Abstract
The goal of the current study is to determine the effects of blocking phosphatidylserine (PS) on the growth of neuroblastoma in mice. PS, an anionic phospholipid restricted to the cytoplasmic surface of plasma membranes in most cells, is externalized to the surface of apoptotic cells. PS has been shown to induce immune tolerance to self-antigens. PS can also be found on the surface of live cells and in particular tumor cells. Annexin-V (AnV) is a protein that specifically binds and blocks PS. To determine the effects of blocking PS with AnV on tumor growth and immunogenicity, mice were inoculated with AGN2a, a poorly immunogenic murine neuroblastoma that expresses high level of PS on the cell surface. Survival and anti-tumor T cell response were determined. AGN2a were engineered to secrete AnV. Secreted protein effectively blocked tumor PS. 40 % of mice inoculated with AnV-expressing AGN2a cells survived free of tumor, whereas none of the mice inoculated with control cells survived (p = 0.0062). The benefits of AnV were lost when mice were depleted of T cells. The findings suggest that AnV could protect mice from tumor challenge through an immune mediated mechanism. Mice were then immunized with irradiated AnV-secreting or control cells, and challenged with wild-type AGN2a cells. AnV-secreting cell vaccine protected 80 % of mice from AGN2a challenge, while control cell vaccine prevented tumor growth in only 30 % of animals (p = 0.012). ELISPOT analysis demonstrated that AnV-secreting cell vaccine induced a greater frequency of interferon-gamma producing splenic T cells. T cells isolated from mice immunized with AnV-secreting but not control vaccine lysed AGN2a. In summary, AnV blocked PS, enhanced T cell mediated tumor immunity, and inhibited tumor growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The immune system has an exquisite ability to recognize and kill tumor cells, leaving the surrounding cells unaffected. Effective tumor immunity depends on the development of an anti-tumor T-cell response [1]. Tumor antigens are taken up, processed, and presented in the context of major histocompatibility complex molecules to T cells by antigen-presenting cells (APC) [2]. T cells recognizing antigen in the presence of appropriate co-stimulation and the cytokine IL-12p70 proliferate and produce interferon (IFN)-gamma [3, 4]. The presence of large numbers of IFN-gamma-secreting, tumor-specific T cells correlate with successful anti-tumor immunity and the destruction of tumor [5–8]. However, tumor cells are able to evade the immune response leading to unpredictable and infrequent responses to cancer immunotherapy. Tumor immune tolerance has been a major hurdle to successful immunotherapy for cancer [9–11]. Elucidating the mechanisms by which tumor cells avoid immune destruction may lead to the development of effective and generally applicable immune-based therapies.
Externalization of phosphatidylserine (PS) is a potential mechanism for regulating the immune response to self-antigens exposed during apoptosis [12–15]. PS is an anionic phospholipid that is most commonly restricted to the cytoplasmic surface of cell membranes. During early apoptosis, PS is externalized to the cell surface where it can be recognized by phagocytic cells [16, 17]. In vitro, PS has been shown to inhibit the activation of macrophages and the maturation of DCs [14, 18]. In vivo, PS was shown to inhibit allogeneic immune responses and contribute to the poor immunogenicity of apoptotic tumor cell vaccines [15, 19]. Recent studies suggest that PS is also present on the surface of some live cells, including tumor cells [20–22]. Despite the fact that PS is an “eat me” signal, it does not appear to promote engulfment of live tumor cells, possibly because additional signals are required for engulfment and because live cells express “don’t eat me signals” that prevent engulfment. The effects of PS on tumor immunity and on tumor growth are not well established.
The current study examined the effects of Annexin-V, a ubiquitous cytoplasmic protein that was previously shown to specifically bind and block PS with high affinity, on neuroblastoma growth and the anti-tumor CD8+ T cells response in vivo [23, 24]. AGN2a is an aggressive murine neuroblastoma that is poorly immunogenic [25, 26]. We have found that PS is highly expressed on live AGN2a cells. To block PS in vivo, AGN2a cells were engineered to secrete AnV. Our findings demonstrated that AnV could prevent AGN2a growth and promote anti-tumor T cell immunity.
Materials and methods
Mice
A/J mice, 6–8 weeks of age, were purchased from Jackson laboratory (Bar Harbor, ME, USA) and housed in a pathogen-free facility. Care was provided and experiments conducted according to institutional guidelines and approved protocols.
Tumor cells and Annexin-V expression
An aggressive variant of Neuro-2a, AGN2a, was derived by sequential in vivo and in vitro passage [27]. AGN2a is syngeneic with A/J mice. The full-length cDNA sequence for mouse AnV was obtained from the Mammalian Gene Collection (NIH, Bethesda, MD, USA). Forward primer tgcagaagcttatggctacgaga and reverse primer tgcagtctagatcagtcatcctc were used to amplify mouse AnV from pCMV-SPORT6. The PCR product was initially cloned into the pCR4-Blunt Topo cloning vector and then transferred to the pFLAG-CMV-3 expression vector behind the pre-pro-trypsin (PPT) signal peptide and FLAG sequences (Sigma, St. Louis, MO, USA) using Hind III and Xba I restriction sites. AGN2a cells were transfected with pFLAG-CMV-3 containing mouse AnV (AnV/pFLAG-CMV-3) or with pFLAG-CMV-3 control vector (i.e., without the AnV gene) using nucleofection (Lonza, Basel, Switzerland). Transfected tumor cells were selected in 1,000 μg/ml geneticin and cloned by limiting dilution. For vaccination experiments, transiently nucleofected AGN2a cells were cultured overnight to allow for optimal expression of AnV and irradiated with 4,000 rads immediately prior to vaccination.
Antibodies, T-cell isolation and analysis of PS expression
Rabbit anti-FLAG antibody and horseradish peroxidase (HRP)-conjugated donkey anti-rabbit antibody were purchased from Sigma-Aldrich (St. Louis, MO, USA). FITC-conjugated AnV was purchased from BD Pharmingen (San Diego, CA, USA). Antibodies against activated caspase-3 were purchased from BD Biosciences (San Diego, CA, USA). Anti-PS antibodies were purchased from US Biological (Swampscott, MA, USA) or generously provided by Dr. Philip Thorpe (UT Southwestern, Dallas, TX, USA). In vivo PS staining was performed as previously reported [28]. To induce apoptosis, cells were treated with 0.5-μM staurosporine. Anti-CD8-conjugated beads (Miltenyi Biotec, Auburn, CA, USA) were used to isolate CD8+ T cells by immunomagnetic cell sorting (AutoMACS, Miltenyi Biotec, Auburn, CA, USA). Surface PS expression was determined using flow cytometry (Becton–Dickinson, San Jose, CA, USA). Tumor sections were examined using the Zeiss 710 confocal fluorescent microscope (Carl Zeiss Microimaging LLC, Thornwood, NY, USA).
Recombinant Annexin-V preparation
For AnV protein purification, Chinese hamster ovary (CHO-1) cells were transfected with AnV/pFLAG-CMV-3 and cloned to obtain a cell line permanently expressing high levels of FLAG–AnV. The FLAG–AnV-expressing CHO-1 cells were placed in protein and serum-free CHO media (CHO PF-AF, Sigma, c8730) and cultured in a Celline AD 1000 bioreactor (Integra Biosciences, Switzerland) to obtain culture supernatants containing high concentrations of AnV. Bioreactor-derived AnV was further concentrated by centrifugation using Amicon Ultra-15 protein concentrators (Millipore, Bedford, MA, USA).
Western blot analysis
To examine AnV levels in cell lysates and culture supernatants of AGN2a, cells were collected 2 days after nucleofection or after plating permanently transfected cells. Culture supernatants from 105 to 106 cells or cell lysates were fractionated by SDS polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride (PVDF) membranes. Total protein was measured prior to loading the gels. The membranes were incubated in blocking solution containing 5 % nonfat dry milk in TBS containing 0.5 % Tween-20 for 1 h and then incubated with antibody against FLAG (diluted 1:1,000) overnight at 4 °C. After washing repeatedly with TBST, membranes were incubated with secondary antibody for 2 h at room temperature. Antibody-bound proteins were visualized using an ECL plus Lumigen PS-3 detection reagent (Amersham Bioscience, Sunnyvale, CA, USA).
Tumor growth and tumor vaccination experiments
To assess tumor cell growth in vivo, A/J mice were injected subcutaneously with viable AnV-secreting or control (transfected with empty vector) AGN2a cells. In some experiments, A/J mice were depleted of T cells by injecting them with 250 micrograms of anti-Thy1.2 monoclonal antibody intraperitoneally on the day of tumor inoculation and every 3 days thereafter.
For tumor vaccination/challenge experiments, AGN2a cells were nucleofected with AnV vector or control vector, collected the next day, and irradiated with 4,000 rad. A/J mice were injected subcutaneously with 2 × 106 of the irradiated cells. The vaccination was repeated 7 days later, and 1 week after the second vaccination, mice were challenged subcutaneously with viable wild-type AGN2a cells. Tumor size was measured every 3 days, and the mice were considered moribund and euthanized when tumor size exceeded 250 mm2.
ELISPOT analysis
A/J mice were vaccinated twice weekly with irradiated AGN2a cells that had been nucleofected to express AnV or nucleofected with control vector (as above). Five days after the second vaccination, spleens were collected and CD8+ T cells purified by immunomagnetic sorting (Milenyi Biotec). The purified CD8+ T cells were analyzed by flow cytometry to assess purity, and the isolated cells were greater than 95 % CD8+ (data not shown). To examine tumor reactivity, the purified CD8+ T cells were tested in interferon-gamma (IFN-gamma) enzyme-linked immunospot (ELISPOT) assays according to the manufacturer’s directions (BD Biosciences Pharmingen). Briefly, 96-well hydrophobic PVDF membrane plates (Millipore, Bedford, MA, USA) were coated with IFN-gamma-specific capture antibody overnight at 4 °C. On the following day, twofold dilutions of purified CD8+ splenocytes were added to triplicate wells starting at a concentration of 2.5 × 105 cells per well. AGN2a “stimulator” cells were added to experimental wells at a concentration of 104 cells per well, and the plates were incubated at 37 °C for 30–36 h. Following incubation, the cells were removed from the wells, and the plates were incubated with biotinylated detection IFN-gamma antibody for 2 h at room temperature. After extensive washing, the plates were incubated with ExtrAvidin-alkaline phosphatase (Sigma-Aldrich), and the spots developed by adding BCIP/NBT substrate (Sigma-Aldrich). The numbers of spots were quantitated with an ImmunoSpot Analyzer using included acquisition and analysis software (CTL Analyzers, LLC, Cleveland, OH, USA).
In vitro lysis assay
Spleens from mice immunized with irradiated AnV-secreting or control AGN2a tumors were homogenized and cultured in the presence of bone marrow derived dendritic cells pulsed with AGN2a tumor lysate. T cells were stimulated 2 times, isolated, washed, and incubated with AGN2a or YAC targets cells in 96-well plates in triplicate. YAC1 is a lymphoma cell line that is syngeneic to A/J mice. It is also a sensitive natural killer (NK) target. Cyto Tox 96® (Promega, Madison, WI, USA) non-radioactive colorimetric assay was used to determine cytotoxicity. Release of LDH from lysed cells into the supernatants is measured in an enzymatic assay that results in the conversion of tetrazolium salt into a red formazan product. The amount of color metabolite formed is proportional to the number of cells lysed. Percent lysis was calculated as follows: %cytotoxicity = experimental–effector spontaneous − target spontaneous/Target Maximum − Target Spontaneous.
Statistical analysis
Statistical comparisons of parametric variables were performed using Student’s t tests. Survival curves were compared using Wilcoxon rank sum test.
Results
Viable AGN2a cells constitutively express PS on the cell surface
We examined the level of PS on the surface of AGN2a, a murine NB cell line. The cells were stained with FITC-conjugated AnV and analyzed by flow cytometry. Mean fluorescence intensity (MFI) of stained cells was 10–30 fold greater than unstained control cells (Fig. 1a). AGN2a cells showed a uniform staining profile suggesting that all cells expressed PS on the cell surface. Coating AGN2a cells with recombinant AnV blocked staining with FITC–AnV, indicating that the staining was specific. AGN2a cells were clearly viable despite expression of PS on the cell surface as indicated by their ability to exponentially expand in culture and ability to exclude trypan blue and other cell viability dyes (not shown). To determine whether AGN2a cells were apoptotic, caspase-3 activation was examined using flow cytometry. Caspase-3 was activated in only 1 % of cells, but could be activated using staurosporine treatment, suggesting that while AGN2a cells were not apoptotic, they could be induced to undergo apoptosis (Fig. 1b). These findings indicate that live AGN2a constitutively express PS on the cell surface.
Live NB cells express PS on the cell surface. a AGN2a NB cells were stained with FITC-conjugated AnV (AnV–FITC) and examined by flow cytometry (blue line). AGN2a cells were pre-incubated with 0.2 micrograms/ml CHO cell-derived FLAG–AnV (see “Materials and methods”) and then stained with AnV–FITC (green line). The red line indicates unstained control cells. The results are representative of at least 3 independent experiments. b AGN2a cells are not apoptotic. AGN2a cells were examined using flow cytometry for caspase-3 activation, a mediator of apoptosis. Only 1 % of AGN2a cells stain for activated caspase-3 (red line). Apoptosis was inducible with staurosporine. Staurosporine exposure for 8 (green line) and 16 (blue line) hours led to caspase-3 activation in 11 and 26 % of AGN2a cells, respectively. The results are representative of 2 independent experiments
Engineered AGN2a cells secrete functional AnV
To examine the effects of AnV on tumor growth in vivo, AGN2a cells were engineered to secrete AnV. The AnV gene was cloned by PCR and inserted into the pFLAG-CMV-3 eukaryotic vector downstream of the sequences encoding the PPT signal peptide and FLAG peptide. The resulting construct was used to transfect AGN2a cells. When the cells were analyzed by Western blot, AnV could be detected in both the cell lysates and culture supernatants suggesting that AnV was produced and secreted (Fig. 2a). We next examined whether the AnV secreted by the cloned AGN2a cells was capable of binding PS. AGN2a cells were incubated with supernatants derived from AGN2a or HEK293 cells transfected with pFLAG-CMV-3. Cells were stained with AnV–FITC and analyzed using flow cytometry. Supernatants blocked PS and diminished AnV–FITC staining (Fig. 2b). Next, we compared the level of PS exposed on the surface of control and FLAG–AnV-secreting AGN2a cells by flow cytometry. As shown in Fig. 2c, the FLAG–AnV-secreting tumor cells bound threefold less AnV–FITC than control tumor cells. Cell surface staining using fluorescent microscopy showed similar findings. Tumor cells were stained with anti-PS monoclonal antibody 2aG4 (generously provided by Dr. Philip Thorpe, UT Southwestern, Dallas, Texas). In FLAG–AnV-secreting AGN2a cells, 2aG4 binding to PS was blocked, and plasma membrane staining with the anti-PS antibody was low compared to control AGN2a cells (Fig. 3a). Nuclear PS was not blocked since secreted AnV did not enter live cells, and nuclear PS staining was similar in both AnV-secreting and control tumor cells. Taken together with the flow cytometry data, the findings suggest that secreted AnV blocked cell surface PS.
Engineered AGN2a cells secrete AnV, which binds and blocks PS. AGN2a cells were engineered to constitutively secrete AnV by transfecting them with pFLAG-CMV-3 construct containing the FLAG–AnV cDNA sequence. a Cell lysates and culture supernatants were examined using Western blot for AnV expression. Anti-FLAG antibody was used to detect transgene expression. C empty vector or wild-type control; AnV FLAG–AnV transfected; Control sarcoma cell line. Actin was examined to control for loading of cell lysates. To generate supernatants, tumor cells were seeded at equal density. After 48 h, supernatants were collected, and an equal amount of protein was loaded into each lane. The results are representative of at least 3 independent experiments. b AGN2a cells were stained with FITC–AnV, and surface PS was measured using flow cytometry. In order to block PS, AGN2a cells were pre-treated with supernatants from wild-type AGN2a (blue line), AGN2a engineered to secreted FLAG–AnV (green line), HEK 293 cells permanently transfected with FLAG–AnV (teal line), or HEK 293 cells transiently transfected with FLAG–AnV (orange line). Unstained cells are depicted in red. The results are representative of at least 3 independent experiments. c Wild-type AGN2a (blue line) cells and AGN2a cells engineered to secrete AnV (orange line) were stained with AnV–FITC and analyzed by flow cytometry. The red and green lines indicate unstained wild-type and AnV-secreting AGN2a cells. The results are representative of at least 3 independent experiments
AnV binds and blocks tumor PS. a AGN2a cells grown in vitro for 48 h were stained with anti-PS monoclonal antibody (2aG4). The cells were examined using fluorescent microscope. Thin white arrows point to the cytoplasm. Large open arrows point to the nuclei. The nuclei of some cells are outlined in black. Neuroblastoma, which are known as small blue cells, have scant cytoplasm. (i) The plasma membrane of FLAG–AnV-secreting AGN2a cells stained poorly with the anti-PS antibody 2aG4 since AnV blocked PS. (ii) Anti-PS antibody 2aG4 bound PS and stained control AGN2a tumors brightly. Similar levels of staining were seen within the nuclei, since nuclear PS was not accessible and was not blocked by AnV in live cells. White arrows point to the cytoplasm. Open arrows point to the nucleus. The nucleus is outlined in black in some cells. b Mice, bearing AGN2a tumors, were injected intravenously with anti-PS monoclonal antibody (2aG4). Tumors were removed, frozen, and sectioned. Sections were stained with a secondary fluorescently labeled antibody. AGN2a is gelatinous and difficult to cut resulting in very thick sections. (i) Fluorescent microscopy demonstrated bright staining of control AGN2a tumor (white arrows). (ii) Only faint staining was seen when isotype control antibody (G44) was injected into mice (open arrow). The findings suggest that 2aG4 binding is specific. c (i) Concentrated supernatant from cells engineered to secrete FLAG–AnV was injected into mice one hour prior to injecting anti-PS antibody (2aG4) in order to block PS in vivo. Tumor staining with 2aG4 was blocked with AnV, suggesting that PS was blocked in vivo (open arrow). (ii) Control supernatant did not prevent tumor staining with 2aG4 (white arrow). d Mice were inoculated with FLAG–AnV-secreting or control AGN2a tumor cells. Anti-PS (2aG4) monoclonal antibody was injected intravenously when 0.5–1 cm3 tumors developed. (i) AnV-secreting tumors stained poorly with 2aG4, suggesting that FLAG–AnV secreted by tumor cells blocked PS in vivo (open arrows). (ii) Control tumors stained brightly with 2aG4 (white arrows), suggesting that AGN2a tumor cells expressed PS in vivo. Representative micrographs are shown. Each pair of micrographs was taken at the same magnification and the same exposure
To determine PS expression in vivo, anti-PS antibody 2aG4 was injected intravenously into mice. Tumors were isolated, and frozen sections examined using fluorescent microscopy. AGN2a tumor stained brightly with anti-PS (2aG4) but not the isotype control (G44) antibody suggesting that the staining was specific (Fig. 3b). To determine whether AnV could block PS staining in vivo, mice were injected intravenously with FLAG–AnV an hour prior to injecting the anti-PS antibody 2aG4. AGN2a tumor from mice treated with exogenous FLAG–AnV stained dimly compared to AGN2a tumor from mice treated with control supernatant (Fig. 3c). The findings suggest that injected FLAG–AnV bound PS and blocked staining with anti-PS antibody 2aG4 in vivo. We then compared PS staining in control and FLAG–AnV-secreting tumors. As shown in Fig. 3d, FLAG–AnV-secreting AGN2a stained poorly compared to control AGN2a. The findings indicate that FLAG–AnV was secreted and blocked tumor PS in vivo.
AnV inhibits AGN2a growth in immunocompetent mice
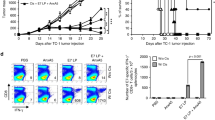
We examined the growth of subcutaneously implanted AnV-secreting or control AGN2a cells in immunocompetent A/J mice. Tumor-free survival was measured. All mice inoculated with control cells developed tumors and none survived (Fig. 4a). In contrast, 40 % of mice inoculated with AnV-secreting tumor cells did not develop tumors and survived (p = 0.0062). To determine whether an intact T-cell immune response was required for AnV to delay or prevent tumor growth, mice were depleted of T cells using anti-Thy1.2 monoclonal antibody. AnV secretion did not alter survival in mice depleted of T cells (Fig. 4b). Furthermore, the growth of AnV-secreting AGN2a and control cells was not significantly different in vitro or in immune-deficient mice (Fig. 4c, d). The findings demonstrating that AnV prevented the growth of neuroblastoma in immune-competent but not immune-deficient animals suggest that the effects of AnV are immune dependent.
Blocking PS in vivo can prevent the growth of AGN2a in immunocompetent but not T-cell-depleted mice. a Immunocompetent A/J mice (n = 15) or b A/J mice depleted of T cells using anti-Thy1.2 monoclonal antibody (n = 12) were subcutaneously inoculated with 104 AGN2a cells engineered to permanently secrete FLAG–AnV (AnV–NB) or AGN2a cells transfected with control vector (Control). Tumor-free survival was determined. The survival curves were generated from the combined results of 2 replicate experiments. c AnV–NB (white) and control (black) cell growth was determined in vitro in triplicate wells. d A/J mice were treated with Thy1.2 to deplete T cells. AnV-secreting (AnV) and control (control) AGN2a tumor growth was determined (n = 5 per group)
Annexin-V enhances the efficacy of cell-based tumor vaccination and increases the frequency of IFN-gamma-producing tumor-reactive CTL
We next tested whether AnV-secreting tumor cells could serve as a cell-based vaccine. AGN2a cells were transiently transfected (nucleofected) with the AnV vector. Nucleofected cells secreted AnV for at least 8 days (Fig. 5a). For vaccination, immunocompetent A/J mice were injected in the flank (subcutaneously) twice, a week apart, with irradiated AnV-secreting or control tumor cells. One week after the second vaccination, the mice were challenged with 104 live AGN2a cells inoculated subcutaneously on the opposite flank (Fig. 5b). The mice were followed for tumor growth and survival. While 30 % of mice vaccinated with control tumor cells survived the tumor challenge, 80 % of the mice vaccinated with AnV-secreting tumor cells survived tumor-free (p = 0.012). These findings suggest that AnV promoted more effective tumor immunity in response to the cell-based tumor vaccine.
Blocking PS in vivo increased the efficacy of tumor vaccination. a AGN2a cells were nucleofected (TT) with either empty vector (C) or FLAG–AnV (AnV) vector. Forty-eight hours after nucleofection, cell lysates and supernatants were prepared and analyzed by Western blot. Permanently transfected (PT) cells were used as controls. Anti-FLAG was used to detect transgene expression. Actin staining, shown in the middle panel, was used as loading control in the lysate lanes. Total protein was quantified, and equal amount of protein was loaded into each lane. AnV was secreted for at least 8 days after nucleofection with FLAG–AnV (AnV), as shown in the lower panel. Production or secretion of AnV was never detected in empty vector (C) group. AGN2a cells were nucleofected with empty vector (C) control or and FLAG–AnV (AnV) coding vector, irradiated and placed in culture. Supernatants were collected on the days indicated. Cells were washed, and media were replaced every 2 days. AnV secretion was detected using Western blot as described above (+ positive control). b Mice (n = 15 per group) were subcutaneously vaccinated twice, a week apart, with 2 × 106 irradiated (4,000 rads) AGN2a cells that had been nucleofected with control or FLAG–AnV (AnV-NB) coding vector. Seven days after the second vaccination, the mice were challenged subcutaneously on the opposite flank with 104 live wild-type AGN2a cells. Survival curves were generated from the combined results of 2 replicate experiments
Tumor immunity is typically dependent upon IFN-gamma production [29]. Therefore, the effect of FLAG–AnV on the frequency of IFN-gamma-producing T cells after tumor vaccination was examined. A/J mice were vaccinated twice, a week apart, with irradiated FLAG–AnV-secreting or control vector-transfected tumor cells. Five days after the second vaccination, CD8+ splenocytes were isolated using immunomagnetic sorting. The purified CD8+ T cells were then tested in ELISPOT assays to determine the frequencies of tumor-reactive, IFN-gamma-producing CD8+ T cells. As compared to mice vaccinated with control cells, mice vaccinated with FLAG–AnV-secreting AGN2a tumor cells had 2–4 times greater frequency of IFN-gamma-producing CD8+ splenic T cells (Fig. 6a). The greater proportion of IFN-gamma-producing CD8+ T cells in the spleen directly correlated with the increased tumor protection in these mice. The findings suggest that vaccination in the presence of AnV led to increase in the frequency of IFN-gamma-producing T cells and protected mice from AGN2a tumor challenge.
Blocking PS in vivo increased the frequency of IFN-gamma-secreting tumor-reactive T cells in response to tumor vaccination. Mice were subcutaneously vaccinated twice, a week apart, with 2 × 106 irradiated (4,000 rads) AnV-secreting or control AGN2a cells. Five days after the second vaccination, splenic CD8+ T cells were isolated using immunomagnetic sorting. a T cells were tested in IFN-gamma ELISPOT assay using AGN2a stimulators. The results are representative of 3 independent experiments (*p < 0.05). b T cells were stimulated twice in vitro with bone marrow derived DCs pulsed with AGN2a cell lysate. Antigen-specific cytolysis was assayed. T cells isolated from mice vaccinated with AnV-secreting cells lysed AGN2a (AnV) preferentially to YAC cells (YAC). Furthermore, T cells isolated from mice vaccinated with AnV-secreting cells (AnV) lysed AGN2a targets more effectively than T cells isolated from control mice (Ctrl). The data represent 3 separate experiments (*p < 0.05)
We next tested the tumor reactivity of the T-cell response to vaccination. Splenic T cells were isolated from mice immunized with FLAG–AnV-secreting or control AGN2a tumor cells and stimulated in vitro with bone marrow derived DCs pulsed with AGN2a tumor cell lysate. In vitro lysis of AGN2a and YAC cells was assayed (Fig. 6b). T cells from mice immunized with FLAG–AnV-secreting cells lysed AGN2a significantly better than T cells from mice immunized with control AGN2a cells, which is consistent with the ELISPOT findings. T cells isolated from mice immunized with AnV-secreting cells also lysed AGN2a significantly better than YAC cells. YAC are derived from H-2a mice and are syngeneic with AGN2a, as well as susceptible to NK cell lysis. The finding suggests that tumor killing was antigen-specific, and immunization with AnV-secreting tumor cells produced tumor-specific T cells.
Discussion
Immune evasion by cancer is a major hurdle to successful immunotherapy [11, 30–33]. A possible mechanism for immune evasion is tumor cells masquerading as apoptotic cells by expressing PS [34]. While PS externalization is commonly associated with apoptosis, recent studies demonstrated that tumors as well as non-neoplastic cells express PS on the cell surface. These include human neuroblastoma and mouse soft tissue sarcoma cells (our unpublished results) as well as gastric carcinoma, colon carcinoma, lymphoma, and proliferating T lymphocytes [2, 20–22, 37–39]. In the current study, we demonstrated that PS was highly expressed on the surface of live AGN2a cells, an aggressive mouse neuroblastoma [35].
We examined the effects of blocking PS with the AnV on tumor growth. To block PS in vivo, AGN2a cells were engineered to secrete mouse AnV. Secreted FLAG–AnV blocked PS in vitro and in vivo. In vitro, FLAG–AnV-secreting AGN2a cells had threefold less PS exposed on the cell surface. In vivo, FLAG–AnV-secreting AGN2a tumors had lower levels of staining with the anti-PS antibody 2aG4. Western blot analysis demonstrated that FLAG–AnV-secreting AGN2a tumors continued to express AnV in vivo for at least 4 weeks (data not shown). These findings suggest that AnV expression persisted in vivo and secreted FLAG–AnV blocked PS.
We found that animals inoculated with FLAG–AnV-secreting AGN2a cells had significantly better survival than those inoculated with control AGN2a cells. The salutary effect of AnV was limited to immune-competent and not T-cell-depleted mice. In the absence of effective T-cell immunity, AnV did not affect tumor growth. Furthermore, mice immunized with irradiated FLAG–AnV-secreting AGN2a cell-based vaccine were protected against tumor challenge, whereas control vaccine was significantly less effective. FLAG–AnV-secreting AGN2a cell vaccine led to a higher frequency of tumor-reactive splenic CD8+ T cells compared with control vaccine. CD8+ T cells isolated from mice treated with AnV-secreting vaccine and expanded ex vivo lysed AGN2a cells better than YAC cells, which are syngeneic to AGN2a as well as sensitive NK cell targets. These findings support the hypothesis that blocking PS with AnV-retarded tumor growth by restoring tumor-specific T-cell-mediated immunity. We did not rule out the possibility that NK cell activity is also enhanced in the presence of AnV.
Beck et al. reported that blocking PS in vivo with an anti-PS antibody, in conjunction with tumor irradiation, can retard tumor growth in mice [40, 41]. In that model, the tumor cells did not constitutively express PS on the cell surface, but radiation treatment induced apoptosis leading to PS expression. In the current study, neuroblastoma cells expressed PS on the cell surface. Secreted FLAG–AnV blocked PS in vivo, promoted anti-tumor CD8+ T-cell immunity, and prevented tumor growth in vivo independently of other interventions. Taken together with previous reports, these findings suggest that tumor PS, either constitutively expressed or induced, promotes immune tolerance and could be targeted for cancer immunotherapy. In future studies, we will test the effects of blocking PS with AnV in a treatment model of established tumors.
The mechanism by which PS promotes tumor immunity is not well described. PS may regulate CD8+ T-cell mediated tumor immunity by inhibiting antigen presentation, co-stimulation, and inflammation. Previous in vivo studies suggested that PS inhibits IL-12 production by APC [38]. More recent reports demonstrated that PS inhibited DC maturation and activation of Th1 response [14]. PS may also inhibit local inflammation by promoting TGF-beta and preventing TNF-alpha production by macrophages [13, 18, 42]. These mechanisms could act synergistically to subvert tumor immunity and promote tumor growth. APCs recognize PS through a receptor(s) that has not been conclusively defined to date [39]. Recent studies suggest that T cell immunoglobulin mucin (TIM)-4 can bind PS and may mediate apoptotic cell engulfment and regulate immunity [43, 44]. Other candidate receptors have been previously suggested, and redundant systems for recognizing PS may exist [39].
In summary, we demonstrated that blocking PS with AnV could lead to T-cell-mediated tumor immunity and improved tumor-free survival. We also showed that AnV-secreting tumor vaccine improved survival in this model of murine neuroblastoma. The findings suggest that by expressing PS, neuroblastoma tumor cells masquerade as apoptotic cells, evade immune recognition, and avoid destruction. The expression of PS on the surface of other human and murine tumor cells as well as non-malignant lymphocytes may possibly point to a more generalized immune escape mechanism. It follows that blocking PS with AnV could be the basis for future clinical immunotherapy applications.
References
Van Mierlo GJ, Boonman ZF, Dumortier HM, den Boer AT, Fransen MF, Nouta J, van der Voort EI, Offringa R, Toes RE, Melief CJ (2004) Activation of dendritic cells that cross-present tumor-derived antigen licenses CD8 + CTL to cause tumor eradication. J Immunol 173(11):6753–6759
Ghiringhelli F, Apetoh L, Housseau F, Kroemer G, Zitvogel L (2007) Links between innate and cognate tumor immunity. Curr Opin Immunol 19(2):224–231
Steitz J, Bruck J, Lenz J, Knop J, Tuting T (2001) Depletion of CD25(+) CD4(+) T cells and treatment with tyrosinase-related protein 2-transduced dendritic cells enhance the interferon alpha-induced, CD8(+) T-cell-dependent immune defense of B16 melanoma. Cancer Res 61(24):8643–8646
Handel-Fernandez ME, Cheng X, Herbert LM, Lopez DM (1997) Down-regulation of IL-12, not a shift from a T helper-1 to a T helper-2 phenotype, is responsible for impaired IFN-gamma production in mammary tumor-bearing mice. J Immunol 158(1):280–286
Barth RJ Jr, Mule JJ, Spiess PJ, Rosenberg SA (1991) Interferon gamma and tumor necrosis factor have a role in tumor regressions mediated by murine CD8 + tumor-infiltrating lymphocytes. J Exp Med 173(3):647–658
Matsui S, Ahlers JD, Vortmeyer AO, Terabe M, Tsukui T, Carbone DP, Liotta LA, Berzofsky JA (1999) A model for CD8 + CTL tumor immunosurveillance and regulation of tumor escape by CD4 T cells through an effect on quality of CTL. J Immunol 163(1):184–193
Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, Schreiber RD (1998) Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci USA 95(13):7556–7561
Dunn GP, Bruce AT, Sheehan KC, Shankaran V, Uppaluri R, Bui JD, Diamond MS, Koebel CM, Arthur C, White JM, Schreiber RD (2005) A critical function for type I interferons in cancer immunoediting. Nat Immunol 6(7):722–729
Rousseau RF, Brenner MK (2005) Vaccine therapies for pediatric malignancies. Cancer J 11(4):331–339
Smyth MJ, Godfrey DI, Trapani JA (2001) A fresh look at tumor immunosurveillance and immunotherapy. Nat Immunol 2(4):293–299
Rabinovich GA, Gabrilovich D, Sotomayor EM (2007) Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol 25:267–296
Kim S, Elkon KB, Ma X (2004) Transcriptional suppression of interleukin-12 gene expression following phagocytosis of apoptotic cells. Immunity 21(5):643–653
Hoffmann PR, Kench JA, Vondracek A, Kruk E, Daleke DL, Jordan M, Marrack P, Henson PM, Fadok VA (2005) Interaction between phosphatidylserine and the phosphatidylserine receptor inhibits immune responses in vivo. J Immunol 174(3):1393–1404
Chen X, Doffek K, Sugg SL, Shilyansky J (2004) Phosphatidylserine regulates the maturation of human dendritic cells. J Immunol 173(5):2985–2994
Bondanza A, Zimmermann VS, Rovere-Querini P, Turnay J, Dumitriu IE, Stach CM, Voll RE, Gaipl US, Bertling W, Poschl E, Kalden JR, Manfredi AA, Herrmann M (2004) Inhibition of phosphatidylserine recognition heightens the immunogenicity of irradiated lymphoma cells in vivo. J Exp Med 200(9):1157–1165
Williamson P, Schlegel RA (2002) Transbilayer phospholipid movement and the clearance of apoptotic cells. Biochim Biophys Acta 1585(2–3):53–63
Asano K, Miwa M, Miwa K, Hanayama R, Nagase H, Nagata S, Tanaka M (2004) Masking of phosphatidylserine inhibits apoptotic cell engulfment and induces autoantibody production in mice. J Exp Med 200(4):459–467
Henson PM, Bratton DL, Fadok VA (2001) The phosphatidylserine receptor: a crucial molecular switch? Nat Rev Mol Cell Biol 2(8):627–633
Gaipl US, Beyer TD, Baumann I, Voll RE, Stach CM, Heyder P, Kalden JR, Manfredi A, Herrmann M (2003) Exposure of anionic phospholipids serves as anti-inflammatory and immunosuppressive signal–implications for antiphospholipid syndrome and systemic lupus erythematosus. Immunobiology 207(1):73–81
Pohl A, Lage H, Muller P, Pomorski T, Herrmann A (2002) Transport of phosphatidylserine via MDR1 (multidrug resistance 1)P- glycoprotein in a human gastric carcinoma cell line. Biochem J 365(Pt 1):259–268
Woehlecke H, Pohl A, Alder-Baerens N, Lage H, Herrmann A (2003) Enhanced exposure of phosphatidylserine in human gastric carcinoma cells overexpressing the half-size ABC transporter BCRP (ABCG2). Biochem J 376(2):489–495
Elliott JI, Surprenant A, Marelli-Berg FM, Cooper JC, Cassady-Cain RL, Wooding C, Linton K, Alexander DR, Higgins CF (2005) Membrane phosphatidylserine distribution as a non-apoptotic signalling mechanism in lymphocytes. Nat Cell Biol 7(8):808–816
Hamon Y, Broccardo C, Chambenoit O, Luciani MF, Toti F, Chaslin S, Freyssinet JM, Devaux PF, McNeish J, Marguet D, Chimini G (2000) ABC1 promotes engulfment of apoptotic cells and transbilayer redistribution of phosphatidylserine. Nat Cell Biol 2(7):399–406
Stach CM, Turnay X, Voll RE, Kern PM, Kolowos W, Beyer TD, Kalden JR, Herrmann M (2000) Treatment with annexin V increases immunogenicity of apoptotic human T-cells in Balb/c mice. Cell Death Differ 7(10):911–915
Yan X, Johnson BD, Orentas RJ (2004) Murine CD8 lymphocyte expansion in vitro by artificial antigen-presenting cells expressing CD137L (4-1BBL) is superior to CD28, and CD137L expressed on neuroblastoma expands CD8 tumour-reactive effector cells in vivo. Immunology 112(1):105–116
Jing W, Orentas RJ, Johnson BD (2007) Induction of immunity to neuroblastoma early after syngeneic hematopoietic stem cell transplantation using a novel mouse tumor vaccine. Biol Blood Marrow Transplant 13(3):277–292
Johnson BD, Yan X, Schauer DW, Orentas RJ (2003) Dual expression of CD80 and CD86 produces a tumor vaccine superior to single expression of either molecule. Cell Immunol 222(1):15–26
He J, Luster TA, Thorpe PE (2007) Radiation-enhanced vascular targeting of human lung cancers in mice with a monoclonal antibody that binds anionic phospholipids. Clin Cancer Res 13(17):5211–5218
Wang RF, Miyahara Y, Wang HY (2008) Toll-like receptors and immune regulation: implications for cancer therapy. Oncogene 27(2):181–189
Chen X, Doffek K, Sugg SL, Shilyansky J (2003) Neuroblastoma cells inhibit the immunostimulatory function of dendritic cells. J Pediatr Surg 38(6):901–905
Shurin GV, Lotze MT, Barksdale EM (2000) Neuroblastoma inhibits dendritic cell differentiation and function. Curr Surg 57(6):637
Horna P, Sotomayor EM (2007) Cellular and molecular mechanisms of tumor-induced T-cell tolerance. Curr Cancer Drug Targets 7(1):41–53
Zhou G, Levitsky HI (2007) Natural regulatory T cells and de novo-induced regulatory T cells contribute independently to tumor-specific tolerance. J Immunol 178(4):2155–2162
Kim R, Emi M, Tanabe K (2005) Cancer cell immune escape and tumor progression by exploitation of anti-inflammatory and pro-inflammatory responses. Cancer Biol Ther 4(9):924–933
Johnson BD, Gershan JA, Natalia N, Zujewski H, Weber JJ, Yan X, Orentas RJ (2005) Neuroblastoma cells transiently transfected to simultaneously express the co-stimulatory molecules CD54, CD80, CD86, and CD137L generate antitumor immunity in mice. J Immunother 28(5):449–460
Kenis H, Hofstra L, Reutelingsperger CP (2007) Annexin A5: shifting from a diagnostic towards a therapeutic realm. Cell Mol Life Sci 64(22):2859–2862
Ran S, Downes A, Thorpe PE (2002) Increased exposure of anionic phospholipids on the surface of tumor blood vessels. Cancer Res 62(21):6132–6140
Calderon C, Huang ZH, Gage DA, Sotomayor EM, Lopez DM (1994) Isolation of a nitric oxide inhibitor from mammary tumor cells and its characterization as phosphatidylserine. J Exp Med 180(3):945–958
Fonseca C, Dranoff G (2008) Capitalizing on the immunogenicity of dying tumor cells. Clin Cancer Res 14(6):1603–1608
Beck AW, Luster TA, Miller AF, Holloway SE, Conner CR, Barnett CC, Thorpe PE, Fleming JB, Brekken RA (2006) Combination of a monoclonal anti-phosphatidylserine antibody with gemcitabine strongly inhibits the growth and metastasis of orthotopic pancreatic tumors in mice. Int J Cancer 118(10):2639–2643
He J, Yin Y, Luster TA, Watkins L, Thorpe PE (2009) Antiphosphatidylserine antibody combined with irradiation damages tumor blood vessels and induces tumor immunity in a rat model of glioblastoma. Clin Cancer Res 15(22):6871–6880
Huynh ML, Fadok VA, Henson PM (2002) Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest 109(1):41–50
Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH (2010) TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev 235(1):172–189
Kobayashi N, Karisola P, Pena-Cruz V, Dorfman DM, Jinushi M, Umetsu SE, Butte MJ, Nagumo H, Chernova I, Zhu B, Sharpe AH, Ito S, Dranoff G, Kaplan GG, Casasnovas JM, Umetsu DT, Dekruyff RH, Freeman GJ (2007) TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity 27(6):927–940
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yan, X., Doffek, K., Yin, C. et al. Annexin-V promotes anti-tumor immunity and inhibits neuroblastoma growth in vivo. Cancer Immunol Immunother 61, 1917–1927 (2012). https://doi.org/10.1007/s00262-012-1250-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-012-1250-4