, a synthetic TLR-9 agonist which is expressed by plasmacytoid dendritic cells but also to a lesser extent by monocytes and macrophages, is being trialled in combination with ipilimumab in patients with metastatic melanoma (NCT02644967). If successful data emerges from these trials it will increasingly turn focus towards the role of innate immune cells in response to CPIs [147].
Data emerging from the phase 3 clinical trial KEYNOTE-252/ECHO-301 suggests that Epacadostat—an IDO inhibitor—in combination with Pembrolizumab can improve outcome for stage III/IV unresectable or metastatic melanoma patients. IDO is primarily secreted by M2 macrophages but can also be produced directly by tumour cells in cancer patients. A phase 3 trial is currently recruiting 600 patients to further test this combination (NCT02752074).
Discussion
Side effects associated with CPIs are dose dependant (Table 4), it appears they are also cumulative to the cycles received and additive with other CPIs [148]. The most recent evidence to emerge from CheckMate 067 examining Ipilimumab and Nivolumab in advanced melanoma, has suggested the side effects are not cumulative but remain high with 58% of patients experiencing grade 3 or 4 adverse events (AEs). Intuitively this has led to a shift in therapeutic design, which has been predominantly focused on engineering or stimulating T cells ex vivo. However, it is uncertain if these cells will be able to overcome the immunosuppressive environment that acts to ‘turn off’ these cells after readministration.
The most notable and promising examples of successful macrophage modulation have been found in murine models on PDAC and these are now beginning to show efficacy in the clinical setting, but the unique composition of the pancreatic cancer TME may not accurately reflect the potential of macrophage modulation in other tumour types. It is hypothesized that the success seen may be due to the restricted flow of cells into and out of the microenvironment resulting in a reduced ability to compensate for a loss of macrophage function and consequent tumour inhibition. It however appears likely that macrophage modulating therapies will compliment CPIs, and it will be of keen interest to see if the reduced AEs seen with GM-CSF and Ipilimumab will be seen with other therapies designed to reduce immunosuppressive factors in the TME.
While some have been quick to suggest that the ability to understand and direct MO behaviour represents an immunotherapy breakthrough it is clear from recent clinical evaluation that manipulation of macrophages as a stand-alone therapy in its current state is insufficient for therapeutic success [149]. However, it appears macrophage depletion may be a more effective strategy than macrophage re-education due to the profound immunosuppressive force exerted by advanced tumours [150].
In addition to the combination of macrophage modulation and immunotherapies, there is significant scope and promise for their combination with other therapies. For example, the anti-tumour effect of BRAF inhibitors was noted to be reliant on host tumour-directed immune responses [151]. 50% of advanced melanomas are BRAF positive and initially respond to therapy, but tumours develop mechanisms of acquired resistance and become refractory [152]. In preliminary studies, inhibiting monocyte and MDSC influx to the TME synergistically enhanced the effect of BRAF inhibition [153, 154]. There is mounting preclinical evidence to justify the use of macrophage modulating therapies with BRAF inhibitors in advanced melanoma.
Preclinical data in murine models has shown that the effect of immunotherapy in mouse models is more effective in the early stages of disease progression, which is generally defined by a low concentration of immunosuppressive elements in the TME. While the reversal of this immunosuppression may restore sensitivity, delineation of the primary immunosuppressive factors responsible for the reduction in efficacy is difficult due to the plethora of interacting factors and systems in the TME. Significant literature is available on many factors, but their relative importance in determining sensitivity to therapy has not been fully elucidated. The clinical prognostic evidence on immunosuppressive factors in patients undergoing treatment is limited, but do suggest that they are the key to the development of systemic and durable anti-cancer responses.
Targeting of macrophages has been shown to profoundly shape the immune response and we now have a range of sophisticated therapeutics that are beginning to make impacts in the clinic. Rational design of immunotherapeutics that will increase their efficacy, response rates and generate systemic and durable response rates will require a holistic mind-set towards understanding the immune system. Given the central role that macrophages play in shaping the immune response they will play an integral role in immunotherapeutic design.
Abbreviations
- 5YS:
-
5-Year survival
- AE:
-
Adverse event
- CPI:
-
Checkpoint inhibitor
- CSF1:
-
Colony-stimulating factor 1
- CSF1R:
-
Colony stimulating factor 1 receptor
- IDO:
-
Indolamine 2,3-dioxygenase
- irAE:
-
Immune related adverse event
- MDSC:
-
Myeloid derived suppressor cell
- MIP-1α:
-
Macrophage inflammatory protein-1α
- NSCLC:
-
Non-small cell lung cancer
- PAMP:
-
Pathogen associated molecular pattern
- PDAC:
-
Pancreatic ductal adenocarcinoma
- PIGF:
-
Phosphatidylinositol-glycan biosynthesis class F protein
- TAM:
-
Tumour associated macrophage
- TLR:
-
Toll-like receptor
- TME:
-
Tumour microenvironment
- uNTX:
-
Urinary N-telopeptide
- VCAM-1:
-
Vascular cell adhesion protein 1
References
Graciotti M, Berti C, Klok HA, Kandalaft L (2017) The era of bioengineering: how will this affect the next generation of cancer immunotherapy? J Transl Med 15(1):142
Farzaneh L, Kasahara N, Farzaneh F (2007) The strange case of TGN1412. Cancer Immunol Immunother 56(2):129–134
Hassel JC, Heinzerling L, Aberle J, Bahr O, Eigentler TK, Grimm MO, Grunwald V, Leipe J, Reinmuth N, Tietze JK, Trojan J, Zimmer L, Gutzmer R (2017) Combined immune checkpoint blockade (anti-PD-1/anti-CTLA-4): evaluation and management of adverse drug reactions. Cancer Treat Rev 57:36–49
Law AM, Lim E, Ormandy CJ, Gallego-Ortega D (2017) The innate and adaptive infiltrating immune systems as targets for breast cancer immunotherapy. Endocr Relat Cancer 24(4):R123–R144
Liu Y, Zeng G (2012) Cancer and innate immune system interactions: translational potentials for cancer immunotherapy. J Immunother 35(4):299–308
Solinas G, Germano G, Mantovani A, Allavena P (2009) Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol 86(5):1065–1073
Mosser DM, Edwards JP (2008) Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8(12):958–969
Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA (2014) Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41(1):14–20
Mills CD, Ley K (2014) M1 and M2 macrophages: the chicken and the egg of immunity. J Innate Immun 6(6):716–726
Mills CD, Lenz LL, Ley K (2015) Macrophages at the fork in the road to health or disease. Front Immunol 6:59
Konig S, Nitzki F, Uhmann A, Dittmann K, Theiss-Suennemann J, Herrmann M, Reichardt HM, Schwendener R, Pukrop T, Schulz-Schaeffer W, Hahn H (2014) Depletion of cutaneous macrophages and dendritic cells promotes growth of basal cell carcinoma in mice. PLoS ONE 9(4):e93555
Nesbit M, Schaider H, Miller TH, Herlyn M (2001) Low-level monocyte chemoattractant protein-1 stimulation of monocytes leads to tumor formation in nontumorigenic melanoma cells. J Immunol 166(11):6483–6490
Sica A, Schioppa T, Mantovani A, Allavena P (2006) Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer 42(6):717–727
Dandekar RC, Kingaonkar AV, Dhabekar GS (2011) Role of macrophages in malignancy. Ann Maxillofac Surg 1(2):150–154
Qian B, Pollard JW (2010) Macrophage diversity enhances tumor progression and metastasis. Cell 141(1):39–51
Mantovani A, Allavena P (2015) The interaction of anticancer therapies with tumor-associated macrophages. J Exp Med 212(4):435–445
Noy R, Pollard JW (2014) Tumor-associated macrophages: from mechanisms to therapy. Immunity 41(1):49–61
DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, Rugo HS, Hwang ES, Jirstrom K, West BL, Coussens LM (2011) Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov 1(1):54–67
Steidl C, Farinha P, Gascoyne RD (2011) Macrophages predict treatment outcome in Hodgkin’s lymphoma. Haematologica 96(2):186–189
Wan T, Liu JH, Zheng LM, Cai MY, Ding T (2009) Prognostic significance of tumor-associated macrophage infiltration in advanced epithelial ovarian carcinoma. Ai Zheng 28(3):323–327
Sconocchia G, Zlobec I, Lugli A, Calabrese D, Iezzi G, Karamitopoulou E, Patsouris ES, Peros G, Horcic M, Tornillo L, Zuber M, Droeser R, Muraro MG, Mengus C, Oertli D, Ferrone S, Terracciano L, Spagnoli GC (2011) Tumor infiltration by FcgammaRIII (CD16) + myeloid cells is associated with improved survival in patients with colorectal carcinoma. Int J Cancer 128(11):2663–2672
Forssell J, Oberg A, Henriksson ML, Stenling R, Jung A, Palmqvist R (2007) High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res 13(5):1472–1479
Zhou Q, Peng RQ, Wu XJ, Xia Q, Hou JH, Ding Y, Zhou QM, Zhang X, Pang ZZ, Wan DS, Zeng YX, Zhang XS (2010) The density of macrophages in the invasive front is inversely correlated to liver metastasis in colon cancer. J Transl Med 8:13
Ohno S, Inagawa H, Dhar DK, Fujii T, Ueda S, Tachibana M, Suzuki N, Inoue M, Soma G, Nagasue N (2003) The degree of macrophage infiltration into the cancer cell nest is a significant predictor of survival in gastric cancer patients. Anticancer Res 23(6d):5015–5022
Medrek C, Ponten F, Jirstrom K, Leandersson K (2012) The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer 12:306
Ohno S, Ohno Y, Suzuki N, Kamei T, Koike K, Inagawa H, Kohchi C, Soma G, Inoue M (2004) Correlation of histological localization of tumor-associated macrophages with clinicopathological features in endometrial cancer. Anticancer Res 24(5c):3335–3342
Gwak JM, Jang MH, Kim DI, Seo AN, Park SY (2015) Prognostic value of tumor-associated macrophages according to histologic locations and hormone receptor status in breast cancer. PLoS ONE 10(4):e0125728
Yuan A, Hsiao Y-J, Chen H-Y, Chen H-W, Ho C-C, Chen Y-Y, Liu Y-C, Hong T-H, Yu S-L, Chen JJW, Yang P-C (2015) Opposite effects of M1 and M2 macrophage subtypes on lung cancer progression. Sci Rep 5:14273
Ma J, Liu L, Che G, Yu N, Dai F, You Z (2010) The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer 10(1):112
Mei J, Xiao Z, Guo C, Pu Q, Ma L, Liu C, Lin F, Liao H, You Z, Liu L (2016) Prognostic impact of tumor-associated macrophage infiltration in non-small cell lung cancer: a systemic review and meta-analysis. Oncotarget 7(23):34217–34228
Shu QH, Ge YS, Ma HX, Gao XQ, Pan JJ, Liu D, Xu GL, Ma JL, Jia WD (2016) Prognostic value of polarized macrophages in patients with hepatocellular carcinoma after curative resection. J Cell Mol Med 20(6):1024–1035
Zhang M, He Y, Sun X, Li Q, Wang W, Zhao A, Di W (2014) A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J Ovarian Res 7:19
Lan C, Huang X, Lin S, Huang H, Cai Q, Wan T, Lu J, Liu J (2013) Expression of M2-polarized macrophages is associated with poor prognosis for advanced epithelial ovarian cancer. Technol Cancer Res Treat 12(3):259–267
Klimp AH, Hollema H, Kempinga C, van der Zee AG, de Vries EG, Daemen T (2001) Expression of cyclooxygenase-2 and inducible nitric oxide synthase in human ovarian tumors and tumor-associated macrophages. Cancer Res 61(19):7305–7309
Colvin EK (2014) Tumor-associated macrophages contribute to tumor progression in ovarian cancer. Front Oncol 4:137
Paulus P, Stanley ER, Schafer R, Abraham D, Aharinejad S (2006) Colony-stimulating factor-1 antibody reverses chemoresistance in human MCF-7 breast cancer xenografts. Cancer Res 66(8):4349–4356
Sugimura K, Miyata H, Tanaka K, Takahashi T, Kurokawa Y, Yamasaki M, Nakajima K, Takiguchi S, Mori M, Doki Y (2015) High infiltration of tumor-associated macrophages is associated with a poor response to chemotherapy and poor prognosis of patients undergoing neoadjuvant chemotherapy for esophageal cancer. J Surg Oncol 111(6):752–759
Mitrofanova I, Zavyalova M, Telegina N, Buldakov M, Riabov V, Cherdyntseva N, Kzhyshkowska J (2017) Tumor-associated macrophages in human breast cancer parenchyma negatively correlate with lymphatic metastasis after neoadjuvant chemotherapy. Immunobiology 222(1):101–109
Matsuoka Y, Yoshida R, Nakayama H, Nagata M, Hirosue A, Tanaka T, Kawahara K, Nakagawa Y, Sakata J, Arita H, Hiraki A, Shinohara M (2015) The tumour stromal features are associated with resistance to 5-FU-based chemoradiotherapy and a poor prognosis in patients with oral squamous cell carcinoma. Apmis 123(3):205–214
Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CM, Pryer N, Daniel D, Hwang ES, Rugo HS, Coussens LM (2014) Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell 26(5):623–637
De Palma M, Lewis CE (2011) Cancer: macrophages limit chemotherapy. Nature 472(7343):303–304
Genin M, Clement F, Fattaccioli A, Raes M, Michiels C (2015) M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer 15:577
Ruffell B, Coussens LM (2015) Macrophages and therapeutic resistance in cancer. Cancer Cell 27(4):462–472
Rolny C, Mazzone M, Tugues S, Laoui D, Johansson I, Coulon C, Squadrito ML, Segura I, Li X, Knevels E, Costa S, Vinckier S, Dresselaer T, Akerud P, De Mol M, Salomaki H, Phillipson M, Wyns S, Larsson E, Buysschaert I, Botling J, Himmelreich U, Van Ginderachter JA, De Palma M, Dewerchin M, Claesson-Welsh L, Carmeliet P (2011) HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell 19(1):31–44
De Palma M, Lewis CE (2013) Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell 23(3):277–286
Germano G, Frapolli R, Belgiovine C, Anselmo A, Pesce S, Liguori M, Erba E, Uboldi S, Zucchetti M, Pasqualini F, Nebuloni M, van Rooijen N, Mortarini R, Beltrame L, Marchini S, Fuso Nerini I, Sanfilippo R, Casali Pilotti S, Galmarini CM, Anichini A, Mantovani A, D’Incalci M, Allavena P (2013) Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell 23(2):249–262
Park B, Yee C, Lee K-M (2014) The effect of radiation on the immune response to cancers. Int J Mol Sci 15(1):927–943
Obeid M, Panaretakis T, Joza N, Tufi R, Tesniere A, van Endert P, Zitvogel L, Kroemer G (2007) Calreticulin exposure is required for the immunogenicity of [gamma]-irradiation and UVC light-induced apoptosis. Cell Death Differ 14(10):1848–1850
Ahn GO, Tseng D, Liao CH, Dorie MJ, Czechowicz A, Brown JM (2010) Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proc Natl Acad Sci USA 107(18):8363–8368
Crittenden MR, Cottam B, Savage T, Nguyen C, Newell P, Gough MJ (2012) Expression of NF-kappaB p50 in tumor stroma limits the control of tumors by radiation therapy. PLoS ONE 7(6):e39295
Martin BJ (2013) Inhibiting vasculogenesis after radiation: a new paradigm to improve local control by radiotherapy. Semin Radiat Oncol 23(4):281–287
Wang SC, Yu CF, Hong JH, Tsai CS, Chiang CS (2013) Radiation therapy-induced tumor invasiveness is associated with SDF-1-regulated macrophage mobilization and vasculogenesis. PLoS ONE 8(8):e69182
Okubo M, Kioi M, Nakashima H, Sugiura K, Mitsudo K, Aoki I, Taniguchi H, Tohnai I (2016) M2-polarized macrophages contribute to neovasculogenesis, leading to relapse of oral cancer following radiation. Sci Rep 6:27548
Crittenden MR, Cottam B, Savage T, Nguyen C, Newell P, Gough MJ (2012) Expression of NF-κB p50 in tumor stroma limits the control of tumors by radiation therapy. PLoS ONE 7(6):e39295
Shiao SL, Ruffell B, DeNardo DG, Faddegon BA, Park CC, Coussens LM (2015) TH2-polarized CD4(+) T cells and macrophages limit efficacy of radiotherapy. Cancer Immunol Res 3(5):518–525
Teresa Pinto A, Laranjeiro Pinto M, Patrícia Cardoso A, Monteiro C, Teixeira Pinto M, Filipe Maia A, Castro P, Figueira R, Monteiro A, Marques M, Mareel M, Dos Santos SG, Seruca R, Adolfo Barbosa M, Rocha S, José Oliveira M (2016) Ionizing radiation modulates human macrophages towards a pro-inflammatory phenotype preserving their pro-invasive and pro-angiogenic capacities. Sci Rep 6:18765
Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, Pfirschke C, Voss RH, Timke C, Umansky L, Klapproth K, Schakel K, Garbi N, Jager D, Weitz J, Schmitz-Winnenthal H, Hammerling GJ, Beckhove P (2013) Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 24(5):589–602
Shaikh S, Noshirwani A, West N, Perry S, Jayne D (2015) Can macrophages within the microenvironment of locally invasive rectal cancers predict response to radiotherapy? Lancet 385(Suppl 1):S87
Greenwald RJ, Freeman GJ, Sharpe AH (2005) The B7 family revisited. Annu Rev Immunol 23:515–548
Che F, Heng X, Zhang H, Su Q, Zhang B, Chen Y, Zhang Z, Du Y, Wang L (2017) Novel B7-H4-mediated crosstalk between human non-Hodgkin lymphoma cells and tumor-associated macrophages leads to immune evasion via secretion of IL-6 and IL-10. Cancer Immunol Immunother 66(6):717–729
Riley JL (2009) PD-1 signaling in primary T cells. Immunol Rev 229(1):114–125
Hutchins AP, Diez D, Miranda-Saavedra D (2013) The IL-10/STAT3-mediated anti-inflammatory response: recent developments and future challenges. Brief Funct Genomics 12(6):489–498
Rodríguez-García M, Porichis F, de Jong OG, Levi K, Diefenbach TJ, Lifson JD, Freeman GJ, Walker BD, Kaufmann DE, Kavanagh DG (2011) Expression of PD-L1 and PD-L2 on human macrophages is up-regulated by HIV-1 and differentially modulated by IL-10. J Leukocyte Biol 89(4):507–515
Hartley G, Regan D, Guth A, Dow S (2017) Regulation of PD-L1 expression on murine tumor-associated monocytes and macrophages by locally produced TNF-alpha. Cancer Immunol Immunother 66(4):523–535
Kohrt HE, Houot R, Marabelle A, Cho HJ, Osman K, Goldstein M, Levy R, Brody J (2012) Combination strategies to enhance antitumor ADCC. Immunotherapy 4(5):511–527
Sliwkowski MX, Mellman I (2013) Antibody therapeutics in cancer. Science 341(6151):1192–1198
Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD, Peggs KS, Ravetch JV, Allison JP, Quezada SA (2013) Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med 210(9):1695–1710
Bulliard Y, Jolicoeur R, Windman M, Rue SM, Ettenberg S, Knee DA, Wilson NS, Dranoff G, Brogdon JL (2013) Activating Fc gamma receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. J Exp Med 210(9):1685–1693
Romano E, Kusio-Kobialka M, Foukas PG, Baumgaertner P, Meyer C, Ballabeni P, Michielin O, Weide B, Romero P, Speiser DE (2015) Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc Natl Acad Sci USA 112(19):6140–6145
Martens A, Wistuba-Hamprecht K, Geukes Foppen M, Yuan J, Postow MA, Wong P, Romano E, Khammari A, Dreno B, Capone M, Ascierto PA, Di Giacomo AM, Maio M, Schilling B, Sucker A, Schadendorf D, Hassel JC, Eigentler TK, Martus P, Wolchok JD, Blank C, Pawelec G, Garbe C, Weide B (2016) Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clin Cancer Res 22(12):2908–2918
Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJM, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A (2014) PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515(7528):568–571
McDermott DF, Sosman JA, Sznol M, Massard C, Gordon MS, Hamid O, Powderly JD, Infante JR, Fassò M, Wang YV, Zou W, Hegde PS, Fine GD, Powles T (2016) Atezolizumab, an anti-programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: long-term safety, clinical activity, and immune correlates from a phase ia study. J Clin Oncol 34(8):833–842
Nicola NA (1987) Granulocyte colony-stimulating factor and differentiation-induction in myeloid leukemic cells. Int J Cell Cloning 5(1):1–15
Helft J, Böttcher J, Chakravarty P, Zelenay S, Huotari J, Schram BU, Goubau D, e Sousa CR (2015) GM-CSF mouse bone marrow cultures comprise a heterogeneous population of CD11c + MHCII + macrophages and dendritic cells. Immunity 42(6):1197–1211
Verreck FAW, de Boer T, Langenberg DML, Hoeve MA, Kramer M, Vaisberg E, Kastelein R, Kolk A, de Waal-Malefyt R, Ottenhoff THM (2004) Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci USA 101(13):4560–4565
Ferlazzo G, Klein J, Paliard X, Wei WZ, Galy A (2000) Dendritic cells generated from CD34 + progenitor cells with flt3 ligand, c-kit ligand, GM-CSF, IL-4, and TNF-alpha are functional antigen-presenting cells resembling mature monocyte-derived dendritic cells. J Immunother 23(1):48–58
Eksioglu EA, Mahmood SS, Chang M, Reddy V (2007) GM-CSF promotes differentiation of human dendritic cells and T lymphocytes toward a predominantly type 1 proinflammatory response. Exp Hematol 35(8):1163–1171
Smith TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, Balducci L, Bennett CL, Cantor SB, Crawford J, Cross SJ, Demetri G, Desch CE, Pizzo PA, Schiffer CA, Schwartzberg L, Somerfield MR, Somlo G, Wade JC, Wade JL, Winn RJ, Wozniak AJ, Wolff AC (2006) 2006 Update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol 24(19):3187–3205
Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF (2010) Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 363(5):411–422
Lutz ER, Kinkead H, Jaffee EM, Zheng L (2014) Priming the pancreatic cancer tumor microenvironment for checkpoint-inhibitor immunotherapy. Oncoimmunology 3(11):e962401
Zheng L (2014) Does vaccine-primed pancreatic cancer offer better candidates for immune-based therapies? Immunotherapy 6(10):1017–1020
Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, Zheng L, Diaz LA Jr, Donehower RC, Jaffee EM, Laheru DA (2013) Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother 36(7):382–389
Adachi N, Yamaguchi K, Morikawa T, Suzuki M, Matsuda I, Abe MK (1994) Constitutive production of multiple colony-stimulating factors in patients with lung cancer associated with neutrophilia. Br J Cancer 69(1):125–129
Oshika Y, Nakamura M, Abe Y, Fukuchi Y, Yoshimura M, Itoh M, Ohnishi Y, Tokunaga T, Fukushima Y, Hatanaka H, Kijima H, Yamazaki H, Tamaoki N, Ueyama Y (1998) Growth stimulation of non-small cell lung cancer xenografts by granulocyte-macrophage colony-stimulating factor (GM-CSF). Eur J Cancer 34(12):1958–1961
Hercus TR, Thomas D, Guthridge MA, Ekert PG, King-Scott J, Parker MW, Lopez AF (2009) The granulocyte-macrophage colony-stimulating factor receptor: linking its structure to cell signaling and its role in disease. Blood 114(7):1289–1298
Chiu HY, Sun KH, Chen SY, Wang HH, Lee MY, Tsou YC, Jwo SC, Sun GH, Tang SJ (2012) Autocrine CCL2 promotes cell migration and invasion via PKC activation and tyrosine phosphorylation of paxillin in bladder cancer cells. Cytokine 59(2):423–432
Craig MJ, Loberg RD (2006) CCL2 (monocyte chemoattractant protein-1) in cancer bone metastases. Cancer Metastasis Rev 25(4):611–619
Saji H, Koike M, Yamori T, Saji S, Seiki M, Matsushima K, Toi M (2001) Significant correlation of monocyte chemoattractant protein-1 expression with neovascularization and progression of breast carcinoma. Cancer 92(5):1085–1091
Kitamura T, Qian BZ, Soong D, Cassetta L, Noy R, Sugano G, Kato Y, Li J, Pollard JW (2015) CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J Exp Med 212(7):1043–1059
Silzle T, Kreutz M, Dobler MA, Brockhoff G, Knuechel R, Kunz-Schughart LA (2003) Tumor-associated fibroblasts recruit blood monocytes into tumor tissue. Eur J Immunol 33(5):1311–1320
Arendt LM, McCready J, Keller PJ, Baker DD, Naber SP, Seewaldt V, Kuperwasser C (2013) Obesity promotes breast cancer by CCL2-mediated macrophage recruitment and angiogenesis. Cancer Res 73(19):6080–6093
Zhao L, Lim SY, Gordon-Weeks AN, Tapmeier TT, Im JH, Cao Y, Beech J, Allen D, Smart S, Muschel RJ (2013) Recruitment of a myeloid cell subset (CD11b/Gr1 mid) via CCL2/CCR2 promotes the development of colorectal cancer liver metastasis. Hepatology 57(2):829–839
Okuma T, Terasaki Y, Kaikita K, Kobayashi H, Kuziel WA, Kawasuji M, Takeya M (2004) C-C chemokine receptor 2 (CCR2) deficiency improves bleomycin-induced pulmonary fibrosis by attenuation of both macrophage infiltration and production of macrophage-derived matrix metalloproteinases. J Pathol 204(5):594–604
Roca H, Varsos ZS, Sud S, Craig MJ, Ying C, Pienta KJ (2009) CCL2 and interleukin-6 promote survival of human CD11b + peripheral blood mononuclear cells and induce M2-type macrophage polarization. J Biol Chem 284(49):34342–34354
Tanaka T, Terada M, Ariyoshi K, Morimoto K (2010) Monocyte chemoattractant protein-1/CC chemokine ligand 2 enhances apoptotic cell removal by macrophages through Rac1 activation. Biochem Biophys Res Commun 399(4):677–682
Lu X, Kang Y (2009) Chemokine (C-C motif) ligand 2 engages CCR2+ stromal cells of monocytic origin to promote breast cancer metastasis to lung and bone. J Biol Chem 284(42):29087–29096
Loberg RD, Ying C, Craig M, Yan L, Snyder LA, Pienta KJ (2007) CCL2 as an important mediator of prostate cancer growth in vivo through the regulation of macrophage infiltration. Neoplasia 9(7):556–562
Loberg RD, Ying C, Craig M, Day LL, Sargent E, Neeley C, Wojno K, Snyder LA, Yan L, Pienta KJ (2007) Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Cancer Res 67(19):9417–9424
Li X, Yao W, Yuan Y, Chen P, Li B, Li J, Chu R, Song H, Xie D, Jiang X, Wang H (2015) Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut 66(1):157–167
Sanford DE, Belt BA, Panni RZ, Mayer A, Deshpande AD, Carpenter D, Mitchem JB, Plambeck-Suess SM, Worley LA, Goetz BD, Wang-Gillam A, Eberlein TJ, Denardo DG, Goedegebuure SP, Linehan DC (2013) Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res 19(13):3404–3415
Qian DZ, Rademacher BLS, Pittsenbarger J, Huang C-Y, Myrthue A, Higano CS, Garzotto M, Nelson PS, Beer TM (2010) CCL2 is induced by chemotherapy and protects prostate cancer cells from docetaxel—induced cytotoxicity. Prostate 70(4):433–442
Nakasone ES, Askautrud HA, Kees T, Park J-H, Plaks V, Ewald AJ, Fein M, Rasch MG, Tan Y-X, Qiu J, Park J, Sinha P, Bissell MJ, Frengen E, Werb Z, Egeblad M (2012) Imaging tumor-stroma interactions during chemotherapy reveals contributions of the microenvironment to resistance. Cancer Cell 21(4):488–503
Geller MA, Bui-Nguyen TM, Rogers LM, Ramakrishnan S (2010) Chemotherapy induces macrophage chemoattractant protein-1 production in ovarian cancer. Int J Gynecol Cancer 20(6):918–925
Moisan F, Francisco EB, Brozovic A, Duran GE, Wang YC, Chaturvedi S, Seetharam S, Snyder LA, Doshi P, Sikic BI (2014) Enhancement of paclitaxel and carboplatin therapies by CCL2 blockade in ovarian cancers. Mol Oncol 8(7):1231–1239
Kalbasi A, Komar C, Tooker GM, Liu M, Lee JW, Gladney WL, Ben-Josef E, Beatty GL (2017) Tumor-derived CCL2 mediates resistance to radiotherapy in pancreatic ductal adenocarcinoma. Clin Cancer Res 23(1):137–148
Gorlov IP, Sircar K, Zhao H, Maity SN, Navone NM, Gorlova OY, Troncoso P, Pettaway CA, Byun JY, Logothetis CJ (2010) Prioritizing genes associated with prostate cancer development. BMC Cancer 10:599
Hu H, Sun L, Guo C, Liu Q, Zhou Z, Peng L, Pan J, Yu L, Lou J, Yang Z, Zhao P, Ran Y (2009) Tumor cell-microenvironment interaction models coupled with clinical validation reveal CCL2 and SNCG as two predictors of colorectal cancer hepatic metastasis. Clin Cancer Res 15(17):5485–5493
Tonouchi H, Miki C, Tanaka K, Kusunoki M (2002) Profile of monocyte chemoattractant protein-1 circulating levels in gastric cancer patients. Scand J Gastroenterol 37(7):830–833
Tao LL, Shi SJ, Chen LB, Huang GC (2014) Expression of monocyte chemotactic protein-1/CCL2 in gastric cancer and its relationship with tumor hypoxia. World J Gastroenterol 20(15):4421–4427
Li X, Yao W, Yuan Y, Chen P, Li B, Li J, Chu R, Song H, Xie D, Jiang X, Wang H (2017) Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut 66(1):157–167
Hembruff SL, Jokar I, Yang L, Cheng N (2010) Loss of transforming growth factor-beta signaling in mammary fibroblasts enhances CCL2 secretion to promote mammary tumor progression through macrophage-dependent and -independent mechanisms. Neoplasia 12(5):425–433
Fujimoto H, Sangai T, Ishii G, Ikehara A, Nagashima T, Miyazaki M, Ochiai A (2009) Stromal MCP-1 in mammary tumors induces tumor-associated macrophage infiltration and contributes to tumor progression. Int J Cancer 125(6):1276–1284
Yoshimura T, Howard OM, Ito T, Kuwabara M, Matsukawa A, Chen K, Liu Y, Liu M, Oppenheim JJ, Wang JM (2013) Monocyte chemoattractant protein-1/CCL2 produced by stromal cells promotes lung metastasis of 4T1 murine breast cancer cells. PLoS ONE 8(3):e58791
Chun E, Lavoie S, Michaud M, Gallini CA, Kim J, Soucy G, Odze R, Glickman JN, Garrett WS (2015) CCL2 promotes colorectal carcinogenesis by enhancing polymorphonuclear myeloid-derived suppressor cell population and function. Cell Rep 12(2):244–257
Snyder L, Kesavan P, Kaiser E, Rudnick K, McCabe F, Millar H, Nakada M, Yan L (2007) Neutralization of CCL2 inhibits tumor angiogenesis and pancreatic tumor growth. Mol Cancer Ther 6(11 Supplement):A69 (Abstract)
Youngs SJ, Ali SA, Taub DD, Rees RC (1997) Chemokines induce migrational responses in human breast carcinoma cell lines. Int J Cancer 71(2):257–266
Roca H, Varsos ZS, Pienta KJ (2009) CCL2 is a negative regulator of AMP-activated protein kinase to sustain mTOR complex-1 activation, survivin expression, and cell survival in human prostate cancer PC3 cells. Neoplasia 11(12):1309–1317
Ueno T, Toi M, Saji H, Muta M, Bando H, Kuroi K, Koike M, Inadera H, Matsushima K (2000) Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res 6(8):3282–3289
Zhang J, Yan Y, Cui X, Zhang J, Yang Y, Li H, Wu H, Li J, Wang L, Li M, Liu X, Wang J, Duan X (2017) CCL2 expression correlates with Snail expression and affects the prognosis of patients with gastric cancer. Pathol Res Pract 213(3):217–221
Wang Z, Xie H, Zhou L, Liu Z, Fu H, Zhu Y, Xu L, Xu J (2016) CCL2/CCR2 axis is associated with postoperative survival and recurrence of patients with non-metastatic clear-cell renal cell carcinoma. Oncotarget 7(32):51525–51534
Sandhu SK, Papadopoulos K, Fong PC, Patnaik A, Messiou C, Olmos D, Wang G, Tromp BJ, Puchalski TA, Balkwill F, Berns B, Seetharam S, de Bono JS, Tolcher AW (2013) A first-in-human, first-in-class, phase I study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 in patients with solid tumors. Cancer Chemother Pharmacol 71(4):1041–1050
Pienta KJ, Machiels JP, Schrijvers D, Alekseev B, Shkolnik M, Crabb SJ, Li S, Seetharam S, Puchalski TA, Takimoto C, Elsayed Y, Dawkins F, de Bono JS (2013) Phase 2 study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 (CCL2), in metastatic castration-resistant prostate cancer. Invest New Drugs 31(3):760–768
Lim SY, Yuzhalin AE, Gordon-Weeks AN, Muschel RJ (2016) Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget 7(19):28697–28710
Bonapace L, Coissieux M-M, Wyckoff J, Mertz KD, Varga Z, Junt T, Bentires-Alj M (2014) Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature 515(7525):130–133
Nywening TM, Wang-Gillam A, Sanford DE, Belt BA, Panni RZ, Cusworth BM, Toriola AT, Nieman RK, Worley LA, Yano M, Fowler KJ, Lockhart AC, Suresh R, Tan BR, Lim K-H, Fields RC, Strasberg SM, Hawkins WG, DeNardo DG, Goedegebuure SP, Linehan DC (2016) Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol 17(5):651–662
Beatty GL, Winograd R, Evans RA, Long KB, Luque SL, Lee JW, Clendenin C, Gladney WL, Knoblock DM, Guirnalda PD, Vonderheide RH (2015) Exclusion of T cells from pancreatic carcinomas in mice is regulated by Ly6C(low) F4/80(+) extratumoral macrophages. Gastroenterology 149(1):201–210
Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, Torigian DA, O’Dwyer PJ, Vonderheide RH (2011) CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 331(6024):1612–1616
Pahler JC, Tazzyman S, Erez N, Chen YY, Murdoch C, Nozawa H, Lewis CE, Hanahan D (2008) Plasticity in tumor-promoting inflammation: impairment of macrophage recruitment evokes a compensatory neutrophil response. Neoplasia 10(4):329–340
Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, Belaygorod L, Carpenter D, Collins L, Piwnica-Worms D, Hewitt S, Udupi GM, Gallagher WM, Wegner C, West BL, Wang-Gillam A, Goedegebuure P, Linehan DC, DeNardo DG (2013) Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res 73(3):1128–1141
Priceman SJ, Sung JL, Shaposhnik Z, Burton JB, Torres-Collado AX, Moughon DL, Johnson M, Lusis AJ, Cohen DA, Iruela-Arispe ML, Wu L (2010) Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: combating tumor evasion of antiangiogenic therapy. Blood 115(7):1461–1471
Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, Wang-Gillam A, Goedegebuure SP, Linehan DC, DeNardo DG (2014) CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res 74(18):5057–5069
Martinez FO, Gordon S, Locati M, Mantovani A (2006) Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol 177(10):7303–7311
Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, Olson OC, Quick ML, Huse JT, Teijeiro V, Setty M, Leslie CS, Oei Y, Pedraza A, Zhang J, Brennan CW, Sutton JC, Holland EC, Daniel D, Joyce JA (2013) CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med 19(10):1264–1272
Swierczak A, Cook AD, Lenzo JC, Restall CM, Doherty JP, Anderson RL, Hamilton JA (2014) The promotion of breast cancer metastasis caused by inhibition of CSF-1R/CSF-1 signaling is blocked by targeting the G-CSF receptor. Cancer Immunol Res 2(8):765–776
Richardsen E, Uglehus RD, Johnsen SH, Busund LT (2015) Macrophage-colony stimulating factor (CSF1) predicts breast cancer progression and mortality. Anticancer Res 35(2):865–874
Aharinejad S, Paulus P, Sioud M, Hofmann M, Zins K, Schafer R, Stanley ER, Abraham D (2004) Colony-stimulating factor-1 blockade by antisense oligonucleotides and small interfering RNAs suppresses growth of human mammary tumor xenografts in mice. Cancer Res 64(15):5378–5384
Cassier PA, Gelderblom H, Stacchiotti S, Thomas D, Maki RG, Kroep JR, van der Graaf WT, Italiano A, Seddon B, Domont J, Bompas E, Wagner AJ, Blay JY (2012) Efficacy of imatinib mesylate for the treatment of locally advanced and/or metastatic tenosynovial giant cell tumor/pigmented villonodular synovitis. Cancer 118(6):1649–1655
Panagopoulos I, Brandal P, Gorunova L, Bjerkehagen B, Heim S (2014) Novel CSF1-S100A10 fusion gene and CSF1 transcript identified by RNA sequencing in tenosynovial giant cell tumors. Int J Oncol 44(5):1425–1432
Dewar AL, Cambareri AC, Zannettino AC, Miller BL, Doherty KV, Hughes TP, Lyons AB (2005) Macrophage colony-stimulating factor receptor c-fms is a novel target of imatinib. Blood 105(8):3127–3132
Blay JY, El Sayadi H, Thiesse P, Garret J, Ray-Coquard I (2008) Complete response to imatinib in relapsing pigmented villonodular synovitis/tenosynovial giant cell tumor (PVNS/TGCT). Ann Oncol 19(4):821–822
Smith BD, Leary CB, Lu W-P, Kaufman MD, Flynn DL (2016) The highly specific CSF1R inhibitor DCC-3014 exhibits immunomodulatory and anti-invasive activities in cancer models. Cancer Res 76(14 Supplement):4889 (Abstract)
Hodi FS, Lee S, McDermott DF, Rao UN, Butterfield LH, Tarhini AA, Leming P, Puzanov I, Shin D, Kirkwood JM (2014) Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma: a randomized clinical trial. JAMA 312(17):1744–1753
Allavena P, Germano G, Belgiovine C, D’Incalci M, Mantovani A (2013) Trabectedin: a drug from the sea that strikes tumor-associated macrophages. Oncoimmunology 2(6):e24614
López-Guerrero JA, Romero I, Poveda A (2015) Trabectedin therapy as an emerging treatment strategy for recurrent platinum-sensitive ovarian cancer. Chin J Cancer 34(1):41–49
Germano G, Frapolli R, Simone M, Tavecchio M, Erba E, Pesce S, Pasqualini F, Grosso F, Sanfilippo R, Casali PG, Gronchi A, Virdis E, Tarantino E, Pilotti S, Greco A, Nebuloni M, Galmarini CM, Tercero JC, Mantovani A, D’Incalci M, Allavena P (2010) Antitumor and anti-inflammatory effects of trabectedin on human myxoid liposarcoma cells. Cancer Res 70(6):2235–2244
Guo Z, Wang H, Meng F, Li J, Zhang S (2015) Combined trabectedin and anti-PD1 antibody produces a synergistic antitumor effect in a murine model of ovarian cancer. J Transl Med 13:247
Kanzler H, Barrat FJ, Hessel EM, Coffman RL (2007) Therapeutic targeting of innate immunity with toll-like receptor agonists and antagonists. Nat Med 13(5):552–559
Tarhini A (2013) Immune-mediated adverse events associated with ipilimumab CTLA-4 blockade therapy: the underlying mechanisms and clinical management. Scientifica 2013:857519
Mills CD, Lenz LL, Harris RA (2016) A breakthrough: macrophage-directed cancer immunotherapy. Cancer Res 76(3):513–516
Riabov V, Kim D, Chhina S, Alexander RB, Klyushnenkova EN (2015) Immunostimulatory early phenotype of tumor-associated macrophages does not predict tumor growth outcome in an HLA-DR mouse model of prostate cancer. Cancer Immunol Immunother 64(7):873–883
Knight DA, Ngiow SF, Li M, Parmenter T, Mok S, Cass A, Haynes NM, Kinross K, Yagita H, Koya RC, Graeber TG, Ribas A, McArthur GA, Smyth MJ (2013) Host immunity contributes to the anti-melanoma activity of BRAF inhibitors. J Clin Invest 123(3):1371–1381
Manzano JL, Layos L, Bugés C, de los Llanos Gil M, Vila L, Martínez-Balibrea E, Martínez-Cardús A (2016) Resistant mechanisms to BRAF inhibitors in melanoma. Ann Transl Med 4(12):237
Ngiow SF, Meeth KM, Stannard K, Barkauskas DS, Bollag G, Bosenberg M, Smyth MJ (2016) Co-inhibition of colony stimulating factor-1 receptor and BRAF oncogene in mouse models of BRAFV600E melanoma. Oncoimmunology 5(3):e1089381
Mok S, Tsoi J, Koya RC, Hu-Lieskovan S, West BL, Bollag G, Graeber TG, Ribas A (2015) Inhibition of colony stimulating factor-1 receptor improves antitumor efficacy of BRAF inhibition. BMC Cancer 15(1):356
Beck AH, Espinosa I, Edris B, Li R, Montgomery K, Zhu S, Varma S, Marinelli RJ, van de Rijn M, West RB (2009) The macrophage colony-stimulating factor 1 response signature in breast carcinoma. Clin Cancer Res 15(3):778–787
Tsutsui S, Yasuda K, Suzuki K, Tahara K, Higashi H, Era S (2005) Macrophage infiltration and its prognostic implications in breast cancer: the relationship with VEGF expression and microvessel density. Oncol Rep 14(2):425–431
Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, Zhao YW, Wei YQ (2012) Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS ONE 7(12):e50946
Miyasato Y, Shiota T, Ohnishi K, Pan C, Yano H, Horlad H, Yamamoto Y, Yamamoto-Ibusuki M, Iwase H, Takeya M, Komohara Y (2017) High density of CD204-positive macrophages predicts worse clinical prognosis in patients with breast cancer. Cancer Sci 108(8):1693–1700
Nabeshima A, Matsumoto Y, Fukushi J, Iura K, Matsunobu T, Endo M, Fujiwara T, Iida K, Fujiwara Y, Hatano M, Yokoyama N, Fukushima S, Oda Y, Iwamoto Y (2015) Tumour-associated macrophages correlate with poor prognosis in myxoid liposarcoma and promote cell motility and invasion via the HB-EGF-EGFR-PI3 K/Akt pathways. Br J Cancer 112(3):547–555
Hammes LS, Tekmal RR, Naud P, Edelweiss MI, Kirma N, Valente PT, Syrjanen KJ, Cunha-Filho JS (2007) Macrophages, inflammation and risk of cervical intraepithelial neoplasia (CIN) progression–clinicopathological correlation. Gynecol Oncol 105(1):157–165
Zijlmans HJ, Fleuren GJ, Baelde HJ, Eilers PH, Kenter GG, Gorter A (2006) The absence of CCL2 expression in cervical carcinoma is associated with increased survival and loss of heterozygosity at 17q11.2. J Pathol 208(4):507–517
Bronkhorst IH, Ly LV, Jordanova ES, Vrolijk J, Versluis M, Luyten GP, Jager MJ (2011) Detection of M2-macrophages in uveal melanoma and relation with survival. Invest Ophthalmol Vis Sci 52(2):643–650
No JH, Moon JM, Kim K, Kim YB (2013) Prognostic significance of serum soluble CD163 level in patients with epithelial ovarian cancer. Gynecol Obstet Invest 75(4):263–267
Lissbrant IF, Stattin P, Wikstrom P, Damber JE, Egevad L, Bergh A (2000) Tumor associated macrophages in human prostate cancer: relation to clinicopathological variables and survival. Int J Oncol 17(3):445–451
Komohara Y, Ohnishi K, Kuratsu J, Takeya M (2008) Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol 216(1):15–24
Ohri CM, Shikotra A, Green RH, Waller DA, Bradding P (2009) Macrophages within NSCLC tumour islets are predominantly of a cytotoxic M1 phenotype associated with extended survival. Eur Respir J 33(1):118–126
Sconocchia G, Zlobec I, Lugli A, Calabrese D, Iezzi G, Karamitopoulou E, Patsouris ES, Peros G, Horcic M, Tornillo L, Zuber M, Droeser R, Muraro MG, Mengus C, Oertli D, Ferrone S, Terracciano L, Spagnoli GC (2011) Tumor infiltration by FcγRIII (CD16) + myeloid cells is associated with improved survival in patients with colorectal carcinoma. Int J Cancer 128(11):2663–2672
Niino D, Komohara Y, Murayama T, Aoki R, Kimura Y, Hashikawa K, Kiyasu J, Takeuchi M, Suefuji N, Sugita Y, Takeya M, Ohshima K (2010) Ratio of M2 macrophage expression is closely associated with poor prognosis for Angioimmunoblastic T-cell lymphoma (AITL). Pathol Int 60(4):278–283
Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, Delaney A, Jones SJ, Iqbal J, Weisenburger DD, Bast MA, Rosenwald A, Muller-Hermelink HK, Rimsza LM, Campo E, Delabie J, Braziel RM, Cook JR, Tubbs RR, Jaffe ES, Lenz G, Connors JM, Staudt LM, Chan WC, Gascoyne RD (2010) Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med 362(10):875–885
Kamper P, Bendix K, Hamilton-Dutoit S, Honoré B, Nyengaard JR, d’Amore F (2011) Tumor-infiltrating macrophages correlate with adverse prognosis and Epstein-Barr virus status in classical Hodgkin’s lymphoma. Haematologica 96(2):269–276
Farinha P, Masoudi H, Skinnider BF, Shumansky K, Spinelli JJ, Gill K, Klasa R, Voss N, Connors JM, Gascoyne RD (2005) Analysis of multiple biomarkers shows that lymphoma-associated macrophage (LAM) content is an independent predictor of survival in follicular lymphoma (FL). Blood 106(6):2169–2174
Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, Rey-Giraud F, Pradel LP, Feuerhake F, Klaman I, Jones T, Jucknischke U, Scheiblich S, Kaluza K, Gorr IH, Walz A, Abiraj K, Cassier PA, Sica A, Gomez-Roca C, de Visser KE, Italiano A, Le Tourneau C, Delord JP, Levitsky H, Blay JY, Ruttinger D (2014) Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell 25(6):846–859
Butowski N, Colman H, De Groot JF, Omuro AM, Nayak L, Wen PY, Cloughesy TF, Marimuthu A, Haidar S, Perry A, Huse J, Phillips J, West BL, Nolop KB, Hsu HH, Ligon KL, Molinaro AM, Prados M (2016) Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an Ivy Foundation Early Phase Clinical Trials Consortium phase II study. Neuro Oncol 18(4):557–564
Moskowitz CH, Younes A, de Vos S, Bociek RG, Gordon LI, Witzig TE, Gascoyne RD, West B, Nolop K, Steidl C (2012) CSF1R inhibition by PLX3397 in patients with relapsed or refractory Hodgkin lymphoma: results from a phase 2 single agent clinical trial. Blood 120(21):Abstract 1638
Author information
Authors and Affiliations
Contributions
Liam Friel Tremble was responsible for the research and writing of this paper. Patrick F Forde was responsible for the conceptual design, paragraph formatting, draft correction, critical analysis of drafts and the inclusion and exclusion of a significant proportion of the review. Declan M Soden was responsible for the choice of review topic, conceptual design, draft correction and the inclusion and exclusion of a significant proportion of the review. All authors have read and approved the finalized version of this review.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
Breakthrough Cancer Research, Cork, funded this review.
Rights and permissions
About this article
Cite this article
Tremble, L.F., Forde, P.F. & Soden, D.M. Clinical evaluation of macrophages in cancer: role in treatment, modulation and challenges. Cancer Immunol Immunother 66, 1509–1527 (2017). https://doi.org/10.1007/s00262-017-2065-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-017-2065-0