Abstract
Background
CD4+CD25+ regulatory T cells (Treg), which constitute about 2–3% of CD4+ human T cells, are the main contributors to the maintenance of immune tolerance. Cancer patients, including glioblastoma patients, bear increased number of circulating and tumor infiltrating Treg that exert functional inhibition on tumor-specific T cells. Temozolomide (TMZ) is one of the most effective chemotherapeutic agents in glioblastoma (GBM). Lymphopenia is a common side effect of TMZ treatment, but to what extent the Treg compartment is affected by this chemotherapy has been poorly investigated. We therefore studied the impact of various TMZ regimens on Treg cell population in a TMZ-resistant rat model of glioma.
Methods
RG2 glioma cells were implanted s.c. in Fischer rats. Twelve days after tumor implantation, TMZ was administered orally with schedules designed to mimic the TMZ regimens currently used in humans: 30 mg/kg per day for 5 days, or 10 mg/kg per day for 21 days. In addition, two metronomic regimens with low-dose TMZ (2 and 0.5 mg/kg per day for 21 days) were evaluated. Splenocytes and tumor infiltrating lymphocytes were analysed by flow cytometry using CD3, CD4, CD25, and Foxp3 mAbs. Statistical significance was determined by the Mann–Whitney U test, the Student’s t test or the ANOVA test.
Results
In the spleen of tumor-bearing animals, low-dose TMZ metronomic regimens (0.5 and 2 mg/kg for 21 days) induced a significant decrease of Treg/CD4+ ratios (13 ± 2; p < 0.01, 14 ± 3; p < 0.05, respectively, vs. 19 ± 5 for controls). On the contrary, high-dose TMZ regimen (10 mg/kg per day for 21 days or 30 mg/kg for 5 days) did not significantly modify the percentage of Treg/CD4+. Within tumors, treatment with the 0.5 mg/kg TMZ regimen induced a slight and nearly significant decrease in the percentage of Treg/CD4+ after a 2 to 3-week treatment (24 ± 9 vs. 35 ± 11; p = 0.06). Treg depletion induced by the low-dose metronomic TMZ regimen was accompanied by a decreased suppressive function of the remaining Treg cells as assessed by an in vitro functional test. Treatment with 0.5 mg/kg metronomic TMZ reduced tumor progression when compared to untreated animals but the effect did not reach statistical significance, indicating that Treg depletion alone is not sufficient to significantly impact tumor growth in our model of fully established tumor.
Conclusions
A low-dose metronomic TMZ regimen, but not a standard TMZ regimen, reduced the number of circulating Tregs. These results can have clinical applications for immunotherapeutic approaches in GBM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Temozolomide (TMZ) is one of the most effective chemotherapeutic agents against glioblastoma (GBM). Concomitant radiotherapy plus TMZ (75 mg/m2 daily) followed by six cycles of TMZ has become the new standard of care for patients with newly diagnosed GBM [1]. For the treatment of recurrent glioma, the approved conventional schedule consists of a daily dose of 150–200 mg/m2 for 5 days every 28 days [2, 3]. However, due to various resistance mechanisms, glial tumors have a strong tendency to relapse, leading to local recurrence and death [4].
CD4+CD25+ regulatory T cells (Treg) contribute to the prevention of autoimmune disorders by suppressing autoreactive T lymphocytes [5, 6]. These cells are also involved in the immune tolerance of cancer [7, 8], and it has been demonstrated in several animal models that the therapeutic depletion of Treg cells can evoke effective tumor immunity and lead to tumor rejection [9, 10]. Patients with malignant glioma express significant immune defects, including CD4 lymphopenia and increased fractions of regulatory T cells in peripheral blood and tumor infiltrating lymphocytes [11, 12]. In vitro, this elevation in Treg fraction correlates with a diminished CD4 proliferation in response to antiCD3, which can be restored by Treg depletion [11].
Treatment by low-dose metronomic cyclophosphamide regimens has been shown to deplete Treg cell population in both rodents [13] and humans [14]. It remains unclear, whether all chemotherapy can induce such Treg depletion. We decided to study the effect of various TMZ regimens on Treg cell population in a rat model. Such Treg cell depletion with a drug commonly used in glioma patients could be of great interest in the therapeutic management of glial tumors, particularly in combination with tumor vaccines. Indeed, several tumor models have highlighted the beneficial effect of Treg depletion for an effective immunization [15–17].
In the present study, the RG2 rat glioma model was chosen because of its known resistance to various standard chemotherapeutic regimens and its poor immunogenicity. The rats were treated with TMZ regimens designed to mimic as close as possible to the standard and extended TMZ regimens currently used in humans. In an attempt to find an optimal metronomic regimen in this model, two metronomic regimens with very low TMZ dosages were evaluated.
Materials and methods
Cell culture
The rat glioma cell line RG2 (American Type Culture Collection no. CRL-2433, LGC Promochem, Molsheim, France) was cultured in Dulbecco’s modified essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 units/ml penicillin, 100 μg/ml streptomycin and 0.25 μg/ml amphotericin B (all reagents from Invitrogen, Cergy Pontoise, France).
Temozolomide (TMZ)
For in vitro experiments, TMZ (Temodal, Schering Plough) was dissolved at 33.3 mM in DMSO and further diluted with culture medium to the indicated concentrations. For in vivo experiments, aqueous suspensions of TMZ were administered by oral gavage (0.5 ml/rat). All TMZ solutions were prepared extemporaneously.
In vitro cytotoxicity assay
RG2 cells were seeded in 24-well plates and treated with TMZ (1–100 μM) for 4 days, 10 days or 17 days. Culture media were replaced daily. At the end of the treatment period, a standard MTT-based cytotoxicity assay (Sigma-Aldrich, Saint-Quentin Fallavier, France) was performed. The viability of treated cells was expressed as a percentage of control cultures (vehicle alone).
Animal models
RG2 tumors were induced by s.c. injection of 100,000 tumor cells in the left flank of 5-week-old female Fischer-344 rats (Charles River Laboratories, L’Abresle, France) and allowed to grow for 12 days before treatment (tumors sizing approximately 4 mm in diameter). The standard (30 mg/kg per day i.e. 175 mg/m2 per day for 5 days) and the daily (10 mg/kg per day i.e. 60 mg/m2 per day, 5 days per week for 3 weeks) TMZ dosages and schedules administered to rats were designed to mimic as close as possible to the standard and extended TMZ regimens currently used in humans, dose calculation being made according to the US Food and Drug Administration recommendations (http://www.fda.gov/cder/cancer/animalframe.htm). Two additional TMZ dosages (2 mg/kg per day i.e. 12 mg/m2 per day, 0.5 mg/kg per day i.e. 3 mg/m2 per day) were evaluated in the metronomic (5 days per week for 3 weeks) schedule. Tumor growth was assessed twice a week by size measurement with a calliper, tumor volume being estimated using the standard formula: π/6 × length × width2. Animal use and handling were performed according to the French laws for animal experimentation.
Preparation of splenocytes
Animals were sacrificed with penthotal. Splenocytes were prepared according to standard procedures. Briefly, each spleen was transferred on to a 100 μm cell strainer. The cells were then gently forced through the meshes with a 5 ml syringe pestle, and collected in RPMI 1640 medium containing 10% FBS. The suspension was then centrifuged and the pellet was resuspended in 1 ml of erythrocyte lysis buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM Na2EDTA, pH 7.2) and incubated for 4 min at room temperature. The reaction was stopped by addition of 10 ml of RPMI 1640 medium containing 10% FBS. The cells were centrifuged and the pellet was resuspended in 5 ml of RPMI 1640 medium containing 10% FBS. Isolated splenocytes were stored at 4°C for a maximum of 2 h until incubation with fluorochrome-conjugated monoclonal antibodies and flow cytometry analysis.
Preparation of tumor cells
Tumors were resected immediately after sacrifice. They were minced with scalpels to obtain fragments of about 1 mm3, and incubated with 0.75 mg/ml collagenase (Sigma-Aldrich, L’Isle d’Abeau Chesnes, France) for 30 min at 37°C under gentle agitation. The digested mixture was filtered through a 100 μm cell strainer. The filtrate containing single cell suspension was washed and resuspended in 1 ml of RPMI 1640 medium containing 10% FBS, while the retentate was submitted to a second digestion in order to improve cell recovery.
Flow cytometry analysis
Surface staining
Mouse monoclonal antibodies against rat CD3 (clone 1F4, APC- or FITC-conjugated), rat CD4 (clone OX-35, FITC- or APC-conjugated) and rat CD25 (clone OX-39, PE-conjugated) were purchased from BD Biosciences (Le Pont de Claix, France). Cells were incubated with fluorochrome-conjugated mAbs for 1 h at 4°C in RPMI 1640 medium containing 10% FBS.
Foxp3 labeling
Rat splenocytes were surface stained with monoclonal antibodies against rat CD3 (FITC-conjugated) and CD4 (APC-conjugated). Cells were subsequently fixed, permeabilized and submitted for intracellular staining with a PE-conjugated monoclonal antibody against Foxp3 (clone FJK-16s) using the Mouse Regulatory T cell Staining Kit (http://www.eBioscience.com), which recognizes Foxp3 from both mouse and rat origins, according to manufacturer’s instructions. Cytometry analyses were performed on FACScan (Beckton Dickinson) using WinMDI software.
Treg functional test
Cell suspensions containing at least 95% T cells after flow cytometry analysis were obtained from rat spleens by dissociation through a stainless-steel wire mesh followed by passage through a nylon wool column. Treg depletion was performed using a CD25 Microbead Kit (Miltenyi Biotech, Paris, France) according to manufacturer’s instructions. Briefly, splenic T cells were sequentially incubated with antiCD25-PE and antiPE microbeads, by which CD25 expressing cells were magnetically labeled. CD25+ cells were then retained on an LD column, while the unlabeled CD25− fraction was collected in flow-through. Subsequently, 2 × 106 splenic T cells depleted or not of Treg were each incubated for 3 days with antiCD3 mAb. Supernatants of these cultures were harvested and IFN-γ concentrations were determined by an ELISA test (BD Pharmingen, Le Pont de Clay, France).
Statistics
Statistical significance was determined by the Mann–Whitney U test, the Student’s t test or the ANOVA test.
Results
Effects of standard and low-dose metronomic TMZ regimens on Treg cells in healthy rats
We first investigated, whether low-dose TMZ could deplete Treg in the spleen of tumor-free animals. Three metronomic 5 days per week regimens (0.5, 2, and 10 mg/kg) administered for 3 weeks were compared to the standard regimen of TMZ (30 mg/kg for 5 days). Animals (n = 5 per group) were sacrificed 72 h after the last dose.
In tumor-free animals, low-dose TMZ regimens (0.5 and 2 mg/kg) did not significantly reduce the percentage of Treg/CD4+ when compared to untreated controls (13% ± 1 in both TMZ regimens vs. 14% ± 1). In contrast, higher doses of TMZ (standard regimen and 10 mg/kg daily regimen) resulted in an increased percentage of Treg/CD4+ when compared to controls (17% ± 1 in both TMZ regimens vs. 14% ± 1; p < 0.01).
Effects of standard and low-dose metronomic TMZ regimens on Treg cells in tumor-bearing rats
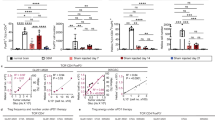
We then examined the effect of the above regimens in tumor-bearing rats, because Treg levels are known to be higher in the spleen of these animals. Indeed, the percentage of Treg/CD4+ (Fig. 1) was higher in the spleen of rats bearing 32-days RG2 tumors [19% ± 5 (n = 8)] than in the spleen of tumor-free control animals [14% ± 4 (n = 9)].
Comparison of the Treg/CD4+ levels in the spleen of tumor-free rats (open circles, n = 9) and tumor-bearing rats (filled circles, n = 8). FACScan analysis of splenocytes was performed 32 days after RG2 tumor implantation. Treg/CD4+ levels were found to be higher in tumor-bearing animals (19 ± 5) than in tumor-free animals (14 ± 4), (p = 0.07)
In the spleen of tumor-bearing animals (Fig. 2), low-dose metronomic TMZ regimens (0.5 and 2 mg/kg) administered for 3 weeks induced a statistically significant decrease of Treg/CD4+ levels (13% ± 2 and 14% ± 3, respectively) when compared to untreated controls (19% ± 5). On the contrary, neither the daily TMZ regimen of 10 mg/kg nor the standard TMZ regimen significantly decreased the Treg/CD4+ ratio in the spleen of tumor-bearing animals (19% ± 5 and 17% ± 3, respectively). A moderate decrease (20%) in spleen weight and cellularity was observed in animals treated with the highest dose (10 mg/kg) of daily TMZ regimen when compared to untreated tumor-bearing rats, whereas other TMZ regimens did not significantly impact these parameters. The proportion of CD4+ cells within spleen lymphocytes was similar in tumor-free and tumor-bearing animals (22 ± 2 and 22 ± 5%, respectively) and was not significantly affected by any of the studied TMZ regimens (26 ± 5, 25 ± 4, 23 ± 6, and 26 ± 3% for standard and various metronomic TMZ regimens) after 3 weeks of treatment. CD4/CD8 ratios, which were increased in tumor-bearing rats when compared to tumor-free animals (range 1.2–2.2 vs. 0.8–0.9, respectively), were not significantly modified by standard or metronomic TMZ treatments (range 1.0–1.9).
Comparison of the Treg/CD4+ levels (mean ± SD) in the spleen of RG2 tumor-bearing rats (7–9 rats per group) treated either by a 5 days/week regimen of TMZ (0.5, 2 or 10 mg/kg per day) for 3 weeks or by a 5-day standard regimen of TMZ (30 mg/kg per day). Tumors were implanted on day-12, 5 days/week regimens were initiated on day 0, and standard regimen of TMZ was initiated on day 14. Animals were sacrificed 72 h after receiving the last dose of treatment. (* p < 0.05; ** p < 0.01)
Altogether, these data suggest that a selective depletion of Treg cells can be achieved with a low-dose (0.5 mg/kg) of metronomic TMZ regimen whereas higher doses are either less effective (2 mg/kg) or result in a non-selective splenocyte depletion (10 mg/kg). In subsequent experiments, we showed that Treg depletion, as assessed by a decreased Treg/CD4+ ratio, occurred as early as 1 week after the initiation of the 0.5 mg/kg metronomic TMZ treatment and was maintained throughout the evaluated treatment period (Table 1).
Effects of low-dose metronomic TMZ regimen on tumor infiltrating lymphocytes
We then examined how the low-dose TMZ metronomic treatment would affect the populations of tumor infiltrating lymphocytes (TILs). After 2–3 weeks of treatment with the 0.5 mg/kg TMZ regimen, whereas the percentage of CD4+ T lymphocytes remained unchanged when compared to untreated controls (23 ± 5 vs. 24 ± 5%, respectively), a decrease in the percentage of Treg/CD4+ was seen (24 ± 9 vs. 35 ± 11%, respectively; n = 7 per group). However, this decrease did not reach the level of statistical significance (p = 0.06).
Correlation between CD25 and intracellular Foxp3 expression in rat splenocytes
To ascertain that the CD3+CD4+CD25+ cells—which were considered as regulatory T cells throughout this work—were indeed Treg cells, a concurrent determination of CD25 and Foxp3 expression (Fig. 3) was performed in three groups of tumor-bearing rats (either treated with standard TMZ, metronomic TMZ or vehicle alone), and one group of tumor-free rats (n = 3 per group). Results obtained with Foxp3 labeling closely paralleled those obtained with CD25 labeling. As previously noticed, spleens of tumor-bearing rats contained a higher percentage of Tregs (either identified as CD4+CD25+ or as CD4+Foxp3+ cells) than those of tumor-free rats. In tumor-bearing rats, the low-dose of metronomic TMZ regimen significantly reduced the Treg/CD4+ ratio when compared to vehicle alone, whereas the standard TMZ regimen did not.
Comparative study of the characterization of Treg splenocytes by CD25 surface staining or by Foxp3 intracellular staining. Four groups of three rats were either inoculated with RG2 cells on day-12 or left tumor-free (Naive). Treatment, which consisted of vehicle alone (Untreated), TMZ 30 mg/kg per day for 5 days (Std TMZ) or TMZ 0.5 mg/kg per day for 3 weeks (Metronomic TMZ) was administered to tumor-bearing rats. Animals were sacrificed on day 21. Isolated splenocytes were stained with antiCD3, antiCD4, and antiCD25 or with antiCD3, antiCD4, and antiFoxp3 mAbs and then submitted to flow cytometry analysis. a Results expressed as mean ± SD of the Treg/CD4+ levels (%) in the four groups of rats, Treg being either characterized as CD4+CD25+ cells (open bars) or as CD4+Foxp3+ cells (filled bars). b FCM analysis of spleen cells isolated from an untreated (left) and a metronomic TMZ-treated rat (right), both bearing RG2 tumors. Cells were surface stained with antiCD3-FITC and antiCD4-APC, then submitted to intracellular staining with antiFoxp3-PE. These plots (gated on the CD3+ population) are representative of the results obtained with three rats in each group (percentage of cells in the CD4+ quadrants are indicated)
Evaluation of the impact of low-dose metronomic TMZ regimens on Treg function
As metronomic TMZ treatment was shown to decrease the Treg/CD4+ ratio in the spleen of tumor-bearing rats, we further investigated the impact of such treatment on splenic T cell function (Fig. 4). T lymphocytes isolated from the spleen of rats bearing RG2 tumors (Untreated) and cultured with antiCD3 mAb (which mimics the MHC class II-peptide complex) produced low IFN-γ levels (175 ± 33 pg/ml) when compared to T splenocytes isolated from tumor-free (Naïve) rats (410 ± 49 pg/ml). A 3-week 0.5 mg/kg per day TMZ regimen (Metronomic TMZ) administered to tumor-bearing rats dramatically increased IFN-γ secretion by antiCD3 stimulated T splenocytes (600 ± 27 pg/ml) when compared to untreated controls (175 ± 33 pg/ml) whereas a 5-day 30 mg/kg per day regimen (Std TMZ) did not (153 ± 27 pg/ml).
Comparison of the impact of standard and metronomic TMZ regimens on Treg function. Four groups of three rats were either inoculated with RG2 cells on day-12 or left tumor-free (Naive). Treatment, which consisted of vehicle alone (Untreated), TMZ 30 mg/kg per day for 5 days (Std TMZ) or TMZ 0.5 mg/kg per day for 3 weeks (Metronomic TMZ) was administered to tumor-bearing rats. Animals were sacrificed on day 21 and T cells were isolated from the collected spleens. 2 x 106 splenic T cells depleted (open bars) or not (filled bars) of Treg were incubated for 3 days with antiCD3 mAb. Supernatants of these cultures were harvested and IFN-γ concentrations were determined by an ELISA test. Results are expressed as mean ± SD of IFN-γ levels measured in three splenic T cell cultures obtained from three different rats. IFN-γ levels observed in cultures depleted or not of Treg were compared within each group. NS not significant; * p < 0.05; paired Student’s t test
In naïve rats, Treg depletion from the T cell population before incubation with antiCD3 mAb resulted in enhanced IFN-γ secretion (Fig. 4, Naïve, Open bar) when compared to undepleted cells (Fig. 4, Naïve, Filled bar). Similarly, Treg depletion significantly increased IFN-γ levels produced by T lymphocytes isolated from untreated and standard TMZ-treated tumor-bearing rats. On the contrary, Treg depletion failed to significantly increase IFN-γ levels produced by T splenocytes isolated from metronomic TMZ-treated tumor-bearing rats.
Altogether, these results indicate that T cells isolated from the spleen of tumor-bearing rats produce abnormally low levels of IFN-γ in response to antiCD3 stimulus (Fig. 4), which can be attributed to an increased percentage of Treg cells in the splenocyte population (Fig. 3), as IFN-γ levels could be restored by Treg depletion before incubation with antiCD3. As standard TMZ treatment failed to decrease the Treg/CD4+ ratio in tumor-bearing rats (Fig. 3), T cells isolated from the spleen of these animals produced low IFN-γ levels, similar to those of untreated animals (Fig. 4). Interestingly, although Treg depletion induced by metronomic TMZ regimen in tumor-bearing rats was not complete (Fig. 3), depleting the remaining CD25+ cells from the T cell population before incubation with antiCD3 failed to further increase IFN-γ levels (Fig. 4). This indicates that the low-dose metronomic TMZ regimen not only reduced Treg numbers in the spleen of tumor-bearing rats but also impacted their function.
Effects of a low-dose metronomic TMZ regimen on tumor growth in vivo
We then investigated whether the Treg depletion observed in the spleen and tumor of low-dose metronomic TMZ-treated animals would be sufficient to impact tumor growth (Fig. 5). In spite of a tendency for the tumor volumes to be consistently lower in 0.5 mg/kg metronomic TMZ-treated animals when compared to untreated controls, this difference did not reach statistical significance (p = 0.23).
Effect of a low-dose metronomic TMZ regimen on tumor growth. Rats were inoculated with RG2 cells on day-12. Treatment, which consisted of vehicle alone (filled circles) or TMZ 0.5 mg/kg per day (open squares) was initiated on day 0. Tumor growth was assessed twice a week by size measurement with a calliper, and tumor volume was estimated using the standard formula: p/6 × length × width2. Results are expressed as mean ± SEM of tumor volumes measured in 30 rats per group, by compilation of several experiments
In vitro study of TMZ toxicity on RG2 tumor cells
To ensure that the observed delay in tumor growth could not be attributed to direct toxicity of the drug, in vitro toxicity of TMZ on RG2 cells was evaluated. After 4- to 17-day exposures, TMZ concentrations ranging from 1 to 100 μM exhibited no significant toxicity, as assessed by MTT viability assay (data not shown).
Discussion
Temozolomide is one of the most effective chemotherapeutic agents for patients with GBM. The conventional doses and schedules can induce a significant lymphopenia. We here report for the first time that a very low-dose metronomic TMZ regimen can induce a selective Treg depletion in vivo.
Continuous administration of doses as low as 0.5 mg/kg per day induced a Treg depletion among the splenocyte and tumor infiltrating lymphocyte populations in our murine glioma model. Although an intracerebral model would have been closer to the clinical situation, the lifespan of rats bearing intra-cranial RG2 tumors is short (less than 21 days), which would have precluded the study of the effects of long-term metronomic TMZ regimens on large tumor-bearing animals. Therefore, a rat model of sub-cutaneously implanted RG2 glioma was chosen. In this model, the low-dose metronomic TMZ regimen both reduced the number of Treg cells and inhibited their suppressive capability. Indeed, in an in vitro functional assay, antiCD3-stimulated T splenocytes isolated from low-dose TMZ-treated rats secreted more IFN-γ than those of untreated tumor-bearing rats, evidence of a lower Treg activity. This lower activity cannot be fully explained by a decreased number of Treg cells because Treg depletion was only around 30%. Actually, this low activity suggests that the remaining Treg have lost their suppressive properties, as shown by the fact that CD25 depletion prior to antiCD3 stimulation did not further increase IFN-γ secretion, as it does in non-treated rats. Such a decrease in both Treg number and function by a metronomic chemotherapeutic regimen has been previously described with low-dose cyclophosphamide (CY) [14, 16, 18]. The mechanism of action of CY on Treg cells is multifactorial and involves enhanced susceptibility to apoptosis, loss of homeostatic proliferation and down-regulation of the expression of genes implicated in regulatory T cell function, such as GITR [18]. Whether the action of metronomic TMZ on Treg cells proceeds through similar mechanisms is probable, because both TMZ and CY are alkylating agents, but this point deserves further investigations.
The efficacy of the TMZ treatment on Treg is dose and schedule dependent. Two low-dose (0.5 and 2 mg/kg per day) of metronomic TMZ regimens administered for 3 weeks induced a significant decrease in Treg/CD4+ ratios in the spleen of tumor-bearing rats, the lowest dose being the most effective. On the contrary, the standard (30 mg/kg per day for 5 days) and daily (10 mg/kg per day for 3 weeks) TMZ regimens failed to significantly impact the Treg/CD4+ ratio in the spleen of tumor-bearing rats, probably because of a non-selective toxicity on lymphocyte population [19]. In our experiments, a moderate decrease in spleen weight and cellularity was indeed observed after 3 weeks of daily TMZ regimen. It is noteworthy that the standard and daily TMZ regimens, which failed to deplete Treg cells, were designed to mimic the standard and extended TMZ regimens currently used in humans (150–200 mg/m2 per day for 5 days and 75 mg/m2 per day daily), whereas the metronomic regimen, which effectively depleted Treg cells, corresponds to a very low dosage (3 mg/m2 per day) and has never been investigated in humans.
In the present study, high-dose of TMZ regimens (30 mg/kg per day for 5 days and 10 mg/kg per day for 3 weeks) failed to delay tumor growth in rats bearing established RG2 tumors. This is not surprising as the RG2 glioma model was chosen because of its known resistance to various standard chemotherapeutic agents [20]. In our hands, TMZ showed no toxicity on RG2 cells at concentration (100 μM) exceeding those achievable in vivo (while IC50 values for sensitive glioma cells which are usually below 75 μM [21]). On the contrary, a slight decrease in tumor growth was observed in low-dose metronomic treated animals when compared to untreated controls. However, this effect did not reach statistical significance, which indicates that Treg depletion alone is not sufficient to significantly impact tumor growth in our tumor model. This is in line with other reports that showed no therapeutic benefit of Treg depletion alone on fully established tumors, in spite of a demonstrated prophylactic efficacy in the same models [9, 22].
In animal models of fully established tumors, Treg depletion has proven efficacy in association with various immunotherapeutic treatments and was even a prerequisite for successful tumor eradication in some of these models [15–17, 23]. Patients with GBM suffer from a well-documented impairment of T and B cell immunity [24], which constitutes a substantial barrier to the activation of antitumor immune response. A major contributor to depressed cellular immunity in these patients is an increased level of regulatory T cells [11]. Treg exert functional inhibition on NK cells [25] and tumor-specific T cells [26], thus blunting both innate and adaptive immunity. It was recently demonstrated that a selective depletion of Treg cells was sufficient to restore T and NK effecter functions in end-stage cancer patients [14]. Therefore, an attractive strategy for the treatment of malignant glioma could involve Treg depletion by a low-dose TMZ regimen in association with a cancer vaccine. Interestingly, a successful association of TMZ chemotherapy and active immunotherapy against EGFRvIII was recently reported [27]. This association was beneficial in spite of an increased Treg ratio in the course of the conventional TMZ chemotherapy. One can speculate that such combination, with lower doses of TMZ aiming at depleting Treg cells, can even be more successful. Concomitant radiotherapy plus TMZ (75 mg/m2 daily) followed by six cycles of TMZ represents the current standard of care for patients with newly diagnosed GBM. Radiation can increase MHC class I expression by tumor cells, increase their vulnerability to CTLs and induces antitumor immune response [28]. However, the immunosuppressive effects of high dose TMZ should be taken into consideration, as repeated cycles of lymphodepletion may destroy the expanding population of tumor-specific immune effectors [29]. Another strategy would therefore be to combine metronomic TMZ at very low-dose and radiotherapy, either in newly diagnosed patients or in a recurrent setting.
In conclusion, an unexpected effect of TMZ on lymphocytes was seen at low doses, with selective depletion of Tregs. To the best of our knowledge, this effect has not been demonstrated for TMZ before. Since regulatory T cells are a major barrier to effective immunotherapy, this is a potentially important observation. Indeed, such a depletion may have clinical applications either alone, or more probably when combined with immunotherapy approaches.
References
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for Research, Treatment of Cancer Brain Tumor, Radiotherapy Groups, National Cancer Institute of Canada Clinical Trials Group (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996
Yung WK, Albright RE, Olson J et al (2000) A phase II study of temozolomide versus procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer 83:588–593
Friedman HS, Kerby T, Calvert H (2000) Temozolomide and treatment of malignant glioma. Clin Cancer Res 6(7):2585–2597
Behin A, Hoang-Xuan K, Carpentier AF, Delattre JY (2003) Primary brain tumours in adults. Lancet 361(9354):323–331
Sakaguchi S (2000) Regulatory T cells: key controllers of immunologic self-tolerance. Cell 101(5):455–458
Gupta S, Shang W, Sun Z (2008) Mechanisms regulating the development and function of natural regulatory T cells. Arch Immunol Ther Exp (Warsz) 56(2):85–102
Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, Kuniyasu Y, Nomura T, Toda M, Takahashi T (2001) Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev 182:18–32
Grauer OM, Nierkens S, Bennink E, Toonen LW, Boon L, Wesseling P, Sutmuller RP, Adema GJ (2007) CD4+FoxP3+ regulatory T cells gradually accumulate in gliomas during tumor growth and efficiently suppress antiglioma immune responses in vivo. Int J Cancer 121(1):95–105
Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E (1999) Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res 59(13):3128–3133
Golgher D, Jones E, Powrie F, Elliott T, Gallimore A (2002) Depletion of CD25+ regulatory cells uncovers immune responses to shared murine tumor rejection antigens. Eur J Immunol 32(11):3267–3275
Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE, Herndon JE 2nd, Bigner DD, Dranoff G, Sampson JH (2006) Increased regulatory T cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res 66(6):3294–3302
El Andaloussi A, Lesniak MS (2006) An increase in CD4+CD25+FOXP3 + regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro Oncol 8(3):234–243
Motoyoshi Y, Kaminoda K, Saitoh O, Hamasaki K, Nakao K, Ishii N, Nagayama Y, Eguchi K (2006) Different mechanisms for anti-tumor effects of low- and high-dose cyclophosphamide. Oncol Rep 16(1):141–146
Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, Solary E, Le Cesne A, Zitvogel L, Chauffert B (2007) Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother 56(5):641–648
Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, Chauffert B, Solary E, Bonnotte B, Martin F (2004) CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol 34(2):336–344
Taieb J, Chaput N, Schartz N, Roux S, Novault S, Ménard C, Ghiringhelli F, Terme M, Carpentier AF, Darrasse-Jèze G, Lemonnier F, Zitvogel L (2006) Chemoimmunotherapy of tumors: cyclophosphamide synergizes with exosome based vaccines. J Immunol 176(5):2722–2729
Grauer OM, Sutmuller RP, van Maren W, Jacobs JF, Bennink E, Toonen LW, Nierkens S, Adema GJ (2008) Elimination of regulatory T cells is essential for an effective vaccination with tumor lysate-pulsed dendritic cells in a murine glioma model. Int J Cancer 122(8):1794–1802
Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H (2005) Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood 105(7):2862–2868
Review of preclinical toxicity studies of temozolomide. http://www.fda.gov/cder/foi/nda/99/21029_Temodar.htm
Barth RF (1998) Rat brain tumor models in experimental neuro-oncology: the 9L, C6, T9, F98, RG2 (D74), RT-2 and CNS-1 gliomas. J Neurooncol 36(1):91–102
Sankar A, Thomas DG, Darling JL (1999) Sensitivity of short-term cultures derived from human malignant glioma to the anti-cancer drug temozolomide. Anticancer Drugs 10(2):179–185
Shimizu J, Yamazaki S, Sakaguchi S (1999) Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol 163(10):5211–5218
Di Paolo NC, Tuve S, Ni S, Hellström KE, Hellström I, Lieber A (2006) Effect of adenovirus-mediated heat shock protein expression and oncolysis in combination with low-dose cyclophosphamide treatment on antitumor immune responses. Cancer Res 66(2):960–969
Dix AR, Brooks WH, Roszman TL, Morford LA (1999) Immune defects observed in patients with primary malignant brain tumors. J Neuroimmunol 100(1–2):216–232
Ghiringhelli F, Ménard C, Terme M, Flament C, Taieb J, Chaput N, Puig PE, Novault S, Escudier B, Vivier E, Lecesne A, Robert C, Blay JY, Bernard J, Caillat-Zucman S, Freitas A, Tursz T, Wagner-Ballon O, Capron C, Vainchencker W, Martin F, Zitvogel L (2005) CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med 202(8):1075–1085
Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W (2004) Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 10(9):942–949
Sampson JH, Aldape KD, Gilbert MR, Hassenbusch SJ, Sawaya R, Schmittling B, Archer GE, Mitchell D, Bigner DD, Reardon DA, Heimberger AB (2007) Temozolomide as a vaccine adjuvant in GBM. J Clin Oncol, 2007 In: Proceedings of the ASCO annual meeting, part I, vol 25, No. 18S (June 20 supplement): 2020
Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, Camphausen K, Luiten RM, de Ru AH, Neijssen J, Griekspoor A, Mesman E, Verreck FA, Spits H, Schlom J, van Veelen P, Neefjes JJ (2006) Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 203(5):1259–1271
Zitvogel L, Apetoh L, Ghiringhelli F, André F, Tesniere A, Kroemer G (2008) The anticancer immune response: indispensable for therapeutic success? J Clin Invest 118(6):1991–2001
Acknowledgments
This work was supported by grants from the Agence Nationale de la Recherche (ANR–06-RIB-005-02) and from the Association Oligocyte.
Conflict of interest statement
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Banissi, C., Ghiringhelli, F., Chen, L. et al. Treg depletion with a low-dose metronomic temozolomide regimen in a rat glioma model. Cancer Immunol Immunother 58, 1627–1634 (2009). https://doi.org/10.1007/s00262-009-0671-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-009-0671-1