Abstract
Non-Hodgkin lymphoma (NHL) is an incurable lymphoproliferative cancer, and patients with NHL have a poor prognosis. The present study explored the regulatory mechanism of expression and possible roles of the immunosuppressive B7-H4 molecule in human NHL. For functional studies, NHL-reactive T cell lines were generated via the isolation of allogeneic CD3+ T cells from healthy donors and repeated in vitro stimulation with irradiated NHL cells isolated from patients. B7-H4 was found to be distributed in NHL cells and tissues, and its surface protein expression levels were further upregulated by the incubation of NHL cells with interleukin (IL)-6, IL-10, or interferon-γ. Additionally, the supernatants of tumor-associated macrophages (tMφs) upregulated B7-H4 surface expression by producing IL-6 and IL-10. B7-H4 expressed in NHL cells inhibited the cytotoxic activity of NHL-reactive T cells. Conversely, the inhibition of B7-H4 in NHL cells promoted T cell immunity and sensitized NHL cells to cytolysis. Furthermore, tMφs induced B7-H4 promoted NHL cell evasion of the T cell immune response. In conclusion, this study shows that NHL-expressed B7-H4 is an important immunosuppressive factor that inhibits host anti-tumor immunity to NHL. Targeting tumor-expressed B7-H4 may thus provide a new treatment strategy for NHL patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-Hodgkin lymphoma (NHL) is a biologically and clinically heterogeneous lymphoproliferative malignancy [1, 2]. It ranks as the sixth most common cancer and seventh leading cause of malignant tumor-associated mortality worldwide [1, 3], affecting >300,000 people each year [4]. Approximately 85% of NHLs are derived from B cells [5]. NHL comprises more than 40 disease entities ranging from indolent to aggressive forms; the most frequent subtypes are follicular lymphoma (FL) and diffuse large B cell lymphoma (DLBCL) [4–6]. Although overall survival rates in NHL patients have improved significantly in recent years, many patients relapse or are refractory after initial therapy [7], and successful therapeutic strategies for such patients remain a major challenge. Thus, novel treatments are required to augment current approaches.
Immunotherapy has emerged as a useful treatment strategy for many cancers, and the induction of anti-tumor immune responses has the potential to provide a cure for NHL, particularly when used in combination with other treatments [5, 8]. The introduction of chemoimmunotherapy with rituximab, an anti-CD20 monoclonal antibody (mAb), has improved the overall survival of patients with NHL [5, 9]. Additionally, the induction of anti-tumor immunity with idiotypic proteins has been demonstrated to improve the clinical outcomes of NHL [10]. For example, a multicenter controlled phase III clinical trial revealed that the administration of specific tumor-derived idiotypic vaccines following chemotherapy improved the disease-free survival of FL patients [11].
Recent studies identifying the mechanisms by which tumors evade the immune response have led to substantial progress in tumor immunotherapy [12]. The programmed death protein (PD)-1/programmed death ligand (PD-L)1 pathway has been identified as a major immune checkpoint and was implicated in the immune evasion of various types of cancer [12, 13]. In patients with relapsed FL, the combination of rituximab and immune checkpoint blockade therapy with anti-PD-1 mAbs induced objective response and complete response rates of 66 and 52%, respectively [14]. However, resistance to immunotherapy remains a problem [5]. Possible mechanisms of therapy resistance include inhibitory signaling by the tumor cells and/or the tumor microenvironment. Therefore, improving the efficacy of immunotherapies in NHL patients will require a more complete understanding of the mechanisms of tumor immune evasion.
B7-H4 was discovered in 2003 and, while our knowledge of its function continues to grow, much remains to be understood [15–17]. B7-H4 is a B7 transmembrane molecule [18] encoded by a gene located on chromosome 1 in humans [15, 19]. This gene is expressed extensively in human peripheral tissues, but protein expression appears to be more limited [20–22]. B7-H4 triggers immunosuppressive signaling in T cells and regulates T cell immunity [16]. Recent studies have shown that the B7-H4 protein is detectable in tumors, including gastric [23–27], colorectal [28], pancreatic [29], breast [30], lung [31, 32] and thyroid cancers [33], renal cell carcinoma [34–37], and melanoma [38]. Moreover, B7-H4 expression levels correlate with tumor stage, metastasis, and disease progression of these malignancies, as well as patient prognosis and survival [20, 27, 30–33, 35, 36, 38–42]. Therefore, tumor-expressed B7-H4 may be a novel mechanism by which the tumor evades the immune system. As such, B7-H4 has been shown to promote the proliferation of esophageal squamous cell carcinoma cells [43]. Additionally, in a murine model of 4T1 metastatic breast cancer, T cells obtained from the lungs of B7-H4-knockout mice produced greater amounts of the inflammatory cytokines interferon (IFN)-γ and tumor necrosis factor (TNF)-α than T cells obtained from the lungs of wild-type mice [44]. In this study, the roles of B7-H4 in the anti-tumor immune response to human NHL were explored.
Materials and methods
Patients and cell lines
Primary NHL cells were collected from tissues, bone marrow, and peripheral blood aspirates from patients with NHL. Peripheral blood aspirates were collected from patients with relapsed NHL and bone marrow involvement. Tissue specimens were obtained by surgical resection from newly diagnosed patients at Linyi People’s Hospital (Linyi, China) between 2009 and 2014. All NHL cases were diagnosed and classified on the basis of standard World Health Organization criteria [45]. Clinical staging was conducted on the basis of the Ann Arbor classification. The study was approved by the Institutional Review Board of Linyi People’s Hospital, and all patients provided their informed consent. Mononuclear cells were isolated as described previously [8]. NHL cells were separated by anti-CD19 (B-cell antigen) or anti-CD3 (T cell antigen) mAbs (Miltenyi Biotec, Bergisch Gladbach, Germany). Isolated NHL cells were kept frozen in liquid nitrogen until use. The K562 human myelogenous leukemia cell line, and Nalmawa (Nal), SP53, Jeko-1, OCI-Ly10 (Ly10), Raji, and U2932 human NHL lines (ATCC, Manassas, VA) were maintained as described previously [8].
Reverse transcription (RT)-PCR
Details of RT-PCR analyses have been described previously [46]. The following primers were designed for RT-PCR: B7-H4 forward 5′-CTTCTGCCTCTCAGCCCTTA-3′, B7-H4 reverse 5′-GAAATAGTTCTGTAGATCCCTGTTG-3′; GAPDH forward 5′-GGGTGTGAACCATGAGAAGT-3′, GAPDH reverse 5′-GACTGTGGTCATGAGTCCT-3′.
Immunohistochemistry
All resected tissues were fixed in 10% neutral-buffered formalin and embedded in paraffin. Several 5-µm-thick serial histological sections were cut for immunohistochemical staining. The primary anti-B7-H4 mAb (clone H74) and the immunoglobulin G (IgG) isotype negative control antibody (both Abcam, Cambridge, UK) were used at a dilution of 1:200. For each stained section, five fields were randomly observed at high power with an optical microscope (400× magnification; B-150, Optika, Ponteranica, Italy) and staining was scored by three independent pathologists who were blinded to clinical data.
Flow cytometric analysis
Antibodies against human B7-H4 (clone H74), perforin (clone dG9), granzyme B (clone GB11), CD8 (Clone OKT8), and Fas ligand (FasL) (Clone NOK-1), and isotype control antibodies were obtained from eBioscience (San Diego, CA). Data were acquired with a Navios flow cytometer (Beckman Coulter, Miami, FL).
Generation of macrophages
Peripheral blood mononuclear cells (PBMCs) obtained from healthy donors were cultured in 12-well plates at 37 °C. After 2 h, non-adherent cells were removed by washing with medium and adherent monocytes were differentiated into normal macrophages (nMφs) by culturing in medium supplemented with macrophage colony-stimulating factor (M-CSF) [47] for 7 days. nMφs were then cultured for 72 h with conditioned medium from NHL cell cultures [48] to generate tumor-associated macrophages (tMφs). tMφs were also generated from NHL patient monocytes by culturing for 7 days with M-CSF.
Establishment of NHL-reactive T cell lines and cytotoxicity assay
NHL-reactive T cell lines were established and expanded as described previously [8]. The nomenclature for the T cell lines was based on the treatment of the NHL cells against which they were generated. Thus, cytotoxic T lymphocyte (CTL)-ctl were generated against untreated NHL cells, CTL-α-IgG were generated against NHL cells pretreated with control IgG, and CTL-α-B7-H4 were generated against NHL cells pretreated with anti-B7-H4 mAb. A CytoTox 96 Non-Radioactive Cytotoxicity Assay kit (Promega Corporation, Madison, WI) was used to measure the cytolytic activity of the T cell lines against target cells.
Enzyme-linked immunosorbent assay (ELISA)
ELISA kits (all from R&D Systems, Minneapolis, MN) were used to examine the level of IL-6, IL-10, and IFN-γ protein secreted into the cell supernatants.
Statistical analysis
Student’s t test was applied to compare groups, and P < 0.05 was regarded as statistically significant. Unless otherwise indicated, data are shown as the mean ± standard deviation.
Results
B7-H4 expression in human NHL
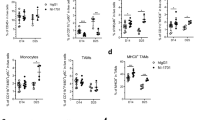
The B7-H4 gene was expressed in all human NHL cells tested (Fig. 1a). However, only the NHL cell line Raji expressed surface B7-H4 protein, while little or no surface expression of B7-H4 was detected in the other five NHL cell lines (Fig. 1b). Nevertheless, B7-H4 was expressed in a significantly higher percentage of primary NHL cells than normal B cells or PBMCs (Fig. 1c, P < 0.01).
Expression of B7-H4 in human NHL cells. a B7-H4 gene expression examined by RT-PCR. b, c Surface protein expression of B7-H4 detected by flow cytometry in NHL cell lines (b red isotype control; green anti-B7-H4), and primary NHL cells obtained from 20 NHL patients, B cells from ten healthy donors, and PBMCs from eight healthy donors (c). d B7-H4 protein expression assessed by IHC staining of tissues from 60 patients with NHL and 15 patients with lymphadenitis. e Representative IHC staining of B7-H4 in NHL tissues. An IgG isotype control was used for negative control staining. Original magnification ×400. f Percentage of B7-H4+ tumor cells among the different NHL subsets: diffuse large B cell lymphoma (DLBCL, n = 29); mantle cell lymphoma (MCL, n = 5); extra-nodal marginal-zone lymphoma of mucosa-associated lymphoid tissue (MALT, n = 2); follicular lymphoma (FL, n = 1); marginal-zone lymphoma (MZL, n = 2); T cell-rich large B cell lymphoma (TRLBCL, n = 1); mature B cell lymphoma, unclassifiable (MBCL-u, n = 6); peripheral T cell lymphoma (PTCL, n = 4); anaplastic large cell lymphoma, ALK-positive (ALCL, n = 1); angioimmunoblastic T cell lymphoma (ATCL, n = 3); natural killer/T cell lymphoma (NK/TCL, n = 5), and T cell lymphoblastic lymphoma (T-LBL, n = 1). Representative samples of B7-H4 expression in NHL cells are shown. NHL non-Hodgkin lymphoma, RT-PCR reverse transcription PCR, PBMC peripheral blood mononuclear cells, IHC immunohistochemical staining, IgG immunoglobulin G
Next, we detected B7-H4 expression in tissues from patients with NHL (n = 60) and lymphadenitis (n = 15) by immunohistochemical staining. B7-H4 expression in NHL tissue was significantly higher than in tissue from lymphadenitis patients (Fig. 1d, P < 0.01). Representative images of B7-H4 immunohistochemical staining in NHL patient tissue are shown in Fig. 1e. Although heterogeneity in the frequency of B7-H4+ tumors was noted for the various NHL subsets (Fig. 1f), the expression of B7-H4 was detected in most NHL tumors examined.
IFN-γ, IL-6, and IL-10 enhance the surface protein expression of B7-H4 in NHL
The mechanisms of regulating B7-H4 expression in NHL are unclear. Therefore, we examined the effects of various inflammatory factors on NHL B7-H4 expression. NHL cell lines and primary NHL cells were cultured for 24 h, stimulated with IL-2, IL-4, IL-6, IL-10 (all at 5 ng/ml), lipopolysaccharide (LPS) (500 ng/ml), TNF-α (10 ng/ml), or IFN-γ (500 IU/ml), and the surface protein expression of B7-H4 was then examined by flow cytometry. We found that whereas IFN-γ, IL-6, and IL-10 upregulated B7-H4 surface protein expression in the NHL cell line Nal (Fig. 2a) and primary NHL cells isolated from three NHL patients (Fig. 2b–d), IL-2, IL-4, LPS, and TNF-α had little or no effect (data not shown).
Induced surface protein expression of B7-H4 in NHL cells. Surface protein expression of B7-H4 in a NHL cell line Nal and primary NHL tumor cells from b PT7, c PT8, and d PT9 following stimulation with IL-6 (5 ng/ml), IL-10 (5 ng/ml), and IFN-γ (500 IU/ml) for 24 h. (red isotype control; green anti-B7-H4). Representative samples of B7-H4 expression in NHL cells are shown. NHL, non-Hodgkin lymphoma, PT patient, IL interleukin, IFN interferon
tMφs upregulate the surface expression levels of B7-H4 in NHL cell lines and primary NHL cells via the secretion of IL-10 and IL-6
The tumor microenvironment is a complex cellular niche containing various cytokines. To determine whether this could affect B7-H4 expression in NHL cells, we incubated the Nal NHL cell line and primary NHL cells isolated from three patients for 24 h with conditioned medium collected from nMφs or tMφs. Surface protein expression levels of B7-H4 were shown to be augmented in Nal and primary NHL cells after stimulation with supernatants from tMφs, but not from nMφs (Fig. 3a).
B7-H4 expression in NHL cells upregulated by tMφs via IL-10 and IL-6 production. a Surface protein expression of B7-H4 (green) in NHL cell line Nal and primary NHL cells from PT7, PT8, and PT9 following stimulation with supernatants from tMφ or nMφ cultures for 24 h (red isotype control). b, c tMφs and nMφs were generated from four healthy donors (1–4) and cultured for 24 h, and supernatants were harvested for quantification of IL-10 (b) and IL-6 (c) by ELISA. d B7-H4 surface expression (green) in Nal cells and primary NHL cells from PT10 following 24 h incubation with supernatants from tMφs in the presence of 20 µg/ml IL-6-neutralizing mAb (α-IL-6), IL-10-neutralizing mAb (α-IL-10), or control IgG (α-IgG) (red isotype control). Representative data from three independent experiments are shown. NHL non-Hodgkin lymphoma, tMφ tumor-associated macrophage, IL interleukin, PT patient, nMφ normal macrophage, ELISA enzyme-linked immunosorbent assay, mAb monoclonal antibody, IgG immunoglobulin G
This finding suggested that tMφs produce a soluble factor that upregulates B7-H4 expression. Therefore, we analyzed the cytokine content of culture supernatants from nMφ and tMφ generated from four healthy donors. IL-10 (Fig. 3b) and IL-6 (Fig. 3c) were expressed at markedly higher levels in the supernatants of tMφs compared with nMφs, suggesting that IL-10 and IL-6 secreted by tMφs contribute to the upregulation of B7-H4 expression in NHL cells. To verify this, neutralizing IL-10 or IL-6 mAbs were added to tMφ-conditioned medium which was then used to incubate NHL cells. Neutralization of IL-10 or IL-6 effectively prevented surface B7-H4 upregulation (Fig. 3d), confirming that IL-10 and IL-6 secreted by tMφs induced surface protein expression of B7-H4 in NHL cells. There was no difference between the growth of NHL cells incubated with tMφ- and nMφ-conditioned medium (data not shown).
Generation of NHL-reactive T cell lines
The potential role of B7-H4 in regulating the host anti-tumor immunity in NHL is unclear. To explore this, we generated NHL-reactive T cell lines by incubating irradiated primary tumor cells from NHL patients with CD3+ T cells isolated from healthy allogeneic donors. Following several cycles of stimulation, NHL-reactive T cell lines were established and analyzed for cytolytic activity. NHL-reactive T cell lines (CTL1–4, generated against NHL cells from patients 1–4, respectively) showed efficient killing of their cognate tumor cells (Fig. 4a). In addition to killing the NHL cells they were raised against, the T cells were able to kill primary NHL cells from other patients, as illustrated for CTL4 in Fig. 4b. However, the myelogenous leukemia line K562 was not killed (Fig. 4b), suggesting that natural killer cells did not participate in cytolysis. Moreover, the T cells showed almost no cytolytic activity against normal B cells or PBMCs (Fig. 4b), indicating that the allogeneic T cell lines were reactive against tumor-specific antigens which are absent in normal blood cells.
Generation of NHL-reactive T cell lines. a Cytotoxicity of CTL lines 1–4. Cell lines were generated from healthy donor CD3+ cells against primary NHL cells from four patients (PT1–4) and tested against cognate tumor cells at E:T ratios of 40:1, 20:1, and 10:1. An E:T ratio of 20:1 was used for subsequent experiments. b Cytotoxicity of CTL4 against primary NHL cells from eight patients (PT1–8), as well as normal B cells and PBMCs from three NHL patients (B1–3 and PBMC1–3, respectively). K562 myelogenous leukemia cells were used to assess natural killer cell activity. c Suppression of CTL4 killing of NHL cells by anti-MHC class I mAb instead of anti-MHC-II or an isotype control mAbs (α-IgG). Similar results to those in b, c were obtained with T cell lines established from the other three healthy donors. **P < 0.01 compared with control T cells. NHL non-Hodgkin lymphoma, CTL cytotoxic T lymphocytes, CD cluster of differentiation, PT patients, E:T effector:target, PBMC peripheral blood mononuclear cells, MHC major histocompatibility complex, mAb monoclonal antibody, IgG immunoglobulin G
To investigate the major histocompatibility complex (MHC) restriction of NHL-specific killing, lysis assays were performed using anti-MHC class I or II mAbs or an isotype control mAb. Lysis of NHL cells was significantly suppressed when using the anti-MHC class I mAb compared with the anti-MHC class II or control IgG mAbs (Fig. 4c, P < 0.01 compared with the control group). These observations suggest that the cytotoxic activity of NHL-reactive T cell lines may depend on MHC class I-restricted CD8+ T cells.
B7-H4 directly inhibits the cytotoxicity of NHL-reactive T cells
We next investigated the cytotoxic activities of various NHL-reactive T cell lines against their target NHL cells. CTL-ctl, CTL-α-IgG, and CTL-α-B7-H4 lines were generated against NHL cells preincubated with medium, isotype control IgG, or anti-B7-H4 mAb, respectively. CTL-α-B7-H4 cells were significantly more cytotoxic than CTL-ctl and CTL-α-IgG lines (Fig. 5a, b, P < 0.05 and P < 0.01, respectively). Furthermore, preincubation of NHL cells with 20 µg/ml anti-B7-H4 mAb immediately prior to the killing assay significantly increased their cytotoxic activity compared with preincubation with control IgG or medium (Fig. 5c, d, P < 0.05 and P < 0.01, respectively). These observations strongly suggest that NHL-expressed B7-H4 directly inhibits the cytotoxic activity of NHL-reactive T cells.
Cytotoxic activity of NHL-reactive T cell lines. CTL2 and CTL11 lines were generated against NHL cells from PT2 and 11, respectively. NHL cells were pretreated with medium alone (ctl) or with 20 µg/ml anti-B7-H4 mAb (α-B7-H4) or control IgG (α-IgG). Cytotoxicity of CTL2-ctl, CTL2-α-IgG, and CTL2-α-B7-H4 against NHL cells from PT2 (a) and CTL11-ctl, CTL11-α-IgG, and CTL11-α-B7-H4 against NHL cells from PT11 (b). Cytotoxicity assay of CTL1 (c) and CTL2 (d) lines against NHL cells from PT1 and PT2, respectively. The killing assay was performed in medium alone (ctl) or in the presence of 20 µg/ml anti-B7-H4 mAb (α-B7-H4) or control IgG (α-IgG). *P < 0.05, **P < 0.01 compared with the control groups. NHL, non-Hodgkin lymphoma CTL cytotoxic T lymphocytes, PT patient, mAb monoclonal antibody, IgG immunoglobulin G
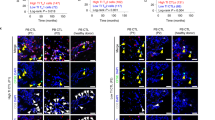
tMφ-induced upregulation of NHL B7-H4 expression confers resistance to NHL-reactive T cell-mediated cytolysis
To examine IFN-γ production by NHL-reactive T cells, the cells were co-cultured with primary NHL cells that were untreated or pretreated with control IgG or anti-B7-H4 mAb. IFN-γ released into the supernatants was then quantified by ELISA. Pretreatment with anti-B7-H4 mAb significantly enhanced IFN-γ secretion in the co-culture system compared with control groups (Fig. 6a, P < 0.05 and P < 0.01, respectively). All of the T cell lines raised against patient NHL cells produced IFN-γ; however, for each donor CTL-patient NHL pair, CTL-α-B7-H4 cells produced significantly higher levels of IFN-γ compared with CTL-ctl or CTL-α-IgG (Fig. 6b, P < 0.05).
B7-H4+ and tMφ-conditioned NHL cells are resistant to NHL-reactive T cell-mediated killing. NHL-reactive T cell lines were co-cultured with various conditioned NHL cells (E:T = 20:1), and IFN-γ in the supernatants was quantified by ELISA. a NHL-reactive T cell lines against target cells treated with medium (-ctl) or pretreated with 20 µg/ml anti-B7-H4 mAb (α-B7-H4) or control IgG (α-IgG). The mAbs were also present in the co-culture system. b Cytotoxicity of three different NHL-reactive T cell lines against NHL cells. c tMφ-conditioned NHL cells were resistant to NHL-reactive T cell-mediated lysis. d NHL-reactive T cell cytotoxicity to tMφ-conditioned NHL cells incubated with or without 20 µg/ml anti-IL-10, anti-IL-6, or anti-B7-H4 mAbs or control IgG. *P < 0.05, **P < 0.01 compared with medium control, control IgG, or nMφs. tMφ tumor-associated macrophages, NHL non-Hodgkin lymphoma, E:T effector:target, IFN interferon, ELISA enzyme-linked immunosorbent assay, mAb monoclonal antibody, IgG immunoglobulin G, IL interleukin, nMφ normal macrophages
We next explored the effects of tMφ-induced upregulation of B7-H4 in NHL cells on the cytotoxic activities of NHL-reactive T cell lines, and found that pretreatment of primary NHL cells with supernatants from tMφ cultures significantly decreased IFN-γ secretion by CTL, consistent with the upregulation of inhibitory B7-H4 (Fig. 6c, P < 0.01 compared with medium control or nMφs). The addition of anti-IL-10, anti-IL-6, or anti-B7-H4 mAbs to tMφ-conditioned medium partially reversed the suppression of IFN-γ production (Fig. 6d, P < 0.05 and P < 0.01 compared with medium control or control IgG). These observations suggest that tMφ-stimulated NHL cells are more resistant to cytolysis by NHL-reactive T cell lines because of the IL-6 and/or IL-10-induced upregulation of inhibitory B7-H4.
NHL-reactive T cell lines express perforin and granzyme B
We next investigated whether NHL-reactive T cell lines express the cytolytic proteins granzyme B and perforin. Flow cytometric analysis indicated that CD8+ T cells obtained from a representative CTL-ctl line expressed both perforin and granzyme B (Fig. 7a, b). Notably, no obvious differences were observed in the expression of either protein among CD8+ T cells isolated from CTL-ctl, CTL-α-IgG, or CTL-α-B7-H4 lines (Fig. 7c, d). Furthermore, none of the NHL-reactive T cell lines expressed FasL (data not shown). These data suggest that CD8+ T cell cytolysis of tumor cells is mediated via pathways involving perforin and/or granzyme B.
Phenotype of NHL-reactive T cell lines. Representative data showing perforin (a) and granzyme B (b) expression in CD8+ T cells of a CTL-ctl line generated from a healthy blood donor. Expression of perforin (c) and granzyme B (d) in CD8+ T cells of CTL-ctl, CTL-α-IgG, and CTL-α-B7-H4 lines generated from three healthy blood donors. NHL non-Hodgkin lymphoma, CD cluster of differentiation, CTL cytotoxic T lymphocyte, IgG immunoglobulin G
Discussion
Recent studies indicate that the growth and progression of malignant tumors are dependent on various immune escape mechanisms, including the downregulation of antigen-presenting MHC proteins [49], the production of inhibitory cytokines [50], the downregulation (or absence) of T cell costimulatory molecules [51], the expression of proteins that promote apoptosis [52], and the recruitment of immune inhibitory cells including regulatory T cells (Tregs), tMφs, and myeloid-derived suppressor cells [53]. However, the precise mechanisms of immune evasion by NHL remain unclear. In the present study, we detected B7-H4 gene and protein expression in NHL cells and tissues from NHL patients. Importantly, NHL-expressed B7-H4 inhibited specific T cell-mediated cytolysis of NHL cells. The identification of this novel mechanism of immune evasion may lead to the development of new agents that enhance the efficacy of treatment in NHL patients.
The function of B7-H4 in malignant tumors has previously been investigated. In cervical cancer patients, the ratio of tumor-infiltrating CD8+ T cells and the level of IFN-γ secretion were lower in patients with B7-H4+ tumors than in those with B7-H4-negative tumors [40]. Moreover, culturing T cells with recombinant B7-H4 reduced IFN-γ secretion and increased IL-10 and transforming growth factor (TGF)-ββ1 production [40]. IFN-γ secretion by CTLs co-cultured with lung cancer cells was increased when using an anti-B7-H4 mAb [54]. Moreover, lung cancer-expressed B7-H4 induced CTL apoptosis, and this effect was inhibited by the addition of a B7-H4-neutralizing antibody [54]. Tregs have been reported to induce IL-10 production, which in turn upregulates the expression of B7-H4 in myeloid dendritic cells and monocytes [55]. Furthermore, Treg-conditioned macrophages significantly inhibited T cell immunity via the upregulation of B7-H4 expression [55, 56]. However, different results are obtained with different experimental approaches. Indeed, other in vitro experiments suggested that the contribution of Tregs to antigen-presenting cell-induced T cell inhibition has not yet been fully elucidated [57].
It was unclear whether B7-H4 is necessary for immune evasion by NHL cells. Therefore, we examined the effect of NHL-expressed B7-H4 on the human immune response to NHL. NHL-reactive T cell lines were generated against allogeneic primary tumor cells pretreated with or without control or anti-B7-H4 mAb. NHL-expressed B7-H4 rendered tumor cells less sensitive to T cell-mediated cytotoxicity, and both killing and IFN-γ secretion were increased in the presence of anti-B7-H4 mAbs compared with control cells. By contrast, NHL-reactive T cells generated against NHL cells pretreated with anti-B7-H4 mAb showed greater cytotoxicity toward their tumor cell targets and produced more IFN-γ than T cells generated against NHL cells pretreated with or without control IgG. The cytotoxic activity was largely reduced by anti-MHC class I molecule mAb, indicating that CD8+ T cells played the most crucial part. We also detected perforin and granzyme B, but not FasL, in CD8+ T cells, suggesting that NHL-reactive T cell lines may kill NHL cells through perforin/granzyme B pathways [58, 59]. These results reveal that B7-H4 inhibits T cell-mediated immunity and imply that NHL-associated B7-H4 may participate in immune evasion in NHL.
Inflammation can promote oncogenesis and facilitate tumor growth and progression [60]. In the tumor microenvironment, inflammatory factors including IL-6, IL-10, vascular endothelial growth factor (VEGF), and TGF-β impair the induction of immunity and participate in tumor immune evasion [50]. Various factors participate in the regulation of B7-H4 expression. The expression of B7-H4 in renal cell carcinoma cells was upregulated after stimulation with IL-2, IFN-α, and IFN-γ [34], and macrophages were induced by ovarian cancer-related Tregs to produce IL-6 and IL-10, which then induced antigen-presenting cells to express B7-H4 [61]. In the present study, we found that IFN-γ, IL-6, and IL-10 enhanced surface protein expression levels of B7-H4 in NHL cells, implicating these cytokines involve in immune surveillance evasion by NHL cells.
Growing evidence indicates that tMφs are components of the inflammatory networks that facilitate tumor progression [54]. The percentage of tMφs in the tumor mass can reach as high as 80% and can predict clinical outcome in some tumor types [61, 62]. tMφs have also been confirmed to be present in NHL. In FL, the ratio of tMφs per high-power magnification field was more than 58%, and patients with high tMφs levels showed a reduced median overall survival compared with those with low tMφs levels [63]. Other studies demonstrated a shorter overall survival for FL patients with tumors expressing macrophage-associated genes compared with T cell-associated genes [64, 65]. Large numbers of tMφs were also related to poor prognosis, survival, and response to treatment in cutaneous T cell lymphoma patients [66], and a high percentage of tMφs suggested poor clinical outcome in DLBCL patients [67]. tMφs produce IL-6, IL-10, IL-12, VEGF, and TGF-β [48, 54]. Here, we detected large quantities of IL-6 and IL-10 in supernatants of tMφ cultures compared with nMφs. Thus, we hypothesized that tMφs affect the surface protein expression of B7-H4 in NHL cells. Indeed, NHL cells stimulated with tMφ supernatants showed upregulated surface protein expression of B7-H4, and this was decreased by neutralizing anti-IL-6 or anti-IL-10 mAbs, confirming that tMφs induced surface protein expression of B7-H4 in NHL cells via secreted IL-6 and IL-10. These results also revealed that tMφ-induced expression of B7-H4 in NHL cells contributed to the inhibition of T cell-mediated cytotoxicity. NHL cells incubated with tMφ-conditioned medium were more resistant to T cell-induced cytolysis than cells incubated with nMφ-conditioned medium. Here, too, anti-IL-6, anti-IL-10, and anti-B7-H4 mAbs were able to partially reverse the suppression of cytolysis.
In summary, this study demonstrates that B7-H4 is expressed by NHL cell lines, primary cells and tissues from NHL patients, and that IL-6, IL-10, and IFN-γ further upregulate the surface protein expression of B7-H4. NHL-associated B7-H4 protected tumor cells against killing by NHL-reactive T cells. Moreover, tMφs augmented the surface protein expression of B7-H4 in NHL cells via the secretion of IL-6 and IL-10, thereby allowing NHL cells to escape killing by T cells. Therefore, the present study implicates NHL-expressed B7-H4 in the suppression of anti-tumor immunity in NHL. Targeting B7-H4 may represent a new immunotherapeutic strategy for patients with B7-H4+ NHL by enhancing tumor-specific T cell-mediated cytolysis.
Abbreviations
- CTL:
-
Cytotoxic T lymphocyte
- DLBCL:
-
Diffuse large B cell lymphoma
- FasL:
-
Fas ligand
- FL:
-
Follicular lymphoma
- IFN:
-
Interferon
- IL:
-
Interleukin
- IgG:
-
Immunoglobulin G
- LPS:
-
Lipopolysaccharide
- Ly10:
-
OCI-Ly10
- mAb:
-
Monoclonal antibody
- M-CSF:
-
Macrophage colony-stimulating factor
- MHC:
-
Major histocompatibility complex
- Nal:
-
Nalmawa
- NHL:
-
Non-Hodgkin lymphoma
- nMφs:
-
Normal macrophages
- PBMCs:
-
Peripheral blood mononuclear cells
- PD:
-
Programmed death protein
- PD-L:
-
Programmed death ligand
- TGF:
-
Transforming growth factor
- tMφs:
-
Tumor-associated macrophages
- TNF:
-
Tumor necrosis factor
- Tregs:
-
Regulatory T cells
- VEGF:
-
Vascular endothelial growth factor
References
Havranek O, Kleiblova P, Hojny J, Lhota F, Soucek P, Trneny M, Kleibl Z (2015) Association of Germline CHEK2 gene variants with risk and prognosis of Non-Hodgkin lymphoma. PLoS One 10:e0140819. doi:10.1371/journal.pone.0140819
Glass S, Phan A, Williams JN, Flowers CR, Koff JL (2016) Integrating understanding of epidemiology and genomics in B-cell non-Hodgkin lymphoma as a pathway to novel management strategies. Discov Med 21:181–188
Grover NS, Park SI (2015) Novel targeted agents in Hodgkin and non-Hodgkin lymphoma therapy. Pharmaceuticals (Basel) 8:607–636. doi:10.3390/ph8030607
Stienen JJ, Hermens RP, Wennekes L et al (2015) Variation in guideline adherence in non-Hodgkin’s lymphoma care: impact of patient and hospital characteristics. BMC Cancer 15:578. doi:10.1186/s12885-015-1547-8
Zappasodi R, de Braud F, Di Nicola M (2015) Lymphoma immunotherapy: current status. Front Immunol 6:448. doi:10.3389/fimmu.2015.00448
Germain C, Guillaudeux T, Galsgaard ED et al. (2015) Lectin-like transcript 1 is a marker of germinal center-derived B-cell non-Hodgkin’s lymphomas dampening natural killer cell functions. Oncoimmunology 4:e1026503. doi:10.1080/2162402X.2015.1026503
Gabellier L, Cartron G (2016) Obinutuzumab for relapsed or refractory indolent non-Hodgkin’s lymphomas. Ther Adv Hematol 7:85–93. doi:10.1177/2040620715622613
Wang L, Qian J, Lu Y et al (2013) Immune evasion of mantle cell lymphoma: expression of B7-H1 leads to inhibited T-cell response to and killing of tumor cells. Haematologica 98:1458–1466. doi:10.3324/haematol.2012.071340
Hollander N (2012) Immunotherapy for B-cell lymphoma: current status and prospective advances. Front Immunol 3:3. doi:10.3389/fimmu.2012.00003
Sakamaki I, Qin H, Kwak LW (2011) Translational development of vaccination strategies in follicular NHL. Best Pract Res Clin Haematol 24:295–304. doi:10.1016/j.beha.2011.03.007
Schuster SJ, Neelapu SS, Gause BL et al (2011) Vaccination with patient-specific tumor-derived antigen in first remission improves disease-free survival in follicular lymphoma. J Clin Oncol 29:2787–2794. doi:10.1200/JCO.2010.33.3005
Kline J, Bishop MR (2015) Update on checkpoint blockade therapy for lymphoma. J Immunother Cancer 3:33. doi:10.1186/s40425-015-0079-8
Bagley SJ, Bauml JM, Langer CJ (2015) PD-1/PD-L1 immune checkpoint blockade in non-small cell lung cancer. Clin Adv Hematol Oncol 13:676–683
Westin JR, Chu F, Zhang M et al (2014) Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol 15:69–77. doi:10.1016/S1470-2045(13)70551-5
Choi IH, Zhu G, Sica GL et al (2003) Genomic organization and expression analysis of B7-H4, an immune inhibitory molecule of the B7 family. J Immunol 171:4650–4654
Sica GL, Choi IH, Zhu G et al (2003) B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity 18:849–861
Salceda S, Tang T, Kmet M, Munteanu A, Ghosh M, Macina R, Liu W, Pilkington G, Papkoff J (2005) The immunomodulatory protein B7-H4 is overexpressed in breast and ovarian cancers and promotes epithelial cell transformation. Exp Cell Res 306:128–141. doi:10.1016/j.yexcr.2005.01.018
Hansen JD, Du Pasquier L, Lefranc MP, Lopez V, Benmansour A, Boudinot P (2009) The B7 family of immunoregulatory receptors: a comparative and evolutionary perspective. Mol Immunol 46:457–472. doi:10.1016/j.molimm.2008.10.007
Wang L, Heng X, Lu Y, Cai Z, Yi Q, Che F (2016) Could B7-H4 serve as a target to activate anti-cancer immunity? Int Immunopharmacol 38:97–103. doi:10.1016/j.intimp.2016.05.020
Cheng C, Qu QX, Shen Y, Lv YT, Zhu YB, Zhang XG, Huang JA (2011) Overexpression of B7-H4 in tumor infiltrated dendritic cells. J Immunoassay Immunochem 32:353–364. doi:10.1080/15321819.2011.578190
Seliger B, Quandt D (2012) The expression, function, and clinical relevance of B7 family members in cancer. Cancer Immunol Immunother 61:1327–1341. doi:10.1007/s00262-012-1293-6
Jeon H, Ohaegbulam KC, Abadi YM, Zang X (2013) B7x and myeloid-derived suppressor cells in the tumor microenvironment: a tale of two cities. Oncoimmunology 2:e24744. doi:10.4161/onci.24744
Arigami T, Uenosono Y, Ishigami S, Hagihara T, Haraguchi N, Natsugoe S (2011) Clinical significance of the B7-H4 coregulatory molecule as a novel prognostic marker in gastric cancer. World J Surg 35:2051–2057. doi:10.1007/s00268-011-1186-4
Shi H, Ji M, Wu J et al (2014) Serum B7-H4 expression is a significant prognostic indicator for patients with gastric cancer. World J Surg Oncol 12:188. doi:10.1186/1477-7819-12-188
Geng Y, Wang H, Lu C, Li Q, Xu B, Jiang J, Wu C (2015) Expression of costimulatory molecules B7-H1, B7-H4 and Foxp3(+) Tregs in gastric cancer and its clinical significance. Int J Clin Oncol 20:273–281. doi:10.1007/s10147-014-0701-7
Matsunaga T, Saito H, Ikeguchi M (2011) Increased B7-H1 and B7-H4 expressions on circulating monocytes and tumor-associated macrophages are involved in immune evasion in patients with gastric cancer. Yonago Acta Med 54:1–10.
Jiang J, Zhu Y, Wu C et al (2010) Tumor expression of B7-H4 predicts poor survival of patients suffering from gastric cancer. Cancer Immunol Immunother 59:1707–1714. doi:10.1007/s00262-010-0900-7
Zhao LW, Li C, Zhang RL, Xue HG, Zhang FX, Zhang F, Gai XD (2014) B7-H1 and B7-H4 expression in colorectal carcinoma: correlation with tumor FOXP3(+) regulatory T-cell infiltration. Acta Histochem 116:1163–1168. doi:10.1016/j.acthis.2014.06.003
Chen Y, Sun J, Zhao H et al. (2014) The coexpression and clinical significance of costimulatory molecules B7-H1, B7-H3, and B7-H4 in human pancreatic cancer. Onco Targets Ther 7:1465–1472. doi:10.2147/OTT.S66809
Mugler KC, Singh M, Tringler B, Torkko KC, Liu W, Papkoff J, Shroyer KR (2007) B7-h4 expression in a range of breast pathology: correlation with tumor T-cell infiltration. Appl Immunohistochem Mol Morphol 15:363–370. doi:10.1097/01.pai.0000213159.79557.71
Li ZY, Zhang XH, Chen Y, Guo JG, Sai K, Yang QY, Chen ZP, Mou YG (2013) Clinical significance of B7-H4 expression in matched non-small cell lung cancer brain metastases and primary tumors. Oncol Targets Ther 6:869–875. doi:10.2147/OTT.S48085
Sun Y, Wang Y, Zhao J, Gu M, Giscombe R, Lefvert AK, Wang X (2006) B7-H3 and B7-H4 expression in non-small-cell lung cancer. Lung Cancer 53:143–151. doi:10.1016/j.lungcan.2006.05.012
Zhu J, Chu BF, Yang YP, Zhang SL, Zhuang M, Lu WJ, Liu YB (2013) B7-H4 expression is associated with cancer progression and predicts patient survival in human thyroid cancer. Asian Pac J Cancer Prev 14:3011–3015
Xu Y, Zhu S, Song M, Liu W, Liu C, Li Y, Wang M (2014) B7-H4 expression and its role in interleukin-2/interferon treatment of clear cell renal cell carcinoma. Oncol Lett 7:1474–1478. doi:10.3892/ol.2014.1961
Jung SG, Choi KU, Lee SD, Lee ZZ, Chung MK (2011) The relationship between B7-H4 expression and clinicopathological characteristics in clinical stage T1 conventional renal cell carcinoma. Korean J Urol 52:90–95. doi:10.4111/kju.2011.52.2.90
Krambeck AE, Thompson RH, Dong H et al (2006) B7-H4 expression in renal cell carcinoma and tumor vasculature: associations with cancer progression and survival. Proc Natl Acad Sci USA 103:10391–10396. doi:10.1073/pnas.0600937103
Zhang L, Wu H, Lu D et al (2013) The costimulatory molecule B7-H4 promote tumor progression and cell proliferation through translocating into nucleus. Oncogene 32:5347–5358. doi:10.1038/onc.2012.600
Quandt D, Fiedler E, Boettcher D, Marsch W, Seliger B (2011) B7-h4 expression in human melanoma: its association with patients’ survival and antitumor immune response. Clin Cancer Res 17:3100–3111. doi:10.1158/1078-0432.CCR-10-2268
Zhang C, Li Y, Wang Y (2015) Diagnostic value of serum B7-H4 for hepatocellular carcinoma. J Surg Res 197:301–306. doi:10.1016/j.jss.2015.04.034
Wang X, Wang T, Xu M, Xiao L, Luo Y, Huang W, Zhang Y, Geng W (2014) B7-H4 overexpression impairs the immune response of T cells in human cervical carcinomas. Hum Immunol 75:1203–1209. doi:10.1016/j.humimm.2014.10.002
Maskey N, Li K, Hu M et al (2014) Impact of neoadjuvant chemotherapy on lymphocytes and co-inhibitory B7-H4 molecule in gastric cancer: low B7-H4 expression associates with favorable prognosis. Tumour Biol 35:11837–11843. doi:10.1007/s13277-014-2410-2
Xu H, Chen X, Tao M, Chen K, Chen C, Xu G, Li W, Yuan S, Mao Y (2016) B7-H3 and B7-H4 are independent predictors of a poor prognosis in patients with pancreatic cancer. Oncol Lett 11:1841–1846. doi:10.3892/ol.2016.4128
Chen X, Wang L, Wang W, Zhao L, Shan B (2016) B7-H4 facilitates proliferation of esophageal squamous cell carcinoma cells through promoting interleukin-6/signal transducer and activator of transcriotion 3 pathway activation. Cancer Sci 107:944–954. doi:10.1111/cas.12949
Abadi YM, Jeon H, Ohaegbulam KC et al (2013) Host b7x promotes pulmonary metastasis of breast cancer. J Immunol 190:3806–3814. doi:10.4049/jimmunol.1202439
Sabattini E, Bacci F, Sagramoso C, Pileri SA (2010) WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica 102:83–87
Wang L, Zhao Y, Qian J et al (2013) Toll-like receptor-4 signaling in mantle cell lymphoma: effects on tumor growth and immune evasion. Cancer 119:782–791. doi:10.1002/cncr.27792
Kikuchi T, Crystal RG (2001) Antigen-pulsed dendritic cells expressing macrophage-derived chemokine elicit Th2 responses and promote specific humoral immunity. J Clin Invest 108:917–927. doi:10.1172/JCI11564
Zheng Y, Cai Z, Wang S et al (2009) Macrophages are an abundant component of myeloma microenvironment and protect myeloma cells from chemotherapy drug-induced apoptosis. Blood 114:3625–3628. doi:10.1182/blood-2009-05-220285
Zindl CL, Chaplin DD (2010) Immunology. Tumor immune evasion. Science 328:697–698. doi:10.1126/science.1190310
Wang S, Yang J, Qian J, Wezeman M, Kwak LW, Yi Q (2006) Tumor evasion of the immune system: inhibiting p38 MAPK signaling restores the function of dendritic cells in multiple myeloma. Blood 107:2432–2439. doi:10.1182/blood-2005-06-2486
Smyth MJ, Godfrey DI, Trapani JA (2001) A fresh look at tumor immunosurveillance and immunotherapy. Nat Immunol 2:293–299. doi:10.1038/86297
Freedman A, Neelapu SS, Nichols C et al (2009) Placebo-controlled phase III trial of patient-specific immunotherapy with mitumprotimut-T and granulocyte-macrophage colony-stimulating factor after rituximab in patients with follicular lymphoma. J Clin Oncol 27:3036–3043. doi:10.1200/JCO.2008.19.8903
Laurent C, Charmpi K, Gravelle P et al. (2015) Several immune escape patterns in non-Hodgkin’s lymphomas. Oncoimmunology 4:e1026530. doi:10.1080/2162402X.2015.1026530
Chen C, Qu QX, Shen Y, Mu CY, Zhu YB, Zhang XG, Huang JA (2012) Induced expression of B7-H4 on the surface of lung cancer cell by the tumor-associated macrophages: a potential mechanism of immune escape. Cancer Lett 317:99–105. doi:10.1016/j.canlet.2011.11.017
Kryczek I, Wei S, Zou L, Zhu G, Mottram P, Xu H, Chen L, Zou W (2006) Cutting edge: induction of B7-H4 on APCs through IL-10: novel suppressive mode for regulatory T cells. J Immunol 177:40–44
Kryczek I, Zou L, Rodriguez P et al (2006) B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med 203:871–881. doi:10.1084/jem.20050930
Mirza N, Gabrilovich D (2007) Comment on “Cutting edge: induction of B7-H4 on APCs through IL-10: novel suppressive mode for regulatory T cells”. J Immunol 178:4705–4706 (author reply 4706)
Kagi D, Vignaux F, Ledermann B, Burki K, Depraetere V, Nagata S, Hengartner H, Golstein P (1994) Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science 265:528–530
Kojima H, Shinohara N, Hanaoka S et al (1994) Two distinct pathways of specific killing revealed by perforin mutant cytotoxic T lymphocytes. Immunity 1:357–364
Rook GA, Dalgleish A (2011) Infection, immunoregulation, and cancer. Immunol Rev 240:141–159. doi:10.1111/j.1600-065X.2010.00987.x
Kryczek I, Wei S, Zhu G, Myers L, Mottram P, Cheng P, Chen L, Coukos G, Zou W (2007) Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Res 67:8900–8905. doi:10.1158/0008-5472.CAN-07-1866
Morantz RA, Wood GW, Foster M, Clark M, Gollahon K (1979) Macrophages in experimental and human brain tumors. Part 2: studies of the macrophage content of human brain tumors. J Neurosurg 50:305–311. doi:10.3171/jns.1979.50.3.0305
Farinha P, Masoudi H, Skinnider BF et al (2005) Analysis of multiple biomarkers shows that lymphoma-associated macrophage (LAM) content is an independent predictor of survival in follicular lymphoma (FL). Blood 106:2169–2174. doi:10.1182/blood-2005-04-1565
Leich E, Hartmann EM, Burek C, Ott G, Rosenwald A (2007) Diagnostic and prognostic significance of gene expression profiling in lymphomas. APMIS 115:1135–1146. doi:10.1111/j.1600-0463.2007.apm_867.xml.x
Alvaro T, Lejeune M, Camacho FI et al (2006) The presence of STAT1-positive tumor-associated macrophages and their relation to outcome in patients with follicular lymphoma. Haematologica 91:1605–1612
Assaf C, Hwang ST (2016) Mac attack: macrophages as key drivers of cutaneous T-cell lymphoma pathogenesis. Exp Dermatol 25:105–106. doi:10.1111/exd.12894
Shen L, Li H, Shi Y, Wang D, Gong J, Xun J, Zhou S, Xiang R, Tan X (2016) M2 tumour-associated macrophages contribute to tumour progression via legumain remodelling the extracellular matrix in diffuse large B-cell lymphoma. Sci Rep 6:30347. doi:10.1038/srep30347
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This work was supported by Funds for Young Scholars of the National Natural Science Foundation of China (Grant No. 81402353), the Medical Health Science and Technology Development Plan of Shandong Province (Grant No. 2014WS0287), the China Postdoctoral Science Foundation (Grant No. 2015M580594), the Postdoctoral Innovation Foundation of Shandong Province (Grant No. 201502008), the Key Research Project program of Shandong Province (Grant No. 2016GSF201056), and the Natural Science Foundation of Shandong Province (Grant No. ZR2014HM077).
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Che, F., Heng, X., Zhang, H. et al. Novel B7-H4-mediated crosstalk between human non-Hodgkin lymphoma cells and tumor-associated macrophages leads to immune evasion via secretion of IL-6 and IL-10. Cancer Immunol Immunother 66, 717–729 (2017). https://doi.org/10.1007/s00262-017-1961-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-017-1961-7