Abstract
Purpose
Dendritic cell (DC)-based tumor vaccination has rendered promising results in relapsed high-grade glioma patients. In the HGG-2006 trial (EudraCT 2006-002881-20), feasibility, toxicity, and clinical efficacy of the full integration of DC-based tumor vaccination into standard postoperative radiochemotherapy are studied in 77 patients with newly diagnosed glioblastoma.
Patients and methods
Autologous DC are generated after leukapheresis, which is performed before the start of radiochemotherapy. Four weekly induction vaccines are administered after the 6-week course of concomitant radiochemotherapy. During maintenance chemotherapy, 4 boost vaccines are given. Feasibility and progression-free survival (PFS) at 6 months (6mo-PFS) are the primary end points. Overall survival (OS) and immune profiling, rather than monitoring, as assessed in patients’ blood samples, are the secondary end points. Analysis has been done on intent-to-treat basis.
Results
The treatment was feasible without major toxicity. The 6mo-PFS was 70.1 % from inclusion. Median OS was 18.3 months. Outcome improved significantly with lower EORTC RPA classification. Median OS was 39.7, 18.3, and 10.7 months for RPA classes III, IV, and V, respectively. Patients with a methylated MGMT promoter had significantly better PFS (p = 0.0027) and OS (p = 0.0082) as compared to patients with an unmethylated status. Exploratory “immunological profiles” were built to compare to clinical outcome, but no statistical significant evidence was found for these profiles to predict clinical outcome.
Conclusion
Full integration of autologous DC-based tumor vaccination into standard postoperative radiochemotherapy for newly diagnosed glioblastoma seems safe and possibly beneficial. These results were used to power the currently running phase IIb randomized clinical trial.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite state-of-the-art oncological therapy, including maximal, safe surgical resection, external beam radiotherapy, and temozolomide (TMZ) chemotherapy, the prognosis of glioblastoma (GBM) remains poor with a median survival of 14.6 months [1, 2]. Therefore, there is a clear need for well-tolerated long-term and tumor-specific treatments. In recent years, many innovative treatment approaches, including targeted therapy, immunotherapy, and combinations of chemotherapeutical agents, have been investigated with variable success. Several reports on dendritic cell (DC)-based tumor vaccination have shown promising clinical results in high-grade glioma (HGG) patients [3–6].
The rationale for immunotherapy in HGG patients has been reviewed extensively, and proof of the principle of DC-based vaccination strategies against HGG has been documented by our and several other groups [3–13].
Already two decades ago, North [14] nicely demonstrated in an experimental rodent brain tumor model that radiation therapy for malignant gliomas successfully reduced or even eliminated the tumor infiltrating “suppressor” T cells and considered this an important immunomanipulative mechanism for sustained production of effector T cells, resulting in immunologically mediated regression of established brain tumors.
Historically and intuitively, chemotherapy has long been regarded as problematic for the patient’s innate and adaptive immunity. Several chemotherapeutic drugs however, depending on dose and timing of administration, seem to facilitate the efficacy of immunotherapeutic strategies [15]. Several synergistic mechanisms have been elucidated to date. The principles of thymic-independent, homeostatically driven T-cell reconstitution after myelosuppressive (chemo-)therapy [16, 17] can result in a higher number of specific, tumor-rejecting T cells if the vaccination is being performed in or immediately before the reconstitution phase. Banissi et al. [18] were able to show that (only) a low-dose metronomic TMZ regimen in a TMZ-resistant rat glioma model decreased the regulatory T-cell fractions in the spleen and within the tumor, resulting in a reduced tumor progression. The mechanism for this reduced migration of regulatory T cells (Treg) to HGG has been investigated by Jordan et al. [19]. The production of the chemokine CCL2 by glioma cells, responsible for Treg recruitment, was mitigated by TMZ but also by carmustine. The importance of cross-priming by DC, initiated by the use of TMZ in glioma-bearing mice has clearly been demonstrated by Park et al. [20], who reported on an improved survival rate of a combination of TMZ and DC vaccination as compared to each strategy alone. Both factors, suppression of Treg and DC cross-priming after tumor cell apoptosis, were identified as responsible mechanisms for an enhanced antitumor immunity if a combination of TMZ chemotherapy and DC-based vaccines was used in a murine glioma model by Kim et al. [21]. A better cross-priming is believed to result from cancer therapies leading to “immunogenic apoptosis” via the exposure or secretion of various damage-associated molecular patterns (DAMP) like calreticulin and heat-shock proteins [22], currently considered to be a promising approach for future immunotherapy applications.
The inverse relation, that is, immunotherapy resulting in an increased efficacy of chemotherapeutic drugs, has also been demonstrated. Wheeler et al. [23] found more frequent and more intense responses to chemotherapy in HGG patients being vaccinated before the chemotherapy as compared to a control group that had not been vaccinated before. The mechanism suggested for this remarkable finding seems to be an increased chemosensitivity of the remaining tumor cells after targeting and cytotoxic clearance of tyrosine-related protein-2 (TRP-2) positive tumor clones after DC vaccination [24]. Till now, the focus of synergy has been on mutual interactions of either chemotherapy and immunotherapy or on radiotherapy and immunotherapeutic strategies, but not on the three modalities together.
Based on the promising results of adjuvant autologous DC-based tumor vaccination in a large group of patients with relapsed malignant glioma and the results of DC vaccination as add-on therapy in a pilot group of 8 patients with newly diagnosed GBM [3, 4], we integrated immunotherapy within the treatment for newly diagnosed GBM in a group of 77 patients in the HGG-2006 phase I/II trial (EudraCT 2006-002881-20), considering putative mutual beneficial effects of the combination of immunotherapeutic strategies with both radiotherapy and chemotherapy. In our pilot report [3], we already reported on the logistic and immunological feasibility of this fully integrated approach, referred to as radiochemoimmunotherapy. Here, we report on clinical feasibility, toxicity, and progression-free survival (PFS) at 6 months (6mo-PFS) as primary end points. Overall survival (OS) and exploration of immune profiles, before the start of the immunotherapy, are secondary end points.
Patients and methods
Patient population
Seventy-seven patients were included between December 2006 and November 2008, with a newly diagnosed primary GBM, confirmed on central review histopathology. Patients were included for DC-based therapy, if they met the inclusion criteria as summarized in Supplementary Table I (online only). Patients’ characteristics are described in Table 1, and patients are further subdivided according to the EORTC RPA classification for newly diagnosed GBM [25]. All patients were operated upon and were off steroids at the time of leukapheresis and during vaccination. Approval by the local ethics committee was obtained as well as patients’ written informed consent before the start of immunotherapy.
Assessment of extent of tumor resection before vaccination
Total resection was defined by the neurosurgical report and the absence of any residual contrast enhancing mass on early postoperative MRI (T1-weighted spin-echo images before and after gadolinium enhancement) performed within 72 h after surgery in all patients. Any resection leaving a measurable tumoral mass less than 2 cm³ was considered subtotal. All solid residual tumor of a measurable size ≥2 cm³ was classified as partial resection.
Vaccine preparation and administration
Tumor cell lysate and autologous DC were prepared as previously published [3, 4]. Quality control of the cell product (monocyte-derived “early mature” dendritic cells loaded with autologous whole tumor cell lysate) being injected intradermally, included viability (trypan blue exclusion), purity based on cell morphology (DC should display cytoplasmic veils) and flow cytometry (DC should express MHC class II and CD86 and should not express CD14), and sterility (bacterial and fungal cultures and mycoplasma testing in a validated clinical microbiology laboratory). The induction vaccines and boost vaccines were administered intradermally in the upper third of both arms as described [3].
Treatment schedule: fully integrated radio–chemo–immunotherapy
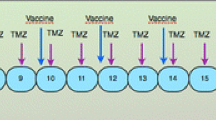
The treatment schedule is depicted in Fig. 1. Leukapheresis was performed 1 week after the withdrawal of corticosteroids and after inclusion criteria were met. Immediately following leukapheresis, patients were treated with radiochemotherapy, as outlined by Stupp et al. [1], with the integration of DC-based immunotherapy. Boost vaccines were given early after maintenance chemotherapy cycles to optimally exploit the principle of homeostatic T-cell reconstitution after chemotherapy [16, 17]. At the time of progression, possible rescue therapy was at the physician’s discretion.
Treatment schedule. Dendritic cell-based immunotherapy was integrated in the state-of-the-art postoperative radiochemotherapy. Leukapheresis to harvest autologous monocytes is performed once, at least 7 days after weaning of steroids and immediately before the start of the concomitant radiochemotherapy (42 days of concomitant temozolomide). After the radiochemotherapy, but before the maintenance chemotherapy with temozolomide (TMZ), four weekly induction vaccines are administered intradermally. Afterward, maintenance chemotherapy (5/28 days) is started and 1 week after the start of the 1st, 2nd, 3rd, and 6th cycle of TMZ, a boost vaccine is administered
Immune profiling
Immune profiling of the patients was performed by flow cytometry. Blood samples were obtained at the times of leukapheresis and vaccine 1, that is, before the start of the actual immunotherapy. Peripheral blood mononuclear cells (PBMC) from each blood sample were cryopreserved and thawed together at the end of the immunotherapy for flow cytometric analysis. For each blood sample, the phenotype of circulating cell populations was determined by fluorescence-activated cell sorting (FACS): CD4+ and CD8+ subpopulations, Treg cells (CD4+CD25+CD127dim) [26], natural killer T cells (NKT) (CD3+CD56+), and natural killer (NK) cells (CD3−CD56+). For this, FITC-, PE-, and PerCP-labeled monoclonal antibodies were purchased from BD Biosciences Pharmingen (San Jose, USA).
Based on the FACS data, relative counts (ratios) of the different cell populations’ frequencies could be determined; CD4+ and CD8+ T-cell ratios were expressed on the total lymphocyte gate, as were NKT and NK cell ratios. Treg cell ratios were expressed on total CD4+ cells. Ratios at the time of the first vaccine (V1) were expressed relative to data at the time of leukapheresis (LF), to evaluate the effect of radiochemotherapy. For this, ratios were derived from the respective frequencies of the studied cell populations, not from absolute numbers, as relative frequencies were believed to reflect the change in immunological microenvironment more accurately because absolute numbers of all T-cell subsets and NK cells clearly went down between LF and V1 and therefore prohibited a discriminative categorization based on the evolution of absolute numbers. This was meant to study a possible global “immunological reset” by radiochemotherapy that might be predictive for the outcome of radiochemoimmunotherapy as presented here. ELISPOT immunomonitoring, as performed in our pilot trial [3], was not performed in the actual trial.
Patient assessment
All patients were followed by clinical examination and MRI (12 weeks after surgery and from then every 3 months). Survival was calculated as time from inclusion (leukapheresis) to death from cancer or any other cause. Within 2–5 weeks after the histological diagnosis of GBM, leukapheresis took place. Radiochemotherapy started within 1 week after leukapheresis.
Data from all the patients that were included in the trial were used for intent-to-treat (ITT) analysis. Patients who had finished radiochemotherapy and received at least 3 induction vaccines were assigned as-treated (AT). Patients who received every part of the outlined treatment, that is, full-dose radiotherapy, concomitant and maintenance TMZ chemotherapy, 4 induction vaccines, and 4 boost vaccines were assigned treated per protocol (PP).
O6-methylguanine-DNA methyltransferase (MGMT) promotor methylation of the tumor was determined using methylation-specific polymerase chain reaction after DNA bisulfite modification [27].
Adverse events were graded according to the National Cancer Institute Common Toxicity Criteria (NCI CTC).
Statistical analysis
For the calculation of the trial sample size, comparison with the historical control group from the EORTC 26981/22981-NCIC CE3 Trial [1] was based on PFS. The power has been calculated to reject the null hypothesis, using a Cox regression, of equal hazard for the historical control group and the intervention group. The alternative hypothesis has been phrased as one-sided, with alpha-level equal to 5 %. With 60 recruited and vaccinated patients in the test group and assuming a 6mo-PFS of 54 and 70 % in, respectively, the control and test group, the study has 91.9 % power to detect a difference.
Two approaches are used to verify whether there is any information in the immunological profiles to predict overall survival (OS) and progression-free survival (PFS).
In a first approach, Cox regression models are used to verify whether the immunological profiles contain any information for the prediction of OS and PFS. Considered predictors were all the ratios at the different set points in time (see “Patients and methods”). Ratios are available for 5 different cell types: CD4 cells, CD8 cells, NK cells, NKT cells, and Treg cells. Univariable as well as various multivariable models are fitted. Due to the high correlation between CD4 and CD8, information from both counts are never combined in the same model. Likelihood ratio tests are used in each model, to verify whether there is any information in the considered predictor(s).
A second approach consists of two steps. Firstly, a k-means cluster analysis on all the available ratios from the relative counts of the various cell types is used as an exploratory tool to group patients. A patient is attributed to a specific cluster because the distance (in multiple dimensions) to the mean of that cluster is the lowest. Due to the low number of subjects, the number of clusters was fixed on three and a single imputation step (assuming multivariate normality) preceding the cluster analysis was used to handle missing values in the ratios. Secondly, it is verified whether there is a relationship between cluster membership and survival (OS/PFS) using log-rank tests.
This method of cluster analysis was explored for any possible combination of predictors (i.e., above-mentioned ratios). As none of the performed clusterings resulted in a clustering membership with a significant relation with PFS or OS, we only present the data of the most “informative” cluster by the way of example. All analyses were performed using SAS software, version 9.2 of the SAS System for Windows (Copyright © 2002 SAS Institute Inc.) SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, NC, USA.
Statistical survival analysis was done by log-rank test on Kaplan–Meier survival estimates.
Results
Feasibility: vaccine preparation
The patients received a median of 8 vaccines (range: 0–17 vaccines); 8 vaccines were scheduled according to the trial protocol. Due to early disease progression, death, or steroid dependence, some ITT patients received less than 8 vaccines. More than 8 vaccines were administered in patients further receiving three monthly boost vaccines if enough lysate was available until disease progression. For the 4 induction vaccines, the median total number of loaded “early mature” DC was 5.2 × 106 per vaccination session (range: 0.24–55 × 106; n = 290). For a more detailed distribution of the number of DC used per vaccine or per patient, we refer to the supplementary figures S1, S2, and S3. The wide variation of DC number per vaccine was not correlated with disease progression. The quality of the cellular product is identical to previously published reports [3, 4]. Further boost vaccines were given with tumor cell lysate, with a median of 1,500 μg (range: 400–1,500 μg) proteins per vaccine injected, in 2 syringes each containing a final volume of 400 μL.
Clinical assessment
The details of the clinical results are given in Table 2: follow-up (FU) period, median PFS and OS, and 6mo-PFS. Analyses were done based on the ITT patients (n = 77), the as-treated patients (n = 71), and the patients treated per protocol (n = 39). In Fig. 2, Kaplan–Meier curves are shown for PFS and OS based on ITT, AT, and PP analysis. PFS of 10.4 months in the ITT group, further increased for the AT group (11.0 months) to 20.4 months in the PP group. The percentage 6-months PFS was 70 % for the ITT group and increased to 100 % for the PP group. With a median FU of more than 2 years, the median OS was 18.3 months in the ITT, 19.4 months in the AT group, and could not yet be determined for the PP group.
Progression-free survival (PFS) and overall survival (OS) based on intent-to-treat (ITT), as-treated (AT) and per protocol (PP) analysis. Median PFS (a) and median OS (b) on ITT were 10.4 and 18.3 months, respectively. Based on AT analysis, median PFS (c) and median OS (d) were 11.0 and 19.4 months, respectively. For PP analysis, median PFS (e) was 20.4 months, and median OS (f) has not yet been reached
Extent of resection was taken into account as prognostic variable, and further analysis based on the EORTC RPA classes was done as well. MGMT status could be determined in 48 of the 77 patients (62 %): in 63 % of patients with a total resection and in 62 % of patients with a subtotal resection. For the subgroups of total and subtotal resection, 53 and 75 % of the patients had a methylated promoter of MGMT, respectively. Figure 3 shows Kaplan–Meier curves for PFS and OS, based on RPA classification, extent of resection, and MGMT status (ITT analysis). Both for RPA classification and MGMT promoter methylation status, a statistically significant difference in PFS and OS was illustrated. Extent of resection did not result in a significant survival difference for this group of patients.
Progression-free survival (PFS) and overall survival (OS) based on RPA classification, grade of resection, and MGMT status. Data depicted are based on ITT analysis. Based on RPA classification, median PFS (a) was 30.5, 11.7, and 5.2 months for classes III, IV, and V, respectively, with 6mo-PFS of 92, 72, and 43 %, respectively. Median OS (b) was 39.7, 18.3, and 10.7 months for RPA classes III, IV, and V, respectively. For total and subtotal resection, median PFS (c) was 9.7 and 10.8 months, respectively. Median OS (d) was 16.6 and 23.5, respectively. Based on MGMT status, median PFS (e) was 14.5 and 6.5 months for methylated and unmethylated MGMT status, respectively. Median OS (f) was 20.0 and 11.1 months for the respective MGMT states (ITT intent-to-treat, MGMT O6-methylguanine-DNA methyltransferase, ns not significant, RPA recursive partitioning analysis, and 6mo-PFS PFS at 6 months)
Toxicity
Adverse events are described in Table 3. Thirty-eight serious adverse events (NCI CTC grade III, IV, and V) were reported in 30 patients (39 %), including 19 hematological adverse events (hematotoxicity) in 18 patients (23 %). One patient died (NCI CTC grade V) due to an overwhelming infection in the early postoperative period after leukapheresis but before radiochemotherapy or vaccination was started.
Immune profiling to detect a “reset-mechanism” by radiochemotherapy
Based on the FACS data, patterns of increase and decrease in the different cell ratios could be determined, providing information on immunological profiles, changing during radiochemotherapy, and thus before the start of the actual vaccination therapy. Combining increase/decrease in different cell ratios led to a further subdivision into subgroups. In this way, subgroups were made with NK, Treg, CD4, and CD8 cells. Next, the patterns of increase/decrease were compared to clinical outcome, to see whether there was any information in the immunological profiles to predict PFS and OS. Univariable and multivariable models were tested. Using all these different models, we found no evidence that the immunological changes contained any predictive information for either PFS or OS. To roughly illustrate the changes in the relative frequencies of the examined subtypes of cells after radiochemotherapy, we report the median, minimum, and maximum ratios (V1/LF): CD4 median = 1.01 (0.47–3.97), CD8 median = 1.08 (0.53–2.04), NK median = 0.89 (0.14–3.45), and Treg median = 1.28 (0.36–5.73). Also, cluster analysis was performed based on all possible combinations of possible predictors, but none of the performed clustering models resulted in a clustering membership with a statistical significant relation with PFS or OS (data not shown). However, cluster analysis based on the 2 variables with the highest R², Treg, and NK cell ratios (V1/LF) resulted in the determination of 3 clusters (supplementary Fig. S4A, online only). A clear trend toward a longer PFS was seen from cluster 1 to cluster 3, although this did not reach statistical significance (supplementary Fig. S4B, online only).
Discussion
We hypothesized that the combination of radio-, chemo-, and immunotherapy could potentiate the cumulative antitumor activity when applied in a well-designed strategy. We here demonstrate such a strategy of autologous DC-based immunotherapy as add-on therapy, fully integrated in (rather than applied after) the multimodal treatment for surgery, radiotherapy, and chemotherapy in patients with newly diagnosed GBM. For this radio–chemo–immunotherapy, we provide data on feasibility, efficacy, and toxicity in this prospective single-arm phase I/II trial.
The integration of immunotherapy within the standard postoperative therapy for patients with a newly diagnosed GBM is based on the presumed mutually beneficial effect of the conventional treatment strategies and immunotherapy, mentioned into more detail in the introduction. Each aspect of the presented concept of radiochemoimmunotherapy is believed to play a major role in the global results of this approach. First of all, maximal, safe surgery is performed to induce a state of minimal residual disease as a starting point for the following therapies. It has been shown that the extent of resection has a major impact on the benefit of postoperative radio(chemo)therapy in GBM [28, 29]. Moreover, extent of resection is a strong, independent predictor of the outcome for patients with relapsed malignant glioma, treated with postoperative, adjuvant DC vaccination [4].
Many antitumor strategies, such as radiotherapy, kill tumor cells by apoptosis, and the resulting apoptotic bodies form a good source of cross-presented antigens, which might further lead to cross-priming of T cells in an appropriate pro-inflammatory environment. In 1984, North [14] already showed an elimination of local regulatory T cells in irradiated brain tumor areas. TMZ chemotherapy during radiotherapy might lead to thymic-independent antigen-driven T-cell regeneration within the context of T-cell homeostasis [16, 17], and the concept of tumor-specific immunization at the time of immune reconstitution after chemotherapy has been demonstrated in several animal models [16, 17]. A decreased intratumoral Treg invasion after TMZ chemotherapy [21] and the increased cross-priming by DC after immunogenic apoptosis of tumor cells [20], for example after several types of chemotherapy, are believed to be major factors contributing to the anticipated synergistic activity of DC vaccines and chemotherapy. Immunotherapy itself can increase the sensitivity of (GBM) tumor cells to chemotherapeutics like TMZ [23, 24, 30–32].
Median OS based on ITT analysis of our series of 77 patients was 18.3 months from leukapheresis, which compares favorably to the survival data reported by Stupp et al. [1] with a median OS of 14.6 months. More recent studies, with other new treatment strategies for GBM (e.g., Talampanel, poly-ICLC, or cilengitide treatment with standard radiochemotherapy, or 5-aminolevulinic acid guided glioma resection) [29, 33, 34], point to a median OS in the range of 18–20 months, in line with our ITT findings. However, only a randomized clinical trial can confirm the possible beneficial effect of immunotherapy integrated in the multimodal primary treatment.
Based on ITT analysis, 6mo-PFS was 70.1 %; as such, this result coincided with the assumption in our power analysis, and these data were then used to power the currently running randomized phase IIb trial HGG2010. When we consider the EORTC RPA classification, 65 % of the patients belonged to RPA class IV, 18 % to class V, and 17 % to class III. As one could expect, outcome improved significantly with lower RPA classification. Median OS was 39.7, 18.3, and 10.7 months for classes III, IV, and V, respectively. These data compare favorably to the RPA class-related survival estimates and RPA class-adjusted outcome in the EORTC 26981/22981-NCIC CE3 Trial [25], in which median OS in the radiotherapy/TMZ arm of the study was 21.4, 16.3, and 10.3 months for the respective classes. The doubling of the OS in RPA class III patients is striking. In our study, 6mo-PFS was 92, 72, and 43 % for RPA classes III, IV, and V, respectively. This shows that the prognostic factors used for the EORTC RPA classification hold true for this new treatment regimen including tumor vaccination. Moreover, tumor vaccination integrated into standard radiochemotherapy seems to improve survival, especially in the patients belonging to RPA class III, in which survival seems to be doubled compared to standard postoperative radiochemotherapy. The reason for the striking effect in especially RPA class III patients remains unexplained but several hypothetical factors could account for this finding: the younger age of these patients might be related to a higher number of circulating naïve T cells that can become specifically primed after vaccination. Moreover, we noticed that less patients from RPA class III were progressive under concomitant radiochemotherapy, probably creating a better substrate for subsequent immunization by the DC vaccine.
Six patients (7.8 %) progressed even before vaccinations could start and constitute the difference between the ITT group (77 patients) and the AT group (71 patients). Further protocol violations (incomplete TMZ administration in 12 patients, steroid use in 6 patients, and less than the scheduled 8 vaccines in 14 patients), mainly due to disease progression, further decreased the number of PP patients to 39, thereby indicating the restrictions of the feasibility to fully implement the protocol in all included patients. Patients treated per protocol had a clearly better clinical outcome than as-treated patients and even more so than ITT patients. This is trivial, reflecting a positive selection of patients receiving the full treatment regimen. Moreover, more patients in RPA class III were treated per protocol as compared to classes IV and V (85, 50, and 21 %, respectively). To avoid commonly introduced bias if only those patients are being vaccinated who do not show any progression at the end of the maintenance chemotherapy according to the Stupp regimen, we fully integrated the DC vaccine into the postoperative radiochemotherapy and analyzed all our data on an ITT basis.
Thirty-eight serious adverse events (NCI CTC grade III, IV, and V) were reported in 30 patients (39 %), including 19 hematological serious adverse events (hematotoxicity) in 18 patients (23 %). Hematological adverse events were most likely the result of concomitant and maintenance TMZ therapy. However, Stupp et al. [1] reported grade III or IV hematological toxic effects in only 16 % of patients. One patient died (NCI CTC grade V) due to an overwhelming infection in the postoperative period, even before radiochemotherapy or vaccination had started.
No clear benefit of total resection over subtotal resection was seen in this group of patients, although the grade of resection was a strong, independent predictor of the outcome in the recurrent HGG patients who were vaccinated [4]. This might be explained by the fact that in the subgroup of patients with subtotal resections, other prognostic factors were more favorable; 75 % had a methylated MGMT promoter in the subgroup of subtotal resection, as compared to only 53 % in the subgroup of total resection. Also, more subtotally resected patients belonged to lower RPA classes.
MGMT promoter methylation is correlated with improved PFS and OS in patients treated with alkylating agent chemotherapy (TMZ) and might even be a general favorable prognostic factor in GBM patients [2, 35–37]. This holds true in our patient population. As the production of the whole tumor cell lysate requires a critical minimal amount of tumor volume of about 3 cm3, and harvesting representative samples for reference pathology was mandatory for inclusion, we did not receive enough tumor volume of all patients to assess the MGMT promoter methylation status of all patients. Of the 48 patients in whom MGMT status was determined, 29 patients (60 %) had a methylated MGMT promoter. This percentage is higher than in the EORTC 26981/22981-NCIC CE3 Trial [35]. In that trial, the MGMT promoter was methylated in 45 % of the subgroup of patients treated with radio- and chemotherapy in whom MGMT promoter methylation status could be determined. However, according to the literature, MGMT methylation frequency in newly diagnosed GBM patients varies from 19 to 68 % as determined by methylation-specific polymerase chain reaction [27].
Exploratory immune profiling before the start of the vaccine, rather than classical immune monitoring during vaccination, was done using flow cytometry on blood samples. All these single-arm immunological parameters pointing to a possible induction of specific antitumor cytotoxic T cells can and did provide proof of the principle but are unlikely to accurately reflect a clinically relevant, complex in vivo immune response. From the different groups using whole tumor cell preparations as a source of glioma-associated antigens, only Wheeler et al. [6] were able to find a correlation between immunological and clinical responses. In our previous reports, interferon-γ ELISPOT [3] nor delayed-type hypersensitivity skin tests [11] could reveal any correlation with clinical outcome. Moreover, the lack of standardization in antitumor immune monitoring to date further confounds the field, preventing a comparison of different immune therapy approaches especially if whole tumor cell lysates are used as a source of antigens. Therefore, we rather wanted to explore a possible impact of the concomitant radiochemotherapy on the resetting of global immunological profiles based on changes in relative cell counts during radiochemotherapy for both effector and suppressor cell populations. These “immunological profiles” were compared to clinical outcome to find predictive immunological response patterns, as they can be available before the start of the immunotherapy. Using different statistical models, no statistical significant evidence was found that the immunological profiles contained any predictive information for either PFS or OS. However, cluster analysis plotted on Treg and NK cell ratios (V1/LF) revealed 3 clusters. A trend to increased PFS was seen from cluster 1 to cluster 3, although this did not reach statistical significance. Since these “response patterns/clusters” are based on changes in cell ratios between leukapheresis and the first vaccine, one could hypothesize on the importance of a differential resetting of the immune system by radiochemotherapy, that is, even before starting the immunotherapy. Further data are being gathered to test this hypothesis.
A possible explanation for the lack of correlation between immunological and clinical responses in the literature might be that the peripheral immune status does not mirror the immune responses that occur in the tumor itself. Also, using tumor lysate as the source of TAA has the disadvantage that there is a lack of specific antigens to be targeted in monitoring assays. The discordance between clinical and immunological data is a well-known problem for this type of treatment. It shows the inherent shortcomings of immune monitoring for these types of treatment. Therefore, although the lack of classical immune monitoring in this study might be a drawback, we believe that the use of surrogate immunological end points as main parameters to build a treatment strategy upon does not cover the full picture.
The full nature of the estimated beneficial effects of DC vaccination is without any doubt much more complex than any immune monitoring or molecular biology tool at this stage can fully capture. We realize that several protocol modifications in this vaccination approach might further improve the possible outcome, for example mixing the whole tumor cell lysate with GM-CSF [38–40] or the use of imiquimod, locally on the injection site (unpublished data). Therefore, we designed the currently running single-center prospective double-blind placebo-controlled phase IIb randomized clinical trial HGG-2010 (EudraCT 2009-018228-14), based on the feasibility, toxicity, and preliminary efficacy data reported here in combination with further theoretical vaccine improvements.
References
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoom MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer EA, Mirimanoff RO (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Eng J Med 352:987–996
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466
Ardon H, Van Gool S, Lopes IS, Maes W, Sciot R, Wilms G, Demaerel P, Bijttebier P, Claes L, Goffin J, Van Calenbergh F, De Vleeschouwer S (2010) Integration of autologous dendritic cell-based immunotherapy in the primary treatment for patients with newly diagnosed glioblastoma multiforme: a pilot study. J Neurooncol 99:261–272
De Vleeschouwer S, Fieuws S, Rutkowski S, Van Calenbergh F, Van Loon J, Goffin J, Sciot R, Wilms G, Demaerel P, Warmuth-Metz M, Soerensen N, Wolff JE, Wagner S, Kaempgen E, Van Gool SW (2008) Postoperative adjuvant dendritic cell-based immunotherapy in patients with relapsed glioblastoma multiforme. Clin Cancer Res 14:3098–3104
Sampson JH, Archer GE, Mitchell DA, Heimberger AB, Herndon JE, Lally-Goss D, McGehee-Norman S, Paolino A, Reardon DA, Friedman AH, Friedman HS, Bigner DD (2009) An epidermal growth factor receptor variant III-targeted vaccine is safe and immunogenic in patients with glioblastoma multiforme. Mol Cancer Ther 8:2773–2779
Wheeler CJ, Black KL, Liu G, Mazer M, Zhang XX, Pepkowitz S, Goldfinger D, Ng H, Irvin D, Yu JS (2008) Vaccination elicits correlated immune and clinical responses in glioblastoma multiforme patients. Cancer Res 68:5955–5964
Ardon H, De Vleeschouwer S, Van Calenbergh F, Claes L, Kramm CM, Rutkowski S, Wolff JE, Van Gool SW (2010) Adjuvant dendritic cell-based tumour vaccination for children with malignant brain tumours. Pediatr Blood Cancer 54:519–525
De Vleeschouwer S, Van Calenbergh F, Demaerel P, Flamen P, Rutkowski S, Kaempgen E, Wolff JEA, Plets C, Sciot R, Van Gool SW (2004) Transient local response and persistent tumor control of recurrent malignant glioma treated with combination therapy including dendritic cell therapy. J Neurosurg (pediatrics) 100:492–497
Grauer OM, Wesseling P, Adema GJ (2009) Immunotherapy of diffuse gliomas: biological background, current status and future developments. Brain Pathol 19:674–693
Liau LM, Prins RM, Kiertscher SM, Odesa SK, Kremen TJ, Giovannone AJ, Lin JW, Chute DJ, Mischel PS, Cloughesy TF, Roth MD (2005) Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res 11:5515–5525
Rutkowski S, De Vleeschouwer S, Kaempgen E, Wolff JEA, Kuhl J, Demaerel P, Warmuth-Metz M, Flamen P, Van Calenbergh F, Plets C, Sorensen N, Opitz A, Van Gool SW (2004) Surgery and adjuvant dendritic cell-based tumour vaccination for patients with relapsed malignant glioma, a feasibility study. Br J Cancer 91:1656–1662
Van Gool S, Maes W, Ardon H, Verschuere T, Van Cauter S, De Vleeschouwer S (2009) Dendritic cell therapy of high-grade gliomas. Brain Pathol 19:694–712
Walker DG, Laherty R, Tomlinson FH, Chuah T, Schmidt C (2008) Results of a phase I dendritic cell vaccine trial for malignant astrocytoma: potential interaction with adjuvant chemotherapy. J Clin Neurosci 15:114–121
North RJ (1984) Gamma-irradiation facilitates the expression of adoptive immunity against established tumors by eliminating suppressor T cells. Cancer Immunol Immunother 16:175–181
Emens LA (2010) Chemoimmunotherapy. Cancer J 16:295–303
Jameson SC (2002) Maintaining the norm: T-cell homeostasis. Nat Rev Immunol 2:547–556
Porter DL, June CH (2005) T-cell reconstitution and expansion after hematopoietic stem cell transplantation: ‘T’ it up! Bone Marrow Transplant 35:935–942
Banissi C, Ghiringhelli F, Chen L, Carpentier AF (2009) Treg depletion with a low-dose metronomic temozolomide regimen in a rat glioma model. Cancer Immunol Immunother 58:1627–1634
Jordan JT, Sun W, Hussain SF, DeAngulo G, Prabhu SS, Heimberger AB (2008) Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Cancer Immunol Immunother 57:123–131
Park SD, Kim CH, Kim CK, Park JA, Sohn HJ, Hong YK, Kim TG (2007) Cross-priming by temozolomide enhances antitumor immunity of dendritic cell vaccination in murine brain tumor model. Vaccine 25:3485–3491
Kim TG, Kim CH, Park JS, Park SD, Kim CK, Chung DS, Hong YK (2010) Immunological factors relating to the antitumor effect of temozolomide chemoimmunotherapy in a murine glioma model. Clin Vaccine Immunol 17:143–153
Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, Agostinis P (2010) Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. Biochim Biophys Acta 1805:53–71
Wheeler CJ, Das A, Liu G, Yu JS, Black KL (2004) Clinical responsiveness of glioblastoma multiforme to chemotherapy after vaccination. Clin Cancer Res 10:5316–5326
Liu G, Akasaki Y, Khong HT, Wheeler CJ, Das A, Black KL, Yu JS (2005) Cytotoxic T cell targeting of TRP-2 sensitizes human malignant glioma to chemotherapy. Oncogene 24:5226–5234
Mirimanoff RO, Gorlia T, Mason W, van den Bent MJ, Kortmann RD, Fisher B, Reni M, Brandes AA, Curschmann J, Villa S, Cairncross G, Allgeier A, Lacombe D, Stupp R (2006) Radiotherapy and temozolomide for newly diagnosed glioblastoma: recursive partitioning analysis of the EORTC 26981/22981-NCIC CE3 phase III randomized trial. J Clin Oncol 24:2563–2569
Ardon H, Verbinnen B, Maes W, Beez T, Van Gool S, De Vleeschouwer S (2010) Technical advancement in regulatory T cell isolation and characterization using CD127 expression in patients with malignant glioma treated with autologous dendritic cell vaccination. J Immunol Methods 352:169–173
Weller M, Stupp R, Reifenberger G, Brandes AA, van den Bent MJ, Wick W, Hegi ME (2010) MGMT promoter methylation in malignant gliomas: ready for personalized medicine? Nat Rev Neurol 6:39–51
Gorlia T, van den Bent MJ, Hegi ME, Mirimanoff RO, Weller M, Cairncross JG, Eisenhauer E, Belanger K, Brandes AA, Allgeier A, Lacombe D, Stupp R (2008) Nomograms for predicting survival of patients with newly diagnosed glioblastoma: prognostic factor analysis of EORTC and NCIC trial 26981-22981/CE.3. Lancet Oncol 9:29–38
Stummer W, Tonn JC, Mehdorn HM, Nestler U, Franz K, Goetz C, Bink A, Pichlmeier U (2010) Counterbalancing risks and gains from extended resections in malignant glioma surgery: a supplemental analysis from the randomized 5-aminolevulinic acid glioma resection study. J Neurosurg 114:613–623
Muller AJ, Prendergast GC (2005) Marrying immunotherapy with chemotherapy: why say IDO? Cancer Res 65:8065–8068
Nowak AK, Robinson BW, Lake RA (2003) Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Res 63:4490–4496
Masucci GV, Mansson-Brahme E, Ragnarsson-Olding B, Nilsson B, Wagenius G, Hansson J (2006) Alternating chemo-immunotherapy with temozolomide and low-dose interleukin-2 in patients with metastatic melanoma. Melanoma Res 16:357–363
Grossman SA, Ye X, Chamberlain M, Mikkelsen T, Batchelor T, Desideri S, Piantadosi S, Fisher J, Fine HA (2009) Talampanel with standard radiation and temozolomide in patients with newly diagnosed glioblastoma: a multicenter phase II trial. J Clin Oncol 27:4155–4161
Grossman SA, Ye X, Piantadosi S, Desideri S, Nabors LB, Rosenfeld M, Fisher J (2010) Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res 16:2443–2449
Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003
Rivera AL, Pelloski CE, Gilbert MR, Colman H, De La CC, Sulman EP, Bekele BN, Aldape KD (2010) MGMT promoter methylation is predictive of response to radiotherapy and prognostic in the absence of adjuvant alkylating chemotherapy for glioblastoma. Neuro Oncol 12:116–121
Hegi ME, Liu L, Herman JG, Stupp R, Wick W, Weller M, Mehta MP, Gilbert MR (2008) Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J Clin Oncol 26:4189–4199
Clavreul A, Piard N, Tanguy JY, Gamelin E, Rousselet MC, Leynia P, Menei P (2010) Autologous tumor cell vaccination plus infusion of GM-CSF by a programmable pump in the treatment of recurrent malignant gliomas. J Clin Neurosci 17:842–848
Sloan AE, Dansey R, Zamorano L, Barger G, Hamm C, Diaz F, Baynes R, Wood G (2000) Adoptive immunotherapy in patients with recurrent malignant glioma: preliminary results of using autologous whole-tumor vaccine plus granulocyte-macrophage colony-stimulating factor and adoptive transfer of anti-CD3-activated lymphocytes. Neurosurg Focus 9:e9
Wallenfriedman MA, Conrad JA, DelaBarre L, Graupman PC, Lee G, Garwood M, Gregerson DS, Jean WC, Hall WA, Low WC (1999) Effects of continuous localized infusion of granulocyte-macrophage colony-stimulating factor and inoculations of irradiated glioma cells on tumor progression. J Neurosurg 90:1064–1071
Acknowledgments
We thank our laboratory collaborators for their excellent technical assistance, the department of hematology for the care provided at time of the leukapheresis, and the clinic for radiotherapy for irradiating the tumor lysate preparations. HSA has been provided by the Belgian Red Cross and Baxter. This project is supported by the Olivia Hendrickx Research Fund (http://www.olivia.be), the Herman Memorial Research Fund (http://www.hmrf.be), and the James E. Kearney Memorial Foundation (http://www.jekfoundation.org). Support was also obtained from CAF Belgium, Baxter, and gifts from private families and service clubs. Additionally, grants were obtained from “Stichting tegen Kanker”, IWT (TBM projects), the Stem Cell Institute Leuven, the Emmanuel van der Schueren Fund, the International Union against Cancer, the Klinisch Onderzoeksfonds UZ Leuven, and the Fund for Scientific Research—Flanders (FWO-V). SDV is supported by the “Klinisch onderzoeksfonds” from the University Hospital Leuven. SWVG is Senior Clinical Investigator of the Fund for Scientific Research—Flanders (Belgium) (F.W.O.-Vlaanderen).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ardon, H., Van Gool, S.W., Verschuere, T. et al. Integration of autologous dendritic cell-based immunotherapy in the standard of care treatment for patients with newly diagnosed glioblastoma: results of the HGG-2006 phase I/II trial. Cancer Immunol Immunother 61, 2033–2044 (2012). https://doi.org/10.1007/s00262-012-1261-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-012-1261-1