Abstract
Despite resection, radiochemotherapy, and maintenance temozolomide chemotherapy (TMZm), the prognosis of patients with glioblastoma multiforme (GBM) remains poor. We integrated immunotherapy in the primary standard treatment for eight pilot adult patients (median age 50 years) with GBM, to assess clinical and immunological feasibility and toxicity in preparation of a phase I/II protocol HGG-2006. After maximum, safe resection, leukapheresis was performed before radiochemotherapy, and four weekly vaccinations with autologous GBM lysate-loaded monocyte-derived dendritic cells were given after radiochemotherapy. Boost vaccines with lysates were given during TMZm. During the course of vaccination, immunophenotyping showed a relative increase in CD8+CD25+ cells in six of the seven patients, complying with the prerequisites for implementation of immunotherapy in addition to postoperative radiochemotherapy. In five patients, a more than twofold increase in tumor antigen-reacting IFN-γ-producing T cells on Elispot was seen at the fourth vaccination compared with before vaccination. In three of these five patients this more than twofold increase persisted after three cycles of TMZm. Quality of life during vaccination remained excellent. Progression-free survival at six months was 75%. Median overall survival for all patients was 24 months (range: 13–44 months). The only serious adverse event was an ischemic stroke eight months postoperatively. We conclude that tumor vaccination, fully integrated within the standard primary postoperative treatment for patients with newly diagnosed GBM, is feasible and well tolerated. The survival data were used to power a currently running phase I/II trial.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite full, state-of-the-art oncological therapy, including maximum safe surgical resection, external beam radiotherapy, and chemotherapy, the prognosis of glioblastoma multiforme (GBM) remains poor with a median survival of 14 months [1]. Even after this maximum treatment, relapse is universal. At the time of relapse, the prognosis is even worse and virtually all patients are dead within 18 months after relapse [2–5]. There is a clear need for well-tolerated long-term treatments that are tumor-specific and able to kill not only all (residual) tumor cells that infiltrate the adjacent areas of the brain [6], but also the recently described radio and chemoresistant CD133+ glioma cancer stem cells [7–9]. The immune system provides us with some promising tools in that regard, and further development of immune therapy and its integration in the current therapeutic concepts is warranted.
Brain tumors are located in the so-called immune distinct environment of the central nervous system and malignant gliomas display local and systemic immune suppressive characteristics [10–13]. Nevertheless, proof of the principle of dendritic cell (DC)-based vaccination strategies against high-grade glioma (HGG) has been documented by our group and several others both in vitro [14–16] and in animal models [17–20]. Early clinical data confirm the safety, feasibility and possible beneficial effects of DC vaccination in patients with this fatal disease (recently reviewed by De Vleeschouwer et al. [21] and Van Gool et al. [22]).
Considering the promising results of surgery and adjuvant autologous DC-based tumor vaccination in a large group of patients with relapsed malignant glioma [23], the next step in the clinical development of DC-based immunotherapy for HGG is to fully integrate immunotherapy within the standard first-line treatment, considering putative mutual beneficial effects of the combination of immunotherapeutic strategies with conventional therapeutic modalities such as radiotherapy and chemotherapy [24–30]. In these reports, immunotherapy was added to either radio or chemotherapy but was never fully integrated in a comprehensive schedule of radio–chemo–immunotherapy, as presented here. Following an initial pilot phase, a phase I/II clinical trial has to demonstrate feasibility, toxicity, and efficacy of such a multimodal treatment approach. As final step in the clinical development, a prospective double-blind randomized clinical trial is needed to demonstrate possible beneficial activity of immunotherapy integrated in the primary multimodal treatment for patients with HGG.
We summarize our experience in a pilot group of eight patients with newly diagnosed GBM treated with immunotherapy (autologous DC loaded with autologous tumor cell lysate) integrated in a standardized fashion in the multimodal standard therapy—surgical resection, radiochemotherapy (RCT), and maintenance temozolomide (TMZm) chemotherapy [1]. The emphasis of this report is on the clinical and immunological feasibility and toxicity of integration of DC vaccination in the conventional therapeutic modalities. The progression free survival data were used to power the currently running phase I/II trial (HGG-2006, EudraCT 2006-003881-20), integrating DC vaccination as an add-on therapy to standard postoperative RCT and maintenance chemotherapy in patients with newly diagnosed GBM.
Materials and methods
Patient population
Eight patients (five males and three females) presented with newly diagnosed primary GBM, confirmed on central review histopathology. Patients were included for DC therapy, if they met the inclusion criteria as summarized in Table 1. Patients’ characteristics are described in Table 2. Their median age was 50 years (range 31–62 years). All patients were operated upon and were off steroids and nonsteroidal anti-inflammatory drugs at the time of leukapheresis (as determined by the exclusion criteria) and during vaccination. Approval by the local ethics committee was obtained, and patients’ written informed consent was obtained before the start of immunotherapy.
Assessment of the extent of tumor resection before vaccination
Total resection was defined by the neurosurgical report and the absence of any residual tumor mass on early postoperative MRI (T1 weighted spin-echo images before and after gadolinium enhancement) performed within 72 h after surgery. Any resection leaving a measurable contrast-enhancing tumoral mass less than 2 cm3 was considered subtotal. All solid residual tumor of a measurable size ≥2 cm3 was classified as partial resection.
Tumor cell lysate
Tumor tissue was transported from the operation room into the laboratory and snap-frozen at −80°C without additives. The tissue was kept frozen at −80°C. For further preparation, the tissue was thawed and put into NaCl 0.9% with 1% human serum albumin (HSA), and was homogenized mechanically. Afterwards, six snap freeze–thaw cycles between liquid N2 and 56°C were performed. The lysate was filtered with a 70 μm Falcon filter (BD Biosciences Europe, Erembodegem, Belgium). The amount of protein was measured using the Coomassie blue staining method and spectrophotometry at 595 nm [31]. After irradiation (60 Gy), the lysate was kept frozen in liquid N2 until use.
Preparation of autologous DC vaccines
In all patients peripheral blood mononuclear cells (PBMC) were obtained from a single leukapheresis and kept frozen in liquid N2 until use. For each vaccination, part of the PBMC was thawed, and adherent cells were differentiated to immature DC as described elsewhere [32]. Immature DC were loaded with 200 μg tumor proteins per million DC as described elsewhere [14]. For the loading procedure, 0.01% autologous plasma was used during the first 2 h, 0.1% for the next 4 h, and finally 1% for the last 20 h. At time of loading, rTNF-α (Strathmann Biotec, Dengelsberg, Germany), rIL-1β (Strathmann Biotec) and PGE2 (Prostin®; Pfizer, Brussels, Belgium) were added at final concentrations of 120, 120 and 20 ng/ml, respectively. After 24 h, early mature loaded DC were resuspended in PBS with 0.5% HSA at a concentration of 2–6 × 106/ml. Tuberculin syringes were filled with the loaded DC in suspension (400 μl), containing 1–2 million mature DC per syringe. The phenotype of the cells (Table 3) was determined by FACS, using FITC-labeled and PE-labeled mAb purchased from BD Biosciences Pharmingen (San Jose, USA).
Treatment schedule: fully integrated radio–chemo–immunotherapy
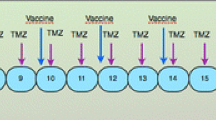
Patients underwent maximum safe surgical resection of the tumor. Peri-operative corticosteroids were withdrawn within one week after resection. Leukapheresis was performed after histological diagnosis was obtained and inclusion criteria were met (Fig. 1). After leukapheresis, patients were treated with limited field external beam radiotherapy (30 × 2 Gy) and concomitant chemotherapy with TMZ (75 mg/m2) during six weeks as outlined by Stupp et al. [1]. After radiochemotherapy, tumor lysate-loaded DC were injected weekly for four weeks. Following these four weeks, TMZm chemotherapy was started. The maintenance 28-day cycles consisted of five days oral intake of TMZ (150 mg/m2 for the first and 200 mg/m2 for the following cycles). During the first, second, third, and sixth cycles, further boost vaccinations with tumor lysate (without DC) were administered at day 8 of the cycle. At the time of progression possible rescue therapy was at the physician’s discretion.
Treatment schedule. DC-based immunotherapy was integrated in the state-of-the art postoperative radiochemotherapy. Leukapheresis to harvest autologous monocytes is performed once, at least seven days after weaning off steroids and immediately before the start of the concomitant radiochemotherapy. After the radiochemotherapy, but before the maintenance chemotherapy with TMZ, four weekly induction vaccines are administered intradermally to the patient. Afterwards, maintenance chemotherapy (5/28 days) is started and one week after the start of the 1st, 2nd, 3rd, and 6th cycles of TMZ, a boost vaccine is administered
Vaccination
Vaccination was performed by intradermal (i.d.) injection of 1–12 million (median 4.1 × 106) DC per lymph node region in the upper third of the arms (left and right) at weeks 1, 2, 3, and 4 (induction vaccines). Further vaccinations were given with tumor lysate with a median of 1,500 μg (range 1,500–4,000 μg) proteins per vaccine injected in two syringes each containing a final volume of 400 μl (boost vaccines).
Immune monitoring
Immune monitoring was performed at three different levels. First, delayed type hypersensitivity (DTH) reaction was tested at the first and fourth vaccinations. For this, 100 μl crude tumor lysate and 100 μl control PBS/HSA were injected intradermally. After 48 and 72 h redness and induration were assessed. DTH reactions were judged as positive if the average perpendicular measurement of the induration exceeded 5 mm.
Blood samples were obtained at times of leukapheresis, vaccine 1, vaccine 4, and vaccine 7. In each whole blood sample, the phenotype of circulating T cell populations was determined by FACS: total CD3+ population, the CD4+ and CD8+ subpopulations, and the activation markers HLA-DR on CD3+ cells and CD25 on both subpopulations. For this, FITC-labeled and PE-labeled mAb were purchased from BD Biosciences Pharmingen.
Finally, PBMC from each blood sample were cryopreserved and thawed together at the end of the immunotherapy for use in an Elispot assay. A positive response was defined as an at least twofold increase in the number of antigen-specific spots after the fourth and seventh vaccinations, as described by Banchereau et al. [33].
The protocol was adapted, based on the manufacturers’ instructions (Mabtech, Nacka Strand, Sweden). In brief, 96-well polyvinylidene difluoride membrane plates (MAIPSWU10; Millipore, Bedford, MA, USA) were treated with 70% ethanol (50 μl per well) for 1 min and washed with PBS before coating. Next, plates were coated overnight (4°C) with coating antibody (1-D1K, 15 μg/ml, Mabtech). After blocking, 2 × 105 viable PBMC per well were seeded in the presence of PHA (1 μg/ml), serum-free medium (CTL-testTM, Cellular Technology, Aalen, Germany) or autologous tumor lysate or protein extracted by ethanol precipitation and incubated for 24 h (37°C, 5% CO2) in a final volume of 100 μl. Cells were washed away and detection antibody (7-B6-1-biotin, 1 μg/ml, Mabtech) was added (overnight, 4°C). Streptavidine-ALP (Mabtech) was added for 1 h after which substrate solution was added (AP conjugate substrate kit, Bio-Rad, Nazareth Eke, Belgium). Spots were counted with Immunoscan and Immunospot software (CTL). Antigen-specific spots were calculated after subtracting the background spots of unstimulated PBMC. All measurements were obtained in triplicate.
Patient assessment
All patients were followed by clinical examination and MRI scanning (12 weeks after surgery and then every 3 months). Upon specific indication methionine PET was performed. At the time of each vaccination, quality of life (QOL) was assessed using the QLQ-C30 and the Fertigkeitenskala Münster-Heidelberg (FMH) [34–36]. Karnofsky performance scale (KPS) was assessed by the patients and registered at each visit [37].
The QLQ-C30 is a 30-item questionnaire composed of multi-item scales and single items. It is designed by the European Organization for Research and Treatment of Cancer (EORTC) for use in international clinical trials in oncology. It is used to assess functional disability, somatic symptoms, global health, and overall quality of life. In this study, only the indices for general health and overall quality of life (single items 29 and 30) were used. The rating scale ranges from 1 (very bad) to 7 (excellent).
The FMH is a 56-item questionnaire used to assess the patients’ ability to carry out daily life activities. In this study, a 53-item version of the instrument was used and a total score ranging from 0 to 53 was calculated.
The KPS was used for assessment of the patients’ level of physical ability; it consists of one rating on a scale from 0 (dead) to 100 (normal functioning).
Results
Feasibility: preparation and characterization of vaccines
The patients received a median of 10 vaccines (range 6–16 vaccines). The details of the vaccination for each patient are described in Table 4. Patients #4 and #8 underwent a re-operation at time of relapse with postoperative inclusion in the HGG-IMMUNO-2003 cohort comparison trial (adjuvant DC-based immunotherapy in patients with relapsed GBM). The median yield of loaded mature DC from freshly isolated PBMC was 8.2 × 106 per vaccination session (range 2–24 × 106; n = 30). Two vaccinations (both in patient #2) could not be administered because the release criteria of the cellular product were not fulfilled.
Feasibility: immune response
An increase in the proportion of CD8+CD25+ T cells was noted during the course of vaccination in six of the seven patients (Table 5). Two of those patients (#3 and #7) showed a positive skin test, and an increase in IFN-γ-producing tumor antigen-reacting T-cells was observed in five patients (#1, #3, #5, #6 and #7).
In six patients a DTH skin test with autologous tumor homogenate was performed at the time of the first and fourth vaccinations (Table 5). In patient #2 the skin test was only performed at the first vaccination and in patient #5 there was not enough tumor material available for the skin test (all tumor lysate was used for vaccination). Two patients (#3 and #7) had a positive reaction within 72 h after i.d. injection at the first and fourth vaccinations. In these patients the control i.d. injections with PBS/HSA were negative. Patient #3 was progression free at the end of the follow-up (FU) period. Patient #7 had a recurrence of the primary tumor at 17 months FU. In patient #8, a delayed positive reaction at multiple injection sites occurred 6 months after the first vaccination. This patient had a recurrence of the primary tumor at 26 months FU.
The results of the Elispot assay (n = 8) showed an increase in IFN-γ-producing tumor antigen-reacting T-cells between the first and fourth vaccinations in five of the eight patients (Table 5; Fig. 2). In three of these five patients (#1, #3 and #5) a more than twofold increase in tumor-specific IFN-γ production persisted after three cycles of TMZm (Table 5; Fig. 2). Two of the five patients with an increase in tumor specific IFN-γ production at the fourth vaccination, had positive skin tests (#3 and #7).
Assessment of the number of specific tumor-antigen reacting T cells using Elispot. The number of IFN-γ producing tumor antigen-reacting T cells is depicted during the vaccination schedule in six of the eight patients. Two patients (#2 and #8) had more spots in the control condition than in the experimental condition at all time points (results not shown). In five patients an increase in tumor-specific IFN-γ production can be seen between the first and fourth vaccinations. In three of them a more than twofold increase in tumor-specific IFN-γ production persisted after three cycles of TMZm (vaccine 7). The fold increase was calculated by use of the formula: ((SFC at time x) − (SFC in control condition at time x))/((SFC before treatment) − (SFC in control condition before treatment))
Toxicity
The clinical data during vaccination are listed in Table 6, including adverse events graded according to the National Cancer Institute (NCI) common toxicity criteria (CTC).
In patient #7 lymphopenia (650/mm3) was diagnosed during TMZm chemotherapy, which did not require cessation or delay of treatment. In patient #2 a focal epileptic insult occurred between the second and third vaccinations. Three patients (#2, #4 and #8) experienced (transient) dysphasia postoperatively and in patient #8 there was a transient recurrence of the dysphasia after the second cycle of adjuvant TMZ chemotherapy. Four patients (#2, #6, #7 and #8) complained of fatigue during the treatment, general malaise and myalgia were each mentioned by one patient (#3 and #6, respectively).
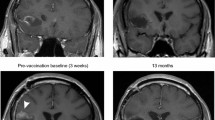
In patient #5, a sudden onset right hemiplegia and aphasia occurred at 8 months FU (after the third boost with tumor lysate). MRI showed hyperintensities in T2 weighted images and heterogenic signals in T1 weighted images at the posteromedial side of the left temporal lobe, near the site of tumor resection (Fig. 3a, b). These MRI data were most compatible with ischemic changes and the episode of hemiplegia and aphasia was diagnosed as an ischemic event. Inflammation, in terms of auto-immunity, could not be ruled out as no biopsy was performed. A repeat MRI three months after the described event showed the same changes at the posteromedial side of the left temporal lobe without any evolution (Fig. 3c–e). We concluded that these changes were most likely to be ischemic, although inflammation or post-radiotherapy sequellae could not be ruled out pathologically in this young patient; the clinical evolution was favorable without administration of steroids. In the same patient the TMZm chemotherapy was stopped because of hematological toxicity (grade 3) and at one year FU this same patient had a status epilepticus which was controlled with fenytoin. Tumor status at that time remained unchanged. No other serious adverse events have been reported.
Patient #5: MRI images at eight and eleven months follow-up (FU). Hyperintensities in T2 weighted images (a) and heterogenic signals in T1 weighted images (b) at the posteromedial side of the left temporal lobe, near the site of tumor resection, one day after sudden onset of right hemiplegia and aphasia (at eight months FU). A repeat MRI three months after the described event shows the same changes at the posteromedial side of the left temporal lobe without any evolution; c T2 weighted images; d T1 weighted images; e T1 weighted images with gadolinium
Quality of life
The results of the QOL and disability assessments revealed that the patient-assessed KPS during the course of vaccination was equal to or exceeded 70 for six patients (median 85; range 70–100) (reflecting a physical condition that enables the patient to care for him/herself). Patients #2 and #5 had KPS scores that varied between 50 and 60 (median 60). Moreover, for all patients the KPS remained stable or even tended to increase throughout the treatment phases.
The FMH score also remained quite high during the course of vaccination: it exceeded 45 (median 52; range 46–53) for all but one patient (#5), indicating that the patients were capable of most daily life activities and did not lose functional ability throughout the treatment phases. In patient #5 median FMH score was 36 (range 30–37).
Six out of seven patients evaluated their general health (item 29) and overall QOL (item 30) on the EORTC QLQ-C30 questionnaire as good (median 5 for both items; range 3–7 for both items) and these evaluations remained stable throughout the treatment phases. Only patient #2 had lower evaluations for general health (median 3; range 2–4) and overall QOL (median 3; range 3–4) during the course of vaccination.
Clinical assessment
The details of the clinical results are given in Table 6. Three patients (37.5%) are still alive at last FU, with median FU of 35 months (34–44 months). The median overall survival (OS) for all patients is 24 months (range 13–44 months). Two patients underwent a total resection of the tumor, in six patients the resection was subtotal. One patient is still free from progression or recurrence at the end of the FU period at 34 months. The median progression-free survival (PFS) in all patients is 18 months (range 2–34 months). PFS at 6 months using this therapeutic regimen was demonstrated in six out of eight patients (75%). In Fig. 4a and b, Kaplan–Meier curves are shown for PFS and OS, respectively.
Progression-free survival (PFS) and overall survival (OS). Kaplan–Meier curves are shown for PFS (a) and OS (b). The median OS for all patients is 24 months (range 13–44 months). The median PFS in all patients is 18 months (range 2–32 months). PFS at 6 months using this therapeutic regimen was demonstrated in six of the eight patients (75%)
Discussion
We have summarized our observations in eight pilot patients with newly diagnosed GBM in whom vaccination with autologous DC loaded with autologous tumor cell lysate was fully integrated in the multimodal standard primary treatment, consisting of maximum safe neurosurgical resection, external beam radiotherapy with concomitant TMZ chemotherapy, and maintenance TMZ chemotherapy [1]. As such, this is the first report of a standardized radio–chemo–immunotherapy approach for patients with newly diagnosed GBM.
As shown in Table 4, DC characteristics and numbers varied substantially amongst patients. As we reported earlier, this is inherent in the nature of autologous cell therapy in an oncological patient population with highly variable baseline characteristics. Nevertheless, Liau et al. [38] did not find any argument for dose-related toxicity or efficacy in DC-based vaccination therapy. It is important to note that there were no serious adverse events (NCI CTC grade 4) except for one patient in whom an ischemic event occurred during the vaccination therapy. This patient made a partial recovery and the extent to which the ischemic event is related to the vaccination therapy remains a point of debate. Important, though, is the fact that post-radiotherapy sequellae or inflammation could not be ruled out completely as no biopsy was performed. At one-year FU the same patient had a status epilepticus which was controlled with fenytoin. Again, the relationship of this event to the vaccination therapy is unclear and the status epilepticus may be linked to the possible ischemic event that the patient suffered earlier. A favorable course of the patient’s clinical symptoms was demonstrated even without the use of steroids, which makes an auto-immune reaction highly improbable. With regard to quality of life and disability assessment during treatment, one can conclude that six patients reported a physical condition that enabled them to take care for themselves and to perform daily life activities during treatment. Also general health and overall quality of life were rated moderate to good by these six patients.
The proof of the principle for DC-based immunotherapy for HGG has been demonstrated in in-vitro experiments [14–16], and in several rodent models [17–20], and justification for clinical use of DC-based immunotherapy for malignant gliomas has recently been reviewed [21, 22]. Several Phase I/II trials or case reports have been published in which patients with malignant glioma have been treated using slightly different variants of DC vaccination [23, 32, 38–50]. Safety, feasibility, and clinical and immunological bioactivity have been demonstrated in these publications. In none of the preclinical in-vivo models nor in patients treated thus far have auto-immune reactions been observed.
In a previous report, we already stressed the importance of minimum residual disease status after surgery before starting the adjuvant DC vaccinations for safety and efficacy reasons [32, 39]. Furthermore, a recently published manuscript from our group describes very promising long-term survival of patients with relapsed HGG treated with postoperative adjuvant DC vaccination [23].
The integration of immunotherapy within the standard postoperative therapy for patients with a newly diagnosed malignant glioma is based on the presumed mutually beneficial effect of the conventional treatment strategies and immunotherapy. Each aspect of the presented concept is believed to play a major role in the global results of this approach. First, maximum safe surgery is performed to induce a state of minimum residual disease as a starting point for subsequent therapy. Not only has it been shown that the extent of resection has a major effect on the benefit of postoperative radiochemotherapy in GBM [51], but the extent of resection is a strong, independent predictor of outcome for patients with relapsed malignant glioma treated with postoperative, adjuvant DC vaccination [23]. Many antitumoral strategies, for example radiotherapy kill tumor cells by apoptosis and the resulting apoptotic bodies form a good source of cross-presented antigens, which might further lead to cross-priming of T cells in an appropriate pro-inflammatory environment. In 1984 North et al. [27] reported elimination of local regulatory T cells (avant la lettre) in irradiated brain tumor areas. Recently, Kjaergaard et al. [25] showed that long-term survivors in a murine brain tumor model occurred only in the group of animals receiving both radiotherapy and DC-based vaccines. The data showed irradiation-induced upregulation of MHC molecules in tumor cells, thereby making them better targets for CTL.
Chemotherapy with TMZ during radiotherapy might be of benefit for subsequent immunotherapy. First, chemotherapy affects the size of the lymphocyte pool slightly, thereby enabling thymic-independent antigen-driven T cell regeneration within the context of T cell homeostasis [24, 29]. In this pilot group of patients, the lymphocyte counts were only moderately reduced. The concept of tumor-specific immunization at the time of immune reconstitution after chemotherapy has been demonstrated in several animal models [24, 29]. Specifically of importance to a regimen with six weeks TMZ, it has been shown that TMZ affects the attraction of tumor-specific regulatory T cells (Treg) into the tumor cavity by blocking CCL2 production by the tumor cells [52]. Although a relative enrichment of Treg in peripheral blood has been described at the end of radiochemotherapy, while the total CD4+ T cell population is diminished [53], one might assume that the pro-inflammatory condition induced by radiotherapy [54] counteracts Treg functionality in the periphery [55, 56].
Immunotherapy itself can increase the sensitivity of GBM tumor cells to chemotherapeutics such as TMZ. Wheeler et al. [30] has already suggested an increased susceptibility of GBM to chemotherapy in patients pre-exposed to DC-based vaccination. Their group even published a presumed molecular mechanism of this synergy based on preferential targeting of tyrosin-related protein 2 (TRP2), a chemoresistance mechanism, by cytotoxic T cells as an explanation [26]. The idea of combining these treatment modalities is not restricted to neuro-oncology [57, 58]. Recently Masucci et al. [59] showed an advantage of combining TMZ with restorative immunotherapy using IL-2 in melanoma patients.
Overall, it is hypothesized that the combination of radiotherapy, chemotherapy, and immunotherapy could potentiate the cumulative antitumoral activity when applied in a well designed strategy. Here, we advocate such a strategy of autologous DC-based immunotherapy as add-on therapy, but fully integrated in the multimodality treatment of surgery, radiotherapy, and chemotherapy in patients with HGG. For this radio-chemo-immunotherapy, we provide early feasibility data in terms of clinical and immunological responses and an acceptable QOL.
The use of boost lysates after the first four induction vaccinations with loaded DC, is based on the data from Jouanneau et al. [60]. They showed that multiple vaccinations with DC efficiently induced an immune response in an orthotopic mouse model, but did not elicit optimum long-term survival. In contrast, injection of DC for priming, followed by boosts with tumor lysate alone generated the most effective anti-tumor effects (CTL and humoral responses). Also, we have shown in relapsed GBM patients that vaccination with boost lysates can result in substantial numbers of long-term survivors after relapse [23].
Immune monitoring was performed with skin tests, provided that enough tumor material was available, Elispot and general immunophenotyping of circulating T cells by FACS analysis. Immune monitoring was used to assess the induction and/or maintenance of vaccine-induced anti-tumoral immunity in the described close temporal relationship with radiochemotherapy. Testing the DTH reaction to the antigen is one monitoring tool to indicate cellular immunity, although it remains controversial whether or not DTH to the autologous tumor can be a reliable correlate of clinical responses. In this small series, we could not find any positive correlation between immune reactivity and clinical outcome. In two of the six patients tested there was a positive skin test at the first and fourth vaccinations, suggesting that radiochemotherapy did not compromise the patients’ specific immunity. One patient had a delayed positive reaction at the injection sites, six months after the first vaccination. The results of the Elispot showed a vaccine-induced increase in IFN-γ-producing tumor antigen-reacting T-cells in five of the eight patients. This points to the induction of a tumor-antigen-directed immune response. In three of these five patients a more than twofold increase in tumor specific IFN-γ production persisted after three cycles of TMZm. These data further support the notion that radiochemotherapy does not interfere negatively with immunotherapy and that these treatments can be combined without losing the therapeutic effect of either. An increase in the proportion of CD8+CD25+ cells within the CD8+ population was noted during the course of vaccination in six of the seven patients. This might suggest a re-expansion of activated CD8+ cytotoxic T cells, which is a prerequisite for implementing tumor vaccination. However, further functional studies should be performed on these cells. To which degree the different immune monitoring tests correlate with clinical responses remains a point of debate, and there was no intention to assess this for this pilot group of patients.
The discordance between clinical and immunological data is a known problem for this type of treatment and is illustrated here by the fact that the two patients with the longest survival (#4 and #8) are the only ones not showing increased IFN-γ production by Elispot. Both patients had had complete resections, in contrast with the other patients. In patient #4 an increase in CTL was seen; the skin test, however, was negative. In contrast, the skin test for patient #8 was delayed positive, but in this patient there was no increase in CTL. This shows the inherent shortcomings of immune monitoring for these types of treatment. Therefore, use of surrogate immunological endpoints as main data on which to build a treatment strategy does not seem to be ideal. The full nature of the estimated beneficial effects of DC vaccination is without any doubt much more complex than any immune monitoring tool at this stage can fully capture.
Three patients (37.5%) are still alive at last FU, with median FU of 35 months (34–44 months). The median OS for all patients is 24 months (range 13–44 months). Considering the fact that seven patients were in RPA class IV and 1 patient in RPA class III, these preliminary data compare favorably with RPA class-related survival estimates and RPA class-adjusted outcome in the EORTC26981/22981-NCIC CE3 trial [61, 62]. MGMT promoter methylation was not assessed in these patients, because it was not an inclusion or exclusion criterion. This warrants an even more cautious interpretation of these survival data.
Tumor vaccination integrated within the multimodal standard therapy, consisting of surgical resection, radiochemotherapy, and maintenance TMZ chemotherapy for patients with newly diagnosed malignant glioma is feasible both clinically and immunologically, is well tolerated, and is possibly beneficial for patients with minimum residual tumor burden. Our findings further underscore the importance of a proper assessment of the potentials of adjuvant DC vaccination in patients with HGG as such and as a fourth oncological treatment modality, integrated in the state-of-the-art conventional therapy for these patients in particular. To further investigate the potential of tumor vaccination as add-on therapy to postoperative radiochemotherapy for newly diagnosed malignant glioma, the PFS rate at 6 months was used to power a currently ongoing phase II clinical trial (HGG-2006). This trial might finally lead to a prospective double-blind randomized clinical trial with add-on vaccination as experimental arm, compared with standard primary treatment.
References
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoom MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Eng J Med 352:987–996
Brada M, Hoang-Xuan K, Rampling R, Dietrich PY, Dirix LY, Macdonald D, Heimans JJ, Zonnenberg BA, Bravo-Marques JM, Henriksson R et al (2001) Multicenter phase II trial of temozolomide in patients with glioblastoma multiforme at first relapse. Ann Oncol 12:259–266
Finlay JL, Boyett JM, Yates AJ, Wisoff JH, Milstein JM, Geyer JR, Bertolone SJ, McGuire P, Cherlow JM, Tefft M et al (1995) Randomized phase III trial in childhood high-grade astrocytoma comparing vincristine, lomustine, and prednisone with the eight-drugs-in-1-day regimen. Children’s Cancer Group. J Clin Oncol 13:112–123
Nieder C, Grosu AL, Molls M (2000) A comparison of treatment results for recurrent malignant gliomas. Cancer Treat Rev 26:397–409
Tamber MS, Rutka JT (2003) Pediatric supratentorial high-grade gliomas. Neurosurg Focus 14. http://www.medscape.com/viewarticle/449870
Kleihues P, Soylemezoglu F, Schauble B, Scheithauer BW, Burger PC (1995) Histopathology, classification, and grading of gliomas. Glia 15:211–221
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN (2006) Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444:756–760
Eramo A, Ricci-Vitiani L, Zeuner A, Pallini R, Lotti F, Sette G, Pilozzi E, Larocca LM, Peschle C, De Maria R (2006) Chemotherapy resistance of glioblastoma stem cells. Cell Death Differ 13:1238–1241
Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, Lu L, Irvin D, Black KL, Yu JS (2006) Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer 5:67
Weller RO, Engelhardt B, Phillips MJ (1996) Lymphocyte targeting of the central nervous system: a review of afferent and efferent CNS-immune pathways. Brain Pathol 6:275–288
Bodey B, Bodey B Jr, Siegel SE, Kaiser HE (2000) Failure of cancer vaccines: the significant limitations of this approach to immunotherapy. Anticancer Res 20:2665–2676
Pawelec G, Engel A, Adibzadeh M (1999) Prerequisites for the immunotherapy of cancer. Cancer Immunol Immunother 48:214–217
Roszman T, Elliott L, Brooks W (1991) Modulation of T-cell function by gliomas. Immunol Today 12:370–374
De Vleeschouwer S, Arredouani M, Ade M, Cadot P, Vermassen E, Ceuppens JL, Van Gool SW (2005) Uptake and presentation of malignant glioma tumor cell lysates by monocyte-derived dendritic cells. Cancer Immunol Immunother 54:372–382
Yoshida S, Morii K, Watanabe M, Saito T, Yamamoto K, Tanaka R (2001) The generation of anti-tumoral cells using dendritic cells from the peripheral blood of patients with malignant brain tumors. Cancer Immunol Immunother 50:321–327
De Vleeschouwer S, Spencer LI, Ceuppens JL, Van Gool SW (2007) Persistent IL-10 production is required for glioma growth suppressive activity by Th1-directed effector cells after stimulation with tumor lysate-loaded dendritic cells. J Neurooncol 84:131–140
Okada H, Tahara H, Shurin MR, Attanucci J, Giezeman-Smits KM, Fellows WK, Lotze MT, Chambers WH, Bozik ME (1998) Bone marrow-derived dendritic cells pulsed with a tumor-specific peptide elicit effective anti-tumor immunity against intracranial neoplasms. Int J Cancer 78:196–201
Siesjo P, Visse E, Sjogren HO (1996) Cure of established, intracerebral rat gliomas induced by therapeutic immunizations with tumor cells and purified APC or adjuvant IFN-gamma treatment. J Immunother Emphas Tumor Immunol 19:334–345
Maes W, Rosas GG, Verbinnen B, Boon L, De Vleeschouwer S, Ceuppens JL, Van Gool SW (2009) DC vaccination with anti-CD25 treatment leads to long-term immunity against experimental glioma. Neuro Oncol 11:529–542
Maes W, Deroose C, Reumers V, Krylyshkina O, Gijsbers R, Baekelandt V, Ceuppens J, Debyser Z, Van Gool SW (2009) In vivo bioluminescence imaging in an experimental mouse model for dendritic cell based immunotherapy against malignant glioma. J Neurooncol 91:127–139
De Vleeschouwer S, Rapp M, Sorg RV, Steiger HJ, Stummer W, Van Gool S, Sabel M (2006) Dendritic cell vaccination in patients with malignant gliomas: current status and future directions. Neurosurgery 59:988–999
Van Gool S, Maes W, Ardon H, Verschuere T, Van Cauter S, De Vleeschouwer S (2009) Dendritic cell therapy of high-grade gliomas. Brain Pathol 19:694–712
De Vleeschouwer S, Fieuws S, Rutkowski S, Van Calenbergh F, Van Loon J, Goffin J, Sciot R, Wilms G, Demaerel P, Warmuth-Metz M et al (2008) Postoperative adjuvant dendritic cell-based immunotherapy in patients with relapsed glioblastoma multiforme. Clin Cancer Res 14:3098–3104
Jameson SC (2002) Maintaining the norm: T-cell homeostasis. Nat Rev Immunol 2:547–556
Kjaergaard J, Wang LX, Kuriyama H, Shu S, Plautz GE (2005) Active immunotherapy for advanced intracranial murine tumors by using dendritic cell-tumor cell fusion vaccines. J Neurosurg 103:156–164
Liu G, Akasaki Y, Khong HT, Wheeler CJ, Das A, Black KL, Yu JS (2005) Cytotoxic T cell targeting of TRP-2 sensitizes human malignant glioma to chemotherapy. Oncogene 24:5226–5234
North RJ (1984) Gamma-irradiation facilitates the expression of adoptive immunity against established tumors by eliminating suppressor T cells. Cancer Immunol Immunother 16:175–181
North RJ (1986) Radiation-induced, immunologically mediated regression of an established tumor as an example of successful therapeutic immunomanipulation. Preferential elimination of suppressor T cells allows sustained production of effector T cells. J Exp Med 164:1652–1666
Porter DL, June CH (2005) T-cell reconstitution and expansion after hematopoietic stem cell transplantation: ‘T’ it up!. Bone Marrow Transplant 35:935–942
Wheeler CJ, Das A, Liu G, Yu JS, Black KL (2004) Clinical responsiveness of glioblastoma multiforme to chemotherapy after vaccination. Clin Cancer Res 10:5316–5326
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Rutkowski S, De Vleeschouwer S, Kaempgen E, Wolff JEA, Kuhl J, Demaerel P, Warmuth-Metz M, Flamen P, Van Calenbergh F, Plets C et al (2004) Surgery and adjuvant dendritic cell-based tumour vaccination for patients with relapsed malignant glioma, a feasibility study. Br J Cancer 91:1656–1662
Banchereau J, Palucka AK, Dhodapkar M, Burkeholder S, Taquet N, Rolland A, Taquet S, Coquery S, Wittkowski KM, Bhardwaj N et al (2001) Immune and clinical responses in patients with metastatic melanoma to CD34(+) progenitor-derived dendritic cell vaccine. Cancer Res 61:6451–6458
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC et al (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365–376
Groenvold M, Klee MC, Sprangers MA, Aaronson NK (1997) Validation of the EORTC QLQ-C30 quality of life questionnaire through combined qualitative and quantitative assessment of patient-observer agreement. J Clin Epidemiol 50:441–450
Wolff JE, Daumling E, Dirksen A, Dabrock A, Hartmann M, Jurgens H (1996) [Munster Heidelberg Abilities Scale—a measuring instrument for global comparison of illness sequelae]. Klin Padiatr 208:294–298
Karnofsky DA, Burchenal BJ (1949) The clinical evaluation of chemotherapeutic agents in cancer. In: MacLeod CM (ed) Evaluation of chemotherapeutic agents. Columbia University Press, New York, pp 191–205
Liau LM, Prins RM, Kiertscher SM, Odesa SK, Kremen TJ, Giovannone AJ, Lin JW, Chute DJ, Mischel PS, Cloughesy TF, Roth MD (2005) Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res 11:5515–5525
De Vleeschouwer S, Van Calenbergh F, Demaerel P, Flamen P, Rutkowski S, Kaempgen E, Wolff JEA, Plets C, Sciot R, Van Gool SW (2004) Transient local response and persistent tumor control of recurrent malignant glioma treated with combination therapy including dendritic cell therapy. J Neurosurg Pediatr 100:492–497
Kikuchi T, Akasaki Y, Irie M, Homma S, Abe T, Ohno T (2001) Results of a phase I clinical trial of vaccination of glioma patients with fusions of dendritic and glioma cells. Cancer Immunol Immunother 50:337–344
Kikuchi T, Akasaki Y, Abe T, Fukuda T, Saotome H, Ryan JL, Kufe DW, Ohno T (2004) Vaccination of glioma patients with fusions of dendritic and glioma cells and recombinant human interleukin 12. J Immunother 27:452–459
Liau LM, Black KL, Martin NA, Sykes SN, Bronstein JM, Jouben-Steele L, Mischel PS, Belldegrun A, Cloughesy TF (2000) Treatment of a glioblastoma patient by vaccination with autologous dendritic cells pulsed with allogeneic major histocompatibility complex class I-matched tumor peptides: case report. Neurosurg Focus 9:e8
Wheeler CJ, Black KL, Liu G, Ying H, Yu JS, Zhang W, Lee PK (2003) Thymic CD8(+) T cell production strongly influences tumor antigen recognition and age-dependent glioma mortality. J Immunol 171:4927–4933
Yamanaka R, Abe T, Yajima N, Tsuchiya N, Homma J, Kobayashi T, Narita M, Takahashi M, Tanaka R (2003) Vaccination of recurrent glioma patients with tumour lysate-pulsed dendritic cells elicits immune responses: results of a clinical phase I/II trial. Br J Cancer 89:1172–1179
Yamanaka R, Homma J, Yajima N, Tsuchiya N, Sano M, Kobayashi T, Yoshida S, Abe T, Narita M, Takahashi M, Tanaka R (2005) Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: results of a clinical phase I/II trial. Clin Cancer Res 11:4160–4167
Yu JS, Wheeler CJ, Zeltzer PM, Ying H, Finger DN, Lee PK, Yong WH, Incardona F, Thompson RC, Riedinger MS et al (2001) Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res 61:842–847
Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ (2004) Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res 64:4973–4979
Okada H, Lieberman FS, Walter KA, Lunsford LD, Kondziolka DS, Bejjani GK, Hamilton RL, Torres-Trejo A, Kalinski P, Cai Q et al (2007) Autologous glioma cell vaccine admixed with interleukin-4 gene transfected fibroblasts in the treatment of patients with malignant gliomas. J Transl Med 5:67
Walker DG, Laherty R, Tomlinson FH, Chuah T, Schmidt C (2008) Results of a phase I dendritic cell vaccine trial for malignant astrocytoma: potential interaction with adjuvant chemotherapy. J Clin Neurosci 15:114–121
Wheeler CJ, Black KL, Liu G, Mazer M, Zhang XX, Pepkowitz S, Goldfinger D, Ng H, Irvin D, Yu JS (2008) Vaccination elicits correlated immune and clinical responses in glioblastoma multiforme patients. Cancer Res 68:5955–5964
Gorlia T, van den Bent MJ, Hegi ME, Mirimanoff RO, Weller M, Cairncross JG, Eisenhauer E, Belanger K, Brandes AA, Allgeier A, Lacombe D, Stupp R (2008) Nomograms for predicting survival of patients with newly diagnosed glioblastoma: prognostic factor analysis of EORTC and NCIC trial 26981-22981/CE.3. Lancet Oncol 9:29–38
Jordan JT, Sun W, Hussain SF, DeAngulo G, Prabhu SS, Heimberger AB (2008) Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Cancer Immunol Immunother 57:123–131
Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE, Herndon JE, Bigner DD, Dranoff G, Sampson JH (2006) Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res 66:3294–3302
Petrini B, Andersson B, Strannegard O, Wasserman J, Blomgren H, Glas U (1992) Monocyte release and plasma levels of interleukin-6 in patients irradiated for cancer. In Vivo 6:531–534
Pasare C, Medzhitov R (2003) Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science 299:1033–1036
Fehervari Z, Sakaguchi S (2004) Control of Foxp3+ CD25+CD4+ regulatory cell activation and function by dendritic cells. Int Immunol 16:1769–1780
Muller AJ, Prendergast GC (2005) Marrying immunotherapy with chemotherapy: why say IDO? Cancer Res 65:8065–8068
Nowak AK, Robinson BW, Lake RA (2003) Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Res 63:4490–4496
Masucci GV, Mansson-Brahme E, Ragnarsson-Olding B, Nilsson B, Wagenius G, Hansson J (2006) Alternating chemo-immunotherapy with temozolomide and low-dose interleukin-2 in patients with metastatic melanoma. Melanoma Res 16:357–363
Jouanneau E, Poujol D, Gulia S, Le Mercier I, Blay JY, Belin MF, Puisieux I (2006) Dendritic cells are essential for priming but inefficient for boosting antitumour immune response in an orthotopic murine glioma model. Cancer Immunol Immunother 55:254–267
Curran WJ Jr, Scott CB, Horton J, Nelson JS, Weinstein AS, Fischbach AJ, Chang CH, Rotman M, Asbell SO, Krisch RE, Nelson DF (1993) Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst 85:704–710
Mirimanoff RO, Gorlia T, Mason W, van den Bent MJ, Kortmann RD, Fisher B, Reni M, Brandes AA, Curschmann J, Villa S et al (2006) Radiotherapy and temozolomide for newly diagnosed glioblastoma: recursive partitioning analysis of the EORTC 26981/22981-NCIC CE3 phase III randomized trial. J Clin Oncol 24:2563–2569
Acknowledgments
We thank Katja Vandenbrande and Goedele Stegen for their excellent technical assistance. We thank the department of hematology for the care provided at time of the leukapheresis. We thank the clinic for radiotherapy for irradiating the tumor lysate preparations. This project is supported by the Olivia Hendrickx Research Fund (www.olivia.be), the Herman Memorial Research Fund (www.hmrf.be), the TBM program of the IWT—Flanders (www.iwt.be), the Belgian Foundation Against Cancer (www.cancer.be), and private initiatives. HSA was provided by the Belgian Red Cross and Baxter. Steven De Vleeschouwer is supported by the “Klinisch onderzoeksfonds” from the University Hospital Leuven. Stefaan Van Gool is Senior Clinical Investigator of the Fund for Scientific Research—Flanders (Belgium) (F.W.O.-Vlaanderen).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ardon, H., Van Gool, S., Lopes, I.S. et al. Integration of autologous dendritic cell-based immunotherapy in the primary treatment for patients with newly diagnosed glioblastoma multiforme: a pilot study. J Neurooncol 99, 261–272 (2010). https://doi.org/10.1007/s11060-010-0131-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-010-0131-y