Abstract
Nasal natural killer/T-cell lymphoma (NNKTL) is an aggressive neoplasm with poor therapeutic responses and prognosis. The programmed death-1/programmed death-ligand 1 (PD-1/PD-L1) pathway plays an important role in immune evasion of tumor cells through T-cell exhaustion. The aim of the present study was to examine the expression of PD-L1 and PD-1 molecules in NNKTL. We detected the expression of PD-L1 in biopsy samples from all of the NNKTL patients studied. PD-L1 was found on both malignant cells and tumor-infiltrating macrophages, while PD-1-positive mononuclear cells infiltrated the tumor tissues in 36% of patients. Most significantly, soluble PD-L1 (sPD-L1) was present in sera of NNKTL patients at higher levels as compared to healthy individuals and the levels of serum sPD-L1 in patients positively correlated with the expression of PD-L1 in lymphoma cells of tumor tissues. In addition, the high-sPD-L1 group of patients showed significantly worse prognosis than the low-sPD-L1 group. Furthermore, we confirmed that membrane and soluble PD-L1 was expressed on the surface and in the culture supernatant, respectively, of NNKTL cell lines. The expression of PD-L1 was observed in tumor tissues and sera from a murine xenograft model inoculated with an NNKTL cell line. Our results suggest that sPD-L1 could be a prognostic predictor for NNKTL and open up the possibility of immunotherapy of this lymphoma using PD-1/PD-L1 axis inhibitors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nasal natural killer (NK)/T-cell lymphoma (NNKTL) is characterized by progressive necrotic lesions in the nasal cavity, palate, and/or nasopharynx [1]. NNKTL cells are derived from two CD56-positive lineages, NK- and γδT-cell, although the NK-cell lineage is the predominant phenotype [2–4]. Our previous studies further showed the presence of Epstein-Barr virus (EBV) DNA, EBV oncogenic proteins, and the clonotypic EBV genome in this lymphoma, indicating that NNKTL is an EBV-related malignancy [2, 3, 5].
It has been established histologically that many inflammatory cells such as lymphocytes, monocytes, and macrophages infiltrate NNKTL tissue [1]. This finding gives rise to the speculation that tumor-reactive cytotoxic T lymphocytes (CTLs) might also exist in NNKTL tissue to attack the lymphoma cells. It is especially possible that EBV-specific CTLs could recognize and destroy NNKTL cells infected by EBV, because EBV-associated proteins are exogenous antigens that induce immune responses [6]. However, NNKTL shows poor prognosis caused by its rapid progression, suggesting that this lymphoma might be able to evade the antitumor immune response using several mechanisms. For example, several amino-acid changes are seen in the sequence encoding the major HLA-A2 restricted CTL epitopes of latent membrane protein (LMP)-1 and LMP-2A, which are EBV oncogenic proteins, resulting in the reduction of CTL recognition for lymphoma cells [7, 8]. In addition, NNKTL cells secrete immune suppressive factors such as interleukin (IL)-10 [9, 10] and transforming growth factor-β [11] that suppress the activity of CTLs, inhibit the effect of tumor-specific helper T cells, and skew naïve helper T cells to regulatory T cells. Although numerous groups including our group are involved in clarifying the immune regulation of NNKTL, details regarding immune evasion are unknown.
Many research groups have recently focused on the programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) axis, which is an immune checkpoint pathway. In general, the PD-1 receptor is expressed on activated T cells, while the PD-L1 ligand is expressed on tumor cells or antigen-presenting cells [12, 13]. The interaction between PD-1 and PD-L1 attenuates T-cell function and proliferation, leading to antitumor immunosuppression through CTL exhaustion [14]. Thus, blockade of the PD-1/PD-L1 pathway with monoclonal antibodies (mAbs) can restore and enhance the CTL response, promoting T-cell survival and proliferation [15]. Furthermore, a number of investigators have reported that a soluble form of PD-L1 can be detected in the blood of patients with some malignant diseases and might be a potent predictive biomarker [16–19]. To further our understanding of the mechanism of immune evasion by NNKTL, it is, therefore, of great interest to examine the expression of PD-L1 and PD-1 in this lymphoma.
In the present study, we aimed to assess the expression of membrane and soluble PD-L1 (sPD-L1) as well as of PD-1 in NNKTL. Using biopsy tissues, cell lines, and a murine xenograft model, we clearly show that PD-L1 is expressed on tumor cells and tumor-infiltrating macrophages. Furthermore, we show that increased sPD-L1 levels are detectable in the blood of patients and correlate with poor prognosis. These results suggest that the PD-1/PD-L1 pathway may play an important role in evasion of the antitumor immune response and that sPD-L1 may be a valuable predictor for prognosis in NNKTL.
Materials and methods
Patients
Seventeen patients with NNKTL were analyzed in this study, all of whom were newly diagnosed according to the World Health Organization classification of hematological malignancies at Asahikawa Medical University (Asahikawa, Japan) between 2000 and 2014. Patient clinicopathological characteristics and follow-up data are summarized in Tables 1 and 2. Serum lactate dehydrogenase (LDH) was considered elevated if >240 IU/mL. The International Prognostic Index (IPI) and the prognostic index of natural killer lymphoma (PINK) were determined based on published criteria [20, 21]. As the primary treatment, all patients received concurrent chemoradiotherapy. Patients were treated with MPVIC-P (ifosfamide, carboplatin, methotrexate, peplomycin, etoposide, and prednisolone) (12 patients) [22], DeVIC (dexamethasone, etoposide, ifosfamide, and carboplatin) (two patients) [23], MTCOP-P (pirarubicin, cyclophosphamide, vincristine, methotrexate, peplomycin, and prednisolone) (one patient) [24], or THP-COP (pirarubicin, cyclophosphamide, vincristine, and prednisolone) (one patient) [25] chemotherapy regimens. One patient received etoposide alone. We also analyzed 23 healthy volunteers as controls. All patients and volunteers signed informed consent forms. This study was conducted with the approval of the Institutional Review Board at Asahikawa Medical University.
Cell lines
The characteristics of the cell lines are summarized in Supplemental Table S1. SNK-6 [4], SNK-1 [26], and SNK-10 [27] were provided by Dr. Norio Shimizu (Tokyo Medical and Dental University, Tokyo, Japan). Raji was established from Burkitt lymphoma [28]. HDLM-2 [29] and Molt-4 [30] were purchased from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany) and the American Type Culture Collection (Manassas, VA), respectively. SNK-6, SNK-1, and SNK-10 were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) and 700 units/mL recombinant human IL-2. Raji, HDLM-2, and Molt-4 were cultured in RPMI 1640 supplemented with 10% FBS. All cell lines were used within ten passages after thawing of frozen cells.
Immunohistochemical staining and in situ hybridization
Formalin-fixed, paraffin-embedded (FFPE) samples were prepared from pretreatment biopsy tissues of NNKTL patients or the subcutaneous tumors of mice transplanted with SNK-6 cells and were cut into 4-µm-thick sections. We used anti-PD-L1 (E1L3N, 1:200, Cell Signaling Technology), anti-PD-1 (NAT105, 1:100, dianova), anti-CD56 (1:50, Novocastra), anti-CD68 (PG-M1, 1:50, DAKO), and anti-CD8 (C8/144B, 1:100, DAKO) mAbs as the primary Ab. The Envision HRP System (DAKO) was used for visualization of the signal. For antigen retrieval, slides were treated with Target Retrieval Solution pH 9 (DAKO) for 15 min in a microwave oven. Serial sections were used for PD-L1 and CD56, PD-L1 and CD68, PD-L1 and PD-1, CD8 and PD-1, or CD56 and CD68 staining. We considered a case CD68-positive if >5% of the cells in tumor cell regions were positive for CD68 staining in three representative high-power (400×) fields [31]. A case was considered as PD-L1 positive if >10% of the CD56-positive or CD68-positive cells were also PD-L1 positive, as previously described by other groups [32, 33]. When >5% of tumor-infiltrating mononuclear immune cells were PD-1 positive, the specimen was defined as PD-1 positive [34, 35]. EBV encoded small RNA (EBER) in FFPE tissue sections was detected by in situ hybridization (ISH) as previously described [3].
Immunofluorescent staining
Double immunofluorescent staining was performed on 2-µm-thick FFPE sections. After antigen retrieval using Target Retrieval Solution High pH (DAKO), the sections were incubated with anti-PD-L1 mAb (E1L3N, 1:500, Cell Signaling Technology) at room temperature (RT) for 60 min. The sections were treated with secondary Abs, Histofine Simple Stain MAX PO (Nichirei Biosciences Inc.), at RT for 30 min, and were then treated with the TSA Cyanine 3 system (PerkinElmer Inc.) at RT for 10 min. A second antigen retrieval was performed using Target Retrieval Solution Citrate pH 6 (DAKO). The sections were incubated with anti-CD56 mAb (1:150, Novocastra) or anti-CD68 mAb (PG-M1, 1:150, DAKO) at 4 °C overnight, and then with anti-mouse AlexaFluor 488 (Abcam) at RT for 120 min. After DAPI (Invitrogen) staining, the sections were embedded in mounting medium.
Measurement of sPD-L1 using ELISA
sPD-L1 in blood from patients or mice was measured using the PDCD1LG1 ELISA Kit (USCN Life Science Inc.). All sera from patients were taken at diagnosis and were frozen at −80 °C. sPD-L1 in cell culture supernatants was measured using the PathScan Total PD-L1 Sandwich ELISA Kit (Cell Signaling Technology). Recombinant human PD-L1 (R&D Systems) was used as the standard. Cell lines (5 × 105/mL) were cultured in 96-well round-bottomed plates, and supernatants of the cell cultures were collected after 24 h. The minimum detectable concentration was 13 pg/mL and the minimum quantitative range was 49 pg/mL. Measurements for serum samples and cell culture supernatants were done in duplicate and triplicate, respectively. The intra-assay and inter-assay variations were below 20%. The results correspond to means ± SEM.
Flow cytometric analysis
Flow cytometric analysis was done using anti-PD-L1 (MIH1) or PD-1 (MIH4) mAb conjugated with PE (eBioscience) as previously described [36]. PE-conjugated mouse IgG1 kappa (eBioscience) was used as an isotype control. The FACS Accuri flow cytometer (BD Biosciences) was used to measure fluorescence and analyze the data.
Western blot analysis
Western blot analysis was performed using anti-PD-L1 (E1L3N, 1:1000, Cell Signaling Technology), anti-PD-1 (D4W2J, 1:1000, Cell Signaling Technology), or control anti-β-Actin (C4, 1:1000, Santa Cruz Biotechnology) mAb as previously described [6]. ImageJ software (National Institute of Health) was used to quantify the amount of PD-L1 protein against β-Actin.
Transplantation of SNK-6 cells into NOD/Shi-scid/IL-2Rγnull (NOG) mice
Six-week old female NOG mice were obtained from the Central Institute for Experimental Animals (Kawasaki, Japan) and were maintained under specific pathogen free conditions in the animal laboratory of the Center for Advanced Research and Education, Asahikawa Medical University (Asahikawa, Japan). Twelve mice were injected subcutaneously with 2 × 106 SNK-6 cells in a shaved flank. Thirty days later, the mice were sacrificed, and the subcutaneous tumors or blood samples were collected. All animal experiments were approved by the Institutional Animal Care and Use Committee of Asahikawa Medical University.
Statistical analysis
The Mann–Whitney U test was used to compare sPD-L1 values between NNKTL patients and healthy controls. The relationship between the serum sPD-L1 values and the expression of molecules including PD-L1 in tissues was evaluated by the Spearman rank correlation. The optimal cut-off value of serum sPD-L1 was determined based on receiver-operating characteristic (ROC) curve analysis for survival (death due to NNKTL or censored). The Kaplan–Meier method was used to estimate survival curves and the statistical significance of differences in survival curves was examined using the log-rank test. Correlations between the sPD-L1 level and the main clinical features were determined using Fisher’s exact test. P < 0.05 was considered statistically significant. All graphics and analyses were done using GraphPad Prism 5 (GraphPad Software) and JMP version 12 (SAS Institute).
Results
Lymphoma cells and tumor-infiltrating macrophages in tissues from NNKTL patients express PD-L1
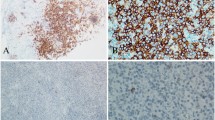
We initially assessed whether the expression of PD-L1 was detected in the tissues from 15 of the 17 NNKTL patients using immunohistological staining (Table 2). Atypical lymphoid cells infiltrated the nasal mucosa in all samples (Fig. 1a). In addition, most tumor cells were positive for EBER (Fig. 1b). Staining using serial sections showed that both CD56 and PD-L1 were expressed in all 15 patients and that CD56-positive lymphoma cells were preferentially localized at PD-L1-expressing areas (Fig. 1c, d). To confirm that tumor cells expressed PD-L1, double immunofluorescent staining for PD-L1 and CD56 was performed. CD56-positive cells coexpressed PD-L1 (Fig. 1e). This result indicated that the malignant cells of all 15 patients showed positive PD-L1 staining.
Expression of PD-L1 on lymphoma cells in biopsy tissues from NNKTL patients. a–d Representative immunohistological features of FFPE samples (Patient 17). a Nasal mucosa is infiltrated by atypical lymphoid cells (H&E staining). b ISH for EBER. Nuclei of EBER-positive cells are stained (brown). c Staining for CD56. d Staining for PD-L1. Serial sections were used for c and d. Scale bar in a–d is 100 µm. e Double immunofluorescent staining of CD56 (green) and PD-L1 (red), counterstained with DAPI (blue). Arrows indicate colocalization of CD56 and PD-L1. Scale bar is 50 µm
It is known that tumor-infiltrating CD68-positive macrophages in several lymphomas such as Hodgkin’s lymphoma and diffuse large B-cell lymphoma strongly express PD-L1 [32, 33]. We, therefore, investigated whether CD68-positive macrophages infiltrated NNKTL tissues. CD68-positive macrophages (Fig. 2a) were observed in all 14 of the patients tested, as shown in Table 2. Furthermore, immunohistochemical staining using serial sections indicated that CD68-positive macrophages were localized at regions corresponding to PD-L1-expressing areas (Fig. 2a, b). Double immunofluorescent staining for PD-L1 and CD68 confirmed that CD68-positive cells coexpressed PD-L1 (Fig. 2c), indicating that tumor-infiltrating CD68-positive macrophages clearly expressed PD-L1 in NNKTL.
Expression of PD-L1 on tumor-infiltrating macrophages in biopsy tissues from NNKTL patients. Representative immunohistological features of FFPE samples (Patient 16) stained for CD68 (a) and PD-L1 (b). Serial sections were used for a and b. Scale bar in a and b is 100 µm. c Double immunofluorescent staining of CD68 (green) and PD-L1 (red), counterstained with DAPI (blue). Arrows indicate colocalization of CD68 and PD-L1. Scale bar is 50 µm
PD-1-positive mononuclear immune cells infiltrate the tissues of patients with NNKTL
We next investigated the expression of PD-1 in NNKTL tissues. Immunohistological staining showed that 5 of 14 samples (36%) were positive for PD-1 (Table 2). Representative immunohistological staining of PD-1-positive mononuclear cells is shown in Fig. 3a. We further examined the infiltration pattern of PD-1-positive cells in NNKTL tissues with PD-L1-positive cells. Staining using consecutive slides showed that PD-1-positive cells mainly infiltrated around PD-L1-positive cells (Fig. 3b, c, and Supplemental Fig. S1) including both CD56-positive lymphoma cells and CD68-positive macrophages, since these cells were present in the same regions of tumor tissue (Supplemental Fig. S2). To assess whether CD8-positive CTLs expressed PD-1, we performed immunohistochemistry for CD8 and PD-1 using serial slides from PD-1-positive patients. As shown in Supplemental Fig. S3, some of the CD8-positive cells were positive for PD-1, suggesting that PD-1-positive CD8 T cells were present in NNKTL tissues.
Localization of PD-1 and PD-L1-positive cells in NNKTL tissues. Representative immunohistological features of NNKTL FFPE samples. a Staining for PD-1 (brown) (Patient 15). Scale bar is 100 µm. Original magnification, ×400. b, c Staining for PD-1 (b) and PD-L1 (c) was performed using serial slides (Patient 17). Right panels show enlargement of the boxed areas that are positive for PD-1 or PD-L1 staining. PD-1 or PD-L1-positive cells are stained brown. Scale bar is 100 µm. Original magnification, ×100 (left) and ×400 (right)
Elevated sPD-L1 levels are detectable in the sera of NNKTL patients and correlate with poor prognosis
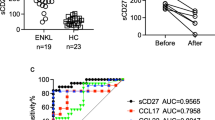
We measured the concentration of sPD-L1 in the sera of NNKTL patients and healthy volunteers. As shown in Fig. 4a, the sPD-L1 serum level was significantly increased in NNKTL patients (mean ± SD = 850 ± 738 pg/mL; median = 639 pg/mL; range = 13-3041 pg/mL) as compared to healthy individuals (mean ± SD = 324 ± 296 pg/mL; median = 221 pg/mL; range = 13–872 pg/mL; p = 0.0074). The serum sPD-L1 levels in patients significantly correlated with the expression of PD-L1 in CD56-positive lymphoma cells (p = 0.0141, Fig. 4b). The correlation between the serum sPD-L1 level and the expression of PD-L1 in CD68-positive cells showed a trend toward statistical significance (p = 0.0746, Supplemental Fig. S4b), while statistical significance was not observed for correlations between the serum sPD-L1 level and the percentage of CD68-positive cells (p = 0.9962, Supplemental Fig. S4a). We further investigated whether the sPD-L1 level correlated with prognosis in NNKTL patients. Based on ROC curve analysis for survival, the optimal sPD-L1 cut-off value was 851 pg/mL, which was almost the same as the mean value of 850 pg/mL (Supplemental Fig. S5). We, therefore, separated the patients into two groups, sPD-L1 low and sPD-L1 high, using a cut-off value of 850 pg/mL. Patients in the low-sPD-L1 concentration group (<850 pg/mL) demonstrated a favorable clinical course with
a 5-year overall survival (OS) rate of 100%, whereas patients in the high concentration group (≥850 pg/mL) showed an unfavorable course with a 5-year OS rate of 57% (Fig. 4c). These results indicate a significant difference between survival rates of the two groups (p = 0.0332). OS according to the main clinical features was also investigated and patients in the late stage (IV), with a higher IPI, and with a higher PINK showed significantly shorter OS (Supplemental Fig. S6). We examined the correlation between the sPD-L1 level and the main clinical features of NNKTL patients; however, no significant correlation was observed (Supplemental Table S2).
Levels of sPD-L1 in sera and correlation with overall survival. a sPD-L1 levels in the sera from 16 NNKTL patients and 23 healthy controls were measured using ELISA. The horizontal lines indicate mean values. Statistical significance was determined using the Mann–Whitney U test. b Correlation between the levels of serum sPD-L1 and the expression levels of PD-L1 in CD56-positive cells of tumor tissues in NNKTL patients. Statistical significance was determined using the Spearman rank correlation. c Kaplan–Meier curves for overall survival of the 16 NNKTL patients. The high-sPD-L1 group (n = 7) showed significantly worse prognosis than the low-sPD-L1 group (n = 9). Statistical significance was determined using the log-rank test
NNKTL cell lines express membrane PD-L1 and produce sPD-L1
To confirm the above findings that were obtained using NNKTL patient materials, we next examined the expression of PD-L1 on the surface of the NNKTL cell lines, SNK-6 and SNK-1, using flow cytometric analysis. We also assayed the SNK-10 cell line, which was established from a patient with chronic active EBV infection (CAEBV) and is of EBV-positive NK-cell lineage, because some CAEBV patients develop from CAEBV to NNKTL with CAEBV. PD-L1 was clearly expressed on the surface of SNK-6, while a low level of expression of membrane PD-L1 was observed on the surface of SNK-1 and SNK-10 (Fig. 5a; Supplemental Fig. S7a). As reported previously [32, 37], HDLM-2 and Raji were positive and negative for the expression of PD-L1, respectively. In addition, we used flow cytometric analysis to investigate whether NNKTL and CAEBV cell lines also expressed PD-1; however, PD-1 was not detected on the surface of the cell lines except for Molt-4, which was used as a positive control for the expression of PD-1 (Fig. 5a). The expression of PD-L1 and PD-1 in cell lines was also assessed by western blot analysis. The PD-L1 protein was expressed in SNK-6, SNK-1, and SNK-10 cells, as well as in the positive control HDLM-2, but it was barely detected in the negative control Raji (Fig. 5b; Supplemental Fig. S7b). On the other hand, the PD-1 protein was not detected in SNK-6, SNK-1, SNK-10, Raji, or HDLM-2 cells, although it was clearly expressed in the positive control Molt-4 (Fig. 5b), confirming that PD-1 is not expressed in these NNKTL cell lines. We further measured the level of sPD-L1 in the supernatants from these cell lines using ELISA (Fig. 5c). sPD-L1 was detectable in the supernatants of SNK-6, SNK-1, SNK-10, and HDLM-2 cells, but was not detectable in the supernatant of the PD-L1 negative cell line Raji.
Expression of PD-L1 in NNKTL cell lines and in tumor tissue and sera from a murine xenograft model inoculated with SNK-6 cells. a Flow cytometric analysis of the surface expression of PD-L1 or PD-1 in the indicated cell lines. Cells were stained with a PE-conjugated anti-PD-L1 or anti-PD-1 mAb (red lines). Black lines, cells stained with isotype control Ab. b PD-L1 or PD-1 expression in cell lines as assessed by western blotting. β-Actin was used to verify the amount of loading. c Supernatants from the indicated cell cultures (5 × 105/mL) in 96-well round-bottomed plates were collected after 24 h, and sPD-L1 production was assessed using ELISA. Columns means of triplicate determinations; bars SEM. In a–c, HDLM-2 and Raji cells were used as positive and negative controls, respectively, for the expression of PD-L1. Molt-4 cells were used as a positive control for the expression of PD-1. d–g Representative immunohistological features of FFPE tumor samples from a murine xenograft model. d H&E-stained section showing tumor tissue formed by highly atypical lymphoid cells. e ISH for EBER. Nuclei of EBER-positive cells are stained brown. f Staining for CD56. g Staining for PD-L1. Scale bar is 100 µm. h sPD-L1 levels in the sera from NOG mice inoculated with SNK-6 cells (NOG-SNK-6, n = 5) and normal NOG mice (NOG, n = 4) were measured using ELISA. Columns means; bars SEM
PD-L1 is detectable in tumor tissue and blood from a murine xenograft model inoculated with an NNKTL cell line
We then extended our investigation to a murine xenograft in vivo model, using NOG mice inoculated subcutaneously with SNK-6, to ascertain the importance of our in vitro findings. Tumor formation at the inoculated site was detectable in all of the NOG mice. Immunohistological staining was performed to analyze PD-L1 expression in FFPE tissue sections prepared from the subcutaneous tumor. Histologically, the tumor was formed by atypical lymphoid cells (Fig. 5d) that were positive for EBER and CD56 (Fig. 5e, f). These characteristics were similar to those of the malignant cells that form the tumor tissue of NNKTL patients. These neoplastic cells clearly expressed PD-L1 (Fig. 5g). Most significantly, high levels of sPD-L1 were detected in the sera from NOG mice inoculated with SNK-6 as compared with the levels in normal NOG mice (Fig. 5h). These results suggest that not only cultured SNK-6 cells but also the SNK-6 tumor in the in vivo xenograft model could express membrane PD-L1 and secrete sPD-L1.
Discussion
In the present study, using serial tissue sections and double immunofluorescent staining, we clearly demonstrated that PD-L1 was expressed by lymphoma cells in the tissue samples from all of the 15 NNKTL patients examined. Chen and colleagues previously reported that the malignant cells of 4 of 6 NNKTL cases (66.7%) were immunohistochemically positive for PD-L1 expression [32]. In recent reports, Kim et al. [38] and Jo et al. [39] further indicated that PD-L1 expression in lymphoma cells was positive in 56.2 and 79.7% of cases, respectively, using immunohistochemistry. Our results and the data of other researchers, therefore, suggest that the majority of NNKTL cases highly express PD-L1 and that the PD-1/PD-L1 pathway might play an important role in immune escape of NNKTL from CTL recognition. However, it is controversial whether PD-L1 expression of lymphoma cells in NNKTL tissues associates with prognosis. In a study of 79 NNKTL biopsy samples, Jo et al. [39] found that PD-L1 positivity in lymphoma cells was not significantly associated with OS. On the other hand, in a study of 73 NNKTL patients, Kim and colleagues [38] found that patients with PD-L1-positive tumors exhibited significantly better OS than patients with PD-L1-negative tumors, although PD-L1 positivity was not significantly correlated with clinicopathological variables. Thus, further studies of tissue samples from NNKTL are needed to clarify the correlation between tissue PD-L1 expression and prognosis. We further found that CD68-positive macrophages abundantly infiltrated NNKTL tissues and that these cells also expressed PD-L1. Other studies have indicated that tumor-infiltrating immune cells expressed PD-L1 in 62–79% of NNKTL cases [38, 39]. Kim et al. reported that these cells morphologically resembled macrophages, although they did not confirm the expression of macrophage markers using serial sections or double-staining methods [38]. These results including ours are similar to those of recent studies on Hodgkin’s lymphoma and diffuse large B-cell lymphoma [32, 33]. Because interferon (IFN)-γ is secreted by NNKTL cells [9, 10] and is known to induce PD-L1 expression on macrophages [40], NNKTL might utilize the PD-L1/PD-1 pathway to inhibit immune suppression via two distinct mechanisms: direct inhibition by PD-L1 expression on tumor cells and indirect inhibition by induction of PD-L1 expression on macrophages via IFN-γ production.
To confirm PD-L1 expression on NNKTL tumor cells, we investigated PD-L1 expression in NNKTL cell lines. We found that PD-L1 is highly expressed in the SNK-6 cell line, which was established from a primary NNKTL lesion, and this result is consistent with that of a recent report [41]. We further demonstrated that not only SNK-6 cells cultured in vitro but also SNK-6 cells inoculated into NOG mice could produce PD-L1, suggesting that SNK-6 cells could maintain the expression of PD-L1 in an in vivo mouse model. We also detected the expression of PD-L1 in SNK-1 and SNK-10 cells, which were isolated from the peripheral blood of NNKTL and CAEBV patients, respectively, although these cell lines weakly expressed cell surface PD-L1 compared with SNK-6 cells. Other studies have indicated that loss of PD-L1 expression could occur during the establishment or the serial passage of human cell lines [37]. Future studies will be required to clarify the reason why there is a difference in the PD-L1 expression level between the cell lines cultured in vitro.
Several recent studies have reported the detection of high concentration levels of sPD-L1 in blood and their association with poor prognosis in some malignancies [17–19]. Because we observed a high number of PD-L1-expressing cells in NNKTL tissues, we speculated that sPD-L1 might be secreted by these cells and, therefore, that higher levels of sPD-L1 might be detected in the blood of NNKTL patients compared to healthy controls. As expected, elevated sPD-L1 levels were detected in the sera of NNKTL patients. Furthermore, the correlation of serum sPD-L1 levels with PD-L1 expression in tissue lymphoma cells and in tumor-infiltrating macrophages was statistically significant and showed a trend towards statistical significance, respectively, and higher sPD-L1 levels were correlated with poor prognosis. In general, our findings correspond with the results reported by Bi et al. [41]. Those authors reported that the concentration of serum sPD-L1 in NNKTL patients with stage I and II was significantly higher than that in healthy controls and positively correlated with the PD-L1 expression level in tumor tissues. They also showed that patients in the high-sPD-L1 group exhibited a significantly worse OS compared to patients in the low-sPD-L1 group. These results presented by Bi et al. and by us suggest that sPD-L1 may be a valuable predictor for prognosis in NNKTL. However, in this study, it should be taken into consideration that all 3 patients who died in the high-sPD-L1 group were late-stage cases that showed significantly shorter OS than early stage cases (Supplemental Fig. S6). In addition, the correlation between the sPD-L1 level and stage showed a trend towards statistical significance (p = 0.0625, Supplemental Table S2). Thus, we cannot rule out the possibility that late-stage might have affected the unfavorable course in the high-sPD-L1 group. We intend to investigate further NNKTL samples including late-stage samples as they become available in the future to address the association between the sPD-L1 level and clinical stage. Although the sources of sPD-L1 remain unknown, it is possible that either tumor cells and/or tumor-infiltrating immune cells could produce sPD-L1. We demonstrated that sPD-L1 levels detected in the sera from SNK-6-bearing NOG mice were elevated compared to those of normal mice, which means that the main source of sPD-L1 in the blood of this mouse model was the inoculated tumor cells. This result suggests that tumor cells are one of the sources of circulating sPD-L1 in the blood of patients with NNKTL, although CD68-positive macrophages might also be able to produce sPD-L1. It has been reported that sPD-L1 that is secreted into the supernatant by tumor cell lines or that is circulating in the sera maintains the ability to bind to the PD-1 receptor [42] and that sPD-L1 from culture supernatants can deliver immunosuppressive signals to T cells [16]. Because high-sPD-L1 levels correlate with poor prognosis in malignant diseases, sPD-L1, similar to membrane PD-L1, might induce immunosuppressive activity through binding to PD-1.
Immunohistochemical analysis using serial sections of NNKTL tissues detected PD-1-positive cells in the tissues of 5 of 14 patients (36%) and these cells were mainly localized around PD-L1-positive lymphoma cells and macrophages. Furthermore, PD-1 was not expressed in NNKTL cell lines. These results suggest that it is infiltrating immune cells including lymphocytes and not lymphoma cells that express PD-1 in NNKTL. We also demonstrated that some of the tumor-infiltrating CD8-positive cells expressed PD-1, indicating that PD-1-positive CD8 T cells are present in NNKTL tissues, although it is possible that other immune cells including CD4 T cells also express PD-1. The association between PD-1 expression on infiltrating lymphocytes and prognosis is controversial. It has been reported that PD-1 expression on infiltrating lymphocytes was correlated with poor prognosis in renal cell carcinoma [34], Hodgkin’s lymphoma [43], and nasopharyngeal carcinoma [44]. On the other hand, other studies have shown that the expression of PD-1 on infiltrating T cells was associated with better survival in follicular lymphoma [35] and human papillomavirus-associated head and neck cancer [45]. Therefore, further study with a greater sample size is required to identify the origin of the PD-1-expressing cells and to evaluate the clinical relevance of PD-1 expression in NNKTL.
In conclusion, our results show that PD-L1 is expressed on malignant cells and tumor-infiltrating macrophages in NNKTL tissues. More importantly, increased sPD-L1 levels were detectable in the sera of patients and correlated with poor prognosis. The expression of PD-L1 was observed in tumor tissues and sera from a murine xenograft model inoculated with an NNKTL cell line, suggesting that tumor cells are one of the sources of circulating sPD-L1 in blood. These findings indicate that a high-sPD-L1 level is a possible valuable predictor of poor prognosis and that the PD-1/PD-L1 pathway may play a crucial role in the escape of NNKTL from the antitumor immune response. We believe that immunotherapy with mAbs targeting PD-1 and PD-L1 could benefit NNKTL patients.
Abbreviations
- CAEBV:
-
Chronic active Epstein–Barr virus infection
- CTLs:
-
Cytotoxic T lymphocytes
- EBER:
-
Epstein–Barr virus encoded small RNA
- EBV:
-
Epstein–Barr virus
- FBS:
-
Fetal bovine serum
- FFPE:
-
Formalin-fixed, paraffin-embedded
- IFN:
-
Interferon
- IL:
-
Interleukin
- IPI:
-
International Prognostic Index
- ISH:
-
In situ hybridization
- LDH:
-
Lactate dehydrogenase
- LMP:
-
Latent membrane protein
- mAbs:
-
Monoclonal antibodies
- NK:
-
Natural killer
- NNKTL:
-
Nasal natural killer/T-cell lymphoma
- OS:
-
Overall survival
- PD-1:
-
Programmed death-1
- PD-L1:
-
Programmed death-ligand 1
- PINK:
-
Prognostic index of natural killer lymphoma
- ROC:
-
Receiver-operating characteristic
- RT:
-
Room temperature
- sPD-L1:
-
Soluble programmed death-ligand 1
References
Harabuchi Y, Takahara M, Kishibe K, Moriai S, Nagato T, Ishii H (2009) Nasal natural killer (NK)/T-cell lymphoma: clinical, histological, virological, and genetic features. Int J Clin Oncol 14:181–190. doi:10.1007/s10147-009-0882-7
Harabuchi Y, Yamanaka N, Kataura A, Imai S, Kinoshita T, Mizuno F, Osato T (1990) Epstein–Barr virus in nasal T-cell lymphomas in patients with lethal midline granuloma. The Lancet 335:128–130
Harabuchi Y, Imai S, Wakashima J, Hirao M, Kataura A, Osato T, Kon S (1996) Nasal T-cell lymphoma causally associated with Epstein–Barr virus: clinicopathologic, phenotypic, and genotypic studies. Cancer 77:2137–2149. doi:10.1002/(SICI)1097-0142(19960515)77:10<2137::AID-CNCR27>3.0.CO;2-V
Nagata H, Konno A, Kimura N, Zhang Y, Kimura M, Demachi A, Sekine T, Yamamoto K, Shimizu N (2001) Characterization of novel natural killer (NK)-cell and gammadelta T-cell lines established from primary lesions of nasal T/NK-cell lymphomas associated with the Epstein–Barr virus. Blood 97:708–713
Minarovits J, Hu LF, Imai S, Harabuchi Y, Kataura A, Minarovits-Kormuta S, Osato T, Klein G (1994) Clonality, expression and methylation patterns of the Epstein–Barr virus genomes in lethal midline granulomas classified as peripheral angiocentric T cell lymphomas. J Gen Virol 75(Pt 1):77–84. doi:10.1099/0022-1317-75-1-77
Kobayashi H, Nagato T, Takahara M et al (2008) Induction of EBV-latent membrane protein 1-specific MHC class II-restricted T-cell responses against natural killer lymphoma cells. Cancer Res 68:901–908. doi:10.1158/0008-5472.CAN-07-3212
Nagamine M, Takahara M, Kishibe K, Nagato T, Ishii H, Bandoh N, Ogino T, Harabuchi Y (2007) Sequence variations of Epstein–Barr virus LMP1 gene in nasal NK/T-cell lymphoma. Virus Genes 34:47–54. doi:10.1007/s11262-006-0008-5
Nagamine M, Kishibe K, Takahara M, Nagato T, Ishii H, Bandoh N, Ogino T, Harabuchi Y (2007) Selected amino acid change encoding Epstein–Barr virus-specific T cell epitope of the LMP2A gene in Japanese nasal NK/T cell lymphoma patients. Intervirology 50:319–322. doi:10.1159/000106462
Ho JW, Liang RH, Srivastava G (1999) Differential cytokine expression in EBV positive peripheral T cell lymphomas. Mol Pathol 52:269–274
Takahara M, Kis LL, Nagy N, Liu A, Harabuchi Y, Klein G, Klein E (2006) Concomitant increase of LMP1 and CD25 (IL-2-receptor alpha) expression induced by IL-10 in the EBV-positive NK lines SNK6 and KAI3. Int J Cancer 119:2775–2783. doi:10.1002/ijc.22139
Kumai T, Matsuda Y, Ohkuri T et al. (2015) c-Met is a novel tumor associated antigen for T-cell based immunotherapy against NK/T cell lymphoma. Oncoimmunology 4:e976077. doi:10.4161/2162402X.2014.976077
Keir ME, Butte MJ, Freeman GJ, Sharpe AH (2008) PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26:677–704. doi:10.1146/annurev.immunol.26.021607.090331
Okazaki T, Honjo T (2007) PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol 19:813–824. doi:10.1093/intimm/dxm057
Blank C, Gajewski TF, Mackensen A (2005) Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother 54:307–314. doi:10.1007/s00262-004-0593-x
Topalian SL, Drake CG, Pardoll DM (2012) Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 24:207–212. doi:10.1016/j.coi.2011.12.009
Frigola X, Inman BA, Lohse CM et al (2011) Identification of a soluble form of B7-H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin Cancer Res 17:1915–1923. doi:10.1158/1078-0432.CCR-10-0250
Rossille D, Gressier M, Damotte D et al (2014) High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-Cell lymphoma: results from a French multicenter clinical trial. Leukemia 28:2367–2375. doi:10.1038/leu.2014.137
Wang L, Wang H, Chen H, Wang WD, Chen XQ, Geng QR, Xia ZJ, Lu Y (2015) Serum levels of soluble programmed death ligand 1 predict treatment response and progression free survival in multiple myeloma. Oncotarget 6:41228–41236. doi:10.18632/oncotarget.5682
Finkelmeier F, Canli O, Tal A et al (2016) High levels of the soluble programmed death-ligand (sPD-L1) identify hepatocellular carcinoma patients with a poor prognosis. Eur J Cancer 59:152–159. doi:10.1016/j.ejca.2016.03.002
Ship MA, Harrington DP, Anderson JR et al (1993) A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med 329:987–994. doi:10.1056/NEJM199309303291402
Kim SJ, Yoon DH, Jaccard A et al (2016) A prognostic index for natural killer cell lymphoma after non-anthracycline-based treatment: a multicentre, retrospective analysis. Lancet Oncol 17:389–400. doi:10.1016/S1470-2045(15)00533-1
Takahara M, Nagato T, Kishibe K, Ueda S, Komabayashi Y, Yamashina M, Takahashi K, Harabuchi Y (2015) Novel treatment for early-stage nasal natural killer/T-cell lymphoma: intra-maxillary arterial infusion chemotherapy with concomitant radiotherapy. Hematol Oncol. doi:10.1002/hon.2273
Yamaguchi M, Tobinai K, Oguchi M et al (2009) Phase I/II study of concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: Japan Clinical Oncology Group Study JCOG0211. J Clin Oncol 27:5594–5600. doi:10.1200/JCO.2009.23.8295
Harabuchi Y, Tsubota H, Ohguro S, Himi T, Asakura K, Kataura A, Ohuchi A, Hareyama M (1997) Prognostic factors and treatment outcome in non-Hodgkin’s lymphoma of Waldeyer’s ring. Acta Oncol 36:413–420
Takamatsu Y, Suzumiya J, Utsunomiya A et al (2010) THP-COP regimen for the treatment of peripheral T-cell lymphoma and adult T-cell leukemia/lymphoma: a multicenter phase II study. Eur J Haematol 84:391–397. doi:10.1111/j.1600-0609.2010.01411.x
Nagata H, Numata T, Konno A, Mikata I, Kurasawa K, Hara S, Nishimura M, Yamamoto K, Shimizu N (2001) Presence of natural killer-cell clones with variable proliferative capacity in chronic active Epstein–Barr virus infection. Pathol Int 51:778–785
Zhang Y, Nagata H, Ikeuchi T et al (2003) Common cytological and cytogenetic features of Epstein–Barr virus (EBV)-positive natural killer (NK) cells and cell lines derived from patients with nasal T/NK-cell lymphomas, chronic active EBV infection and hydroa vacciniforme-like eruptions. Br J Haematol 121:805–814
Epstein MA, Achong BG, Barr YM, Zajac B, Henle G, Henle W (1966) Morphological and virological investigations on cultured Burkitt tumor lymphoblasts (strain Raji). J Natl Cancer Inst 37:547–559
Drexler HG, Gaedicke G, Lok MS, Diehl V, Minowada J (1986) Hodgkin’s disease derived cell lines HDLM-2 and L-428: comparison of morphology, immunological and isoenzyme profiles. Leuk Res 10:487–500
Minowada J, Onuma T, Moore GE (1972) Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J Natl Cancer Inst 49:891–895
Steidl C, Lee T, Shah SP et al (2010) Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med 362:875–885. doi:10.1056/NEJMoa0905680
Chen BJ, Chapuy B, Ouyang J et al (2013) PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res 19:3462–3473. doi:10.1158/1078-0432.CCR-13-0855
Kwon D, Kim S, Kim PJ et al (2016) Clinicopathological analysis of programmed cell death 1 and programmed cell death ligand 1 expression in the tumour microenvironments of diffuse large B cell lymphomas. Histopathology 68:1079–1089. doi:10.1111/his.12882
Thompson RH, Dong H, Lohse CM, Leibovich BC, Blute ML, Cheville JC, Kwon ED (2007) PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res 13:1757–1761. doi:10.1158/1078-0432.CCR-06-2599
Carreras J, Lopez-Guillermo A, Roncador G et al (2009) High numbers of tumor-infiltrating programmed cell death 1-positive regulatory lymphocytes are associated with improved overall survival in follicular lymphoma. J Clin Oncol 27:1470–1476. doi:10.1200/JCO.2008.18.0513
Kumai T, Nagato T, Kobayashi H et al (2015) CCL17 and CCL22/CCR4 signaling is a strong candidate for novel targeted therapy against nasal natural killer/T-cell lymphoma. Cancer Immunol Immunother 64:697–705. doi:10.1007/s00262-015-1675-7
Andorsky DJ, Yamada RE, Said J, Pinkus GS, Betting DJ, Timmerman JM (2011) Programmed death ligand 1 is expressed by non-hodgkin lymphomas and inhibits the activity of tumor-associated T cells. Clin Cancer Res 17:4232–4244. doi:10.1158/1078-0432.CCR-10-2660
Kim WY, Jung HY, Nam SJ, Kim TM, Heo DS, Kim CW, Jeon YK (2016) Expression of programmed cell death ligand 1 (PD-L1) in advanced stage EBV-associated extranodal NK/T cell lymphoma is associated with better prognosis. Virchows Arch 469:581–590. doi:10.1007/s00428-016-2011-0
Jo JC, Kim M, Choi Y, Kim HJ, Kim JE, Chae SW, Kim H, Cha HJ (2017) Expression of programmed cell death 1 and programmed cell death ligand 1 in extranodal NK/T-cell lymphoma, nasal type. Ann Hematol 96:25–31. doi:10.1007/s00277-016-2818-4
Zou W, Chen L (2008) Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol 8:467–477. doi:10.1038/nri2326
Bi XW, Wang H, Zhang WW et al (2016) PD-L1 is upregulated by EBV-driven LMP1 through NF-kappaB pathway and correlates with poor prognosis in natural killer/T-cell lymphoma. J Hematol Oncol 9:109. doi:10.1186/s13045-016-0341-7
Chen Y, Wang Q, Shi B, Xu P, Hu Z, Bai L, Zhang X (2011) Development of a sandwich ELISA for evaluating soluble PD-L1 (CD274) in human sera of different ages as well as supernatants of PD-L1+ cell lines. Cytokine 56:231–238. doi:10.1016/j.cyto.2011.06.004
Muenst S, Hoeller S, Dirnhofer S, Tzankov A (2009) Increased programmed death-1+ tumor-infiltrating lymphocytes in classical Hodgkin lymphoma substantiate reduced overall survival. Hum Pathol 40:1715–1722. doi:10.1016/j.humpath.2009.03.025
Hsu MC, Hsiao JR, Chang KC, Wu YH, Su IJ, Jin YT, Chang Y (2010) Increase of programmed death-1-expressing intratumoral CD8 T cells predicts a poor prognosis for nasopharyngeal carcinoma. Mod Pathol 23:1393–1403. doi:10.1038/modpathol.2010.130
Badoual C, Hans S, Merillon N et al (2013) PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res 73:128–138. doi:10.1158/0008-5472.CAN-12-2606
Acknowledgements
The authors thank Dr. Norio Shimizu (Tokyo Medical and Dental University) for generously providing cell lines, Mr. Toshiyuki Hayakawa (Animal Laboratory for Medical Research, Center for Advanced Research and Education, Asahikawa Medical University) for devotedly maintaining mice, and Ms. Rie Matsumoto (Department of Pathology, Asahikawa Medical University) and Ms. Keiko Nishikura (Department of Dermatology, Asahikawa Medical University) for technical assistance. This study was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI [Grant Numbers 15K20172 (Nagato T), 26462576 (Kishibe K), 15H04986 (Harabuchi Y), and 16K15244 (Kobayashi H)].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no financial conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nagato, T., Ohkuri, T., Ohara, K. et al. Programmed death-ligand 1 and its soluble form are highly expressed in nasal natural killer/T-cell lymphoma: a potential rationale for immunotherapy. Cancer Immunol Immunother 66, 877–890 (2017). https://doi.org/10.1007/s00262-017-1987-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-017-1987-x