Abstract
A better understanding of the risks and benefits of extracolonic findings and radiation dose will aid in the safe and proper implementation of CT colonography in clinical practice. The majority of extracolonic findings in screening patients are benign and can be ignored by referring physicians. Radiologists also need to be responsible in reporting extracolonic findings. Referring providers must be knowledgeable about the theoretic risks and controversies regarding the use of ionizing radiation. Screening CT colonography imparts a low-level of radiation to patients that is equivalent or less than annual background dose.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
This article will review two hot topics of debate in CT colonography (CTC), namely extracolonic findings and radiation dose. A better understanding of risks and benefits of these issues will illustrate the safe and proper implementation of CT colonography in clinical practice.
Part I: extracolonic findings (ECF)
CT colonography provides additional screening of organs within the lower thorax, abdomen, and pelvis, detecting in some cases, additional extracolonic disease. The ability of CTC to evaluate these extracolonic findings (ECF) outside of the colonic lumen can be beneficial when clinically significant; extracolonic disease is diagnosed at an early stage and reduces morbidity, mortality, or cost, such as abdominal aortic aneurysms or early-stage lung cancer. However, the controversy is fueled by additional costs and anxiety generated from detection of less significant extracolonic findings that lead to unnecessary tests or complications for these tests or late-stage disease that does not improve patient outcome, such as pancreatic cancer. Extracolonic findings and their potential cost burden continue to be a point of controversy that CMS has cited to negate expanded coverage of CTC. This section will cover how ECF are categorized, the recent healthcare policy decisions of CTC, the prevalence of these findings in screening vs symptomatic cohorts, their impact in generating additional studies and subsequent costs, the pros and cons of the debate, and the standardization and quality assurance efforts in clinical practice.
Categorization of extracolonic findings
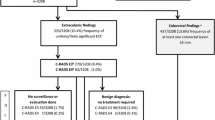
Consistency and standardization in reporting of findings at CT colonography have been aided by a reporting structure, called C-RADS, developed in 2005 [1]. This reporting structure was modeled after the successful development of Bi-RADS used in mammography. C-RADS describes how to report both individual colorectal findings and a per patient category scale summarizing all colorectal findings, ranging from C0 (incomplete/limited study) to C4 (suspected or known cancer). In addition to the colorectal scores, a similar scale of the extracolonic findings was developed, including E0 (incomplete/limited exam), E1 (normal exam or anatomic variant), E2 (clinically unimportant, no workup needed) (Fig. 1), E3 (likely unimportant, incompletely characterized, may need work-up), E4 (potentially important work-up may be needed) (Fig. 2, Table 1). The C-RADS reporting structure has become widely accepted in clinical practice. Furthermore, several studies have used the E-scores for extracolonic findings at screening CTC, which provides standardization in reporting of study results.
Public policy decisions of colorectal screening, 2008–2016
In 2008, five years after the publication of the first large screening trial for CTC [2], the American Cancer Society with the US Multi-Society Task Force and the American College of Radiology included CT colonography as part of the recommended screening tests for colorectal cancer [3]. In that same year, this progress was negated by the indeterminate rating for CT colonography by the US Preventative Task Force (USPTF) [4]. Closely linked to the negative response by the task force decision, CMS gave a non-coverage decision later in 2009 [5], pointing out a paucity of data and outcomes studies for incidental extracolonic findings in asymptomatic patients for screening. Their final conclusion was that the balance between cost/harms and benefit was unknown. Since this CMS decision, a large body of evidence now exists in the literature, reflecting over a decade of clinical experience.
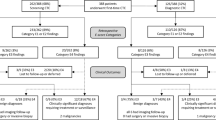
Most recently, USPTF in 2016 did include CT colonography in the list of accepted tests for colorectal screening, with no differences shown between modalities [6]. However, they did continue to state concerns for extracolonic findings. Specifically, the 2016 USPTF evidence report for CRC screening cited 22 original research articles (n=38,293 patients) published from 2000 to 2012 to base its discussion on the extracolonic findings at CTC [7]. These studies ranged in patient cohorts from less than 100 to over 1000 patients, including asymptomatic screening (16 studies) to higher-risk patients. Although some studies were published before or did not incorporate C-RADS, they reported prevalence across a wide range of extracolonic findings, from low clinical significance (E2) to high clinical significance (E4). As such, USPTF stated that extracolonic findings are common, occurring in 27–69% of screening tests, with 5–37% having E3 or E4 findings, 1.4–11% requiring diagnostic follow-up and 3% needing medical or surgical treatment [7]. In summary, the report concluded that extracolonic findings have the potential for both benefit and harm, with potential harms including extra costs, anxiety, and morbidity from additional diagnostic testing or treatment.
Prevalence of extracolonic findings, including cancer and aortic aneurysms
To accurately understand the prevalence of extracolonic findings is important to appreciate the clinical impact. To date, this continues to be debated and greatly misunderstood and misquoted. The prevalence of extracolonic findings varies widely with the type of patient cohort, from screening [2, 8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24] to diagnostic studies [25,26,27,28], and with the type of extracolonic finding, from low to high clinical significance. In symptomatic patients including colon cancer patients with metastatic disease in patient cohorts of 102–111 patients, the prevalence of extracolonic findings has ranged up to 85–89% [26, 27]. In asymptomatic screening cohorts of over 1200 patients, the prevalence of extracolonic findings with high clinical significance has been reported as low as 4.5% [2] (Table 2).
In clinical practice, the reporting of benign extracolonic findings do not lead to additional tests or patient anxiety. These E1 and E2 findings are common with no clinical consequence. These, however, can be included in studies of CT colonography, which falsely elevates prevalence of findings which in reality are dismissed clinically. On the other hand, the incidence of E3 and E4 findings have not only potential clinical significance but also can lead to extra costs due to additional imaging or treatment. As will be later discussed, it is critical, however, to understand that not all of these findings do lead to additional imaging costs, depending on their significance within the clinical context of the patient’s care or whether they represent new vs already known the disease.
An important landmark series to report the rate of E3 and E4 extracolonic findings was in an analysis of 1,410 Medicare-aged population (mean age 75) who underwent screening or surveillance CTC from 2004 to 2009 [21]. In this cohort, 196 patients (13.6%) had E3 findings. Of the patients with E3 findings, most common findings were pulmonary nodules needing follow-up in 24.7%, renal cyst or nodule in 23.4%, vascular aneurysmal atherosclerosis in 12.6%, liver lesion in 5.6%, and gallbladder or biliary abnormality in 5.6%. In addition, 41 patients (2.9%) had E4 findings, with most common findings in these patients of renal mass or cyst in 28.6%, pulmonary nodule needing follow-up in 23.8% and abdominal aortic aneurysm in 19.1%. This article helped to establish that the prevalence of extracolonic findings in Medicare-aged patients for combined E3 and E4 categories of 16.8%, far lower than the upper range of 37% stated by USPTF [7].
Larger series have more recently reported rates of extracolonic findings in average-risk screening cohorts aged 50 years and older in clinical practice. At the University of Wisconsin, the largest screening and research program of CTC in the USA, two publications reported that the combined prevalence of E3 and E4 findings in their clinical practice was 11.7% [23, 24]. This is consistent with prior studies that have reported combined rates of E3 and E4 findings to range from 11 to 16% [13, 17, 20, 22]. In the Wisconsin studies, a cohort of 7952 consecutive patients who underwent first time CTC screening examinations over an eight-year period from 2004 to 2012 were evaluated for E3 and E4 extracolonic findings. In one study, a total of 9.1% (725/7952) had E3 findings; consideration for further imaging was suggested in 84% (608/725) [24]. Of the 660 patients who were able to be followed, 8.3% (55/660) required treatment or follow-up, including eight malignancies (three renal cell carcinomas, three lymphoma, one ovarian adenocarcinoma, and one metastatic breast cancer) (Fig. 3). In the other study of the same cohort, 2.5% (202/7952) of patients had C-RADS category of E4, of which 58% (113/202) of patients underwent further imaging and 44% (89/202) had clinical follow-up [23]. A total of 180/202 were able to be followed, with 68% (123/180) proven to have clinically significant disease including 23% with a malignant or potentially malignant neoplasm and 32% with abdominal aortic or visceral artery aneurysms requiring treatment or surveillance.

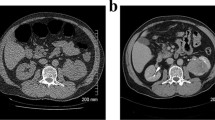
Reproduced with permission from the American Journal of Roentgenology (Pooler et al. [35])
A Incidental discovered adenocarcinoma at right lung base, B Indeterminate ovarian mass (white arrow), which was shown to be a fibroadenoma. Ovarian masses can lead to more additional imaging tests and surgeries.
The detection of abdominal aortic aneurysms deserves specific attention. The overall prevalence of abdominal aortic aneurysms in this asymptomatic cohort of 7952 patients was 0.6% (46 patients), or about 1 in 175 patients [23]. It has been established that screening for abdominal aortic aneurysms can provide high clinical efficacy at a low cost [29, 30]. In one study, screening for aortic aneurysms at age 65 years with ultrasonography resulted in a cost per life years gained under $20,000 [29]. A modeling study of CTC cost-effectiveness in a hypothetical cohort of 100,000 patients simulated the occurrence of colorectal cancer, extracolonic cancer, and abdominal aortic aneurysms [31]. Simulated screening with CTC, using a 6 mm threshold for polyp reporting was compared to optical colonoscopy both with and without ultrasound for aortic imaging. In this simulated population, CTC was the dominant screening strategy, gaining an additional 1458 and 462 life years gained, compared to optical colonoscopy alone and optical colonoscopy with ultrasound, respectively. The additional gains for CTC were largely due to a decrease in abdominal aortic aneurysmal deaths, whereas extracolonic cancer detection was a more minor influence. Specifically, detection of the abdominal aortic aneurysms contributed to 16% of the total life years gained and only 6% of the costs, whereas extracolonic findings contributed to only 2% of the total life years gained but 55% of the costs. A commentary on this article raised concerns that quality of life, including anxiety, was not accounted for and could result in overestimation of the value of aneurysm detection, particularly in patients less than 65 years old [32]. Despite these concerns, the detection of abdominal aortic aneurysms at CTC likely represents one of the most objective benefits in the debate of extracolonic findings.
The incidence of extracolonic cancers at screening CTC has been reported to be greater than the incidence of colorectal cancer. In a large retrospective analysis of 10,286 outpatients (mean age 60 years) undergoing prospective screening CTC from 2004 to 2008 at two institutions (the University of Wisconsin and Bethesda Naval Center), unsuspected neoplasia was found in 0.56% (58 patients) [16]. Colorectal cancer was found in about 1/500 cases (22 patients), whereas extracolonic cancer was found in about 1/300 patients (36 patients). The most common extracolonic cancers were renal cell carcinoma (11 patients), lung cancer (8 patients), and Non-Hodgkin’s lymphoma (6 patients). The clinical extent of disease at diagnosis of all cancers was Stage I or localized in 53.4% (31/58 of the cancers). Regarding treatment, 79.3% (46/58 patients) underwent surgery or endoscopy, 33% (19/58 patients) had chemotherapy, and 13% (7/58 patients) had radiation. Thus, the finding that over half of all unsuspected cancers detected were Stage I in this large series of over 10,000 patients undergoing screening CTC would raise hope that morbidity, mortality, or cost could be reduced with earlier intervention, depending on cancer type.
Additional imaging and costs
In clinical practice, it is critical to discern the difference in the incidence of reporting of extracolonic findings versus the smaller incidence of additional imaging and subsequent costs which results from some of these findings. For example, reporting of many common findings, such as gallstones or kidney stones, which may or may not be already known, typically do not require further testing or intervention in asymptomatic patients. In contrast, extracolonic findings that are new or increased in symptomatic patients can lead to additional studies or procedures for further characterization or treatment.
An important retrospective study which illustrates the issue of radiological additional imaging in CTC was performed as part of a low-dose CT colonography study in both screening examinations and after incomplete colonoscopy in 204 non-senior patients (mean age 52) and 250 senior patients (mean age 69) [19]. In this study, the percentage of patients with at least one reported extracolonic finding was 55.4% in non-seniors and 74.0% in seniors; however, the percentage of patients who were recommended for additional imaging tests based on these findings was 4.4% in non-seniors and 6.0% in seniors. Overall 92% of seniors and 91.8% of non-seniors had extracolonic findings that were of low clinical significance which did not undergo additional examinations. As previously stated, the systematic review by USPTF noted that the work-up rates for extracolonic findings ranged from 1.4 to 11% [7].
Consistent results have been reported in studies evaluating costs for additional imaging. Large screening studies have reported the mean cost for additional imaging associated with extracolonic findings has consistently ranged from $24 to $34 per patient [8,9,10,11, 15]. One study reported costs for both imaging and surgical interventions. Specifically, in a retrospective review of 2195 patients undergoing screening CTC, additional imaging or invasive procedures were performed in 6.1% (133 patients), including ultrasound in 64 patients, CT in 59 patients, MR in 11 patients, and other diagnostic tests in 11 patients [15]. The large number of pelvic ultrasounds in women for adnexal findings (25 patients) accounted for the higher work-up rates in women compared to men. Non-surgical invasive procedures were performed in 19 patients (predominantly the US- and CT-guided biopsies), and invasive surgical procedures were performed in 22 patients. Laparoscopic salpingo-oophorectomy was the most frequent surgical procedure (9 patients). The mean cost per patient for non-surgical procedures was $31.02 and $67.54 for surgical procedures [15].
Debates—pros and cons
Arguments against extracolonic findings include concerns that detection of the unsuspected disease may not be clinically important or patients can suffer harms from anxiety, costs, or complications of additional testing and treatment [32]. Furthermore, untargeted screening of cancers other than colorectal cancer that do not meet public health criteria for screening at a population level has been scrutinized, including lack of evidence that earlier detection and treatment improves patient outcomes [33]. A philosophical argument posed in the early 1970s emphasized that the moral obligation of screening is different than everyday patient care [34]. Namely when a patient seeks out medical care for a symptom, the doctor does the best they can to diagnose and treat the patient, recognizing that medical knowledge is not perfect. However, when a patient undergoes screening, there is a very different assumption that conclusive evidence is present that screening for a given disease can alter the natural history of the disease in the majority of patients being screened. So although screening for abdominal aortic aneurysms in high-risk cardiovascular patients likely has an added benefit, detection of other unsuspected diseases has less certain outcomes and yet is difficult to ignore once found [32].
What are the pros to offset these criticisms? As some have asked, is the glass half empty or half full? One important but often overlooked benefit of CTC is that close to 90% of patients (namely the 84–89% of patients with E1 and E2 findings) have no clinically significant extracolonic findings, with no additional tests needed. No other colorectal screening exam can comprehensively evaluate the entire abdomen and pelvis and provide the reassurance to exclude other diseases [35]. Of the remaining 11–16% of patients with E3 and E4 findings [13, 17, 20, 22,23,24], additional imaging averages 6% [15], with reasonable additional imaging costs averaging about $30 per patient [8,9,10,11, 15]. A total of 0.6% of patients will have an abdominal aortic aneurysm [23], shown to improve outcomes in 65-year-olds [29]. In addition, 0.35% will have an extracolonic cancer of which just over 50% will be Stage I [16]. Although we know that lung cancer detection in high-risk cohorts has met strict criteria for screening [36], early detection of lung cancer in average-risk patients, and other early-stage cancers may also improve patient outcomes on a case by case basis. It is important to recognize that just under 50% of remaining cancers found, based on later stage or cancer type such as pancreatic or ovarian cancer [16], may not improve outcomes and could increase costs for the subset that undergo treatment; however, this represents less than 0.18% of screening patients. To model cost savings for early-stage disease vs cost burdens for later stage disease is difficult, but these patients represent less than 0.5% of the screening population. Lastly, as will be discussed below, quality metrics that track extracolonic finding rates can provide a safeguard and give important feedback for quality improvement.
Standardization and quality assurance
Like all of the radiology, standardization of terminology can help improve quality of reports and clarify management to follow. Use of the C-RADS reporting structure, including the E categories for extracolonic findings, is very helpful in both clinical practice and reporting study results.
In addition to C-RADS, the ACR incidental findings committee has published several comprehensive white papers regarding the management of incidental findings at abdominal CT [37,38,39,40,41]. In 2010, the first manuscript discussed how to report incidental findings in the liver, pancreas, kidneys, and adrenal glands. Important algorithms were outlined of how to follow or ignore common incidental findings based on size and morphology in both the general population and patients with limited life expectancy and/or comorbidity [37]. Since its publication, several other articles have been produced across other organ systems, including adnexal [38], splenic and nodal [39], and gallbladder and biliary [40] systems. One additional topic discussed was the importance of reducing recommendations for additional imaging by a comprehensive review of prior imaging, which certainly is very relevant to CTC [41]. The results of these efforts have been promoted at national meetings in radiology, such as the Society of Computed Body Tomography and Magnetic Resonance (SCBT/MR), Society of Abdominal Imaging (SAR), and Radiological Society of North America (RSNA).
In 2008, the National Radiology Diagnostic Registry (NRDR) at the American College of Radiology established quality metrics for CT colonography [42]. These included both process measures of adequacy of exam quality of bowel preparation, insufflation, and low-dose protocol, along with outcomes measures of positive predictive value of large polyps, rate of perforation, and significant extracolonic findings that require additional work-up. In 2017, NRDR has been approved as a Qualified Clinical Data Registry (QCDR) for the CMS Merit-based Incentive Payment System (MIPS) [42]. This platform will allow continual monitoring and feedback to participating centers to help monitor quality improvement.
Part II: low-radiation dose CT colonography
As computed tomography (CT) scanner technology has improved, there has been an increase in the use of CT for various clinical applications. Concurrently there has been an increase in awareness of the exposure to ionizing radiation from CT. It is important that referring physicians understand that multiple methods are now available to imaging specialists to help achieve the goal of acceptable image quality while decreasing radiation dose to patients. This is particularly important for CT colonography (CTC) which is recommended at repeated intervals for colorectal cancer screening at every five years by the American Cancer Society, the US Multi-Society Task Force, and the American College of Radiology [3]. Over the past 5–10 years, there has been concerted and committed efforts from professional organizations, radiologists, providers of medical imaging services, and CT scanner manufacturers to work together to reduce radiation dose to patients according to the As Low As Reasonable Achievable (ALARA) principle.
History
The risks associated with radiation exposure from medical imaging are theoretic, although it is generally agreed that high levels of radiation exposure typically above what is associated with most imaging tests are carcinogenic through a stochastic effect. A high dose of radiation can also cause cataracts and tissue damage such as skin burns. Various states have instituted regulations such as California though Senate Bill 1237 requiring that estimated CT dose be included in the patient report [43]. This legislation has helped to increase dissemination of dose monitoring programs throughout the imaging community.
The linear, no-threshold (LNT) theory for estimating health effects from radiation exposure is heavily debated since it assumes that even very small amounts of radiation dose can induce malignancy. This model proposes that harmful health effects occur in proportion to the amount of radiation received. However, no harmful effects have been identified or proven for low-radiation dose imaging tests, but rather are theoretic and extrapolated from the effects of high-level radiation exposure. The typical effective radiation dose of CT colonography is low and ranges from ≤ 3 to 6 mSv [44] (Table 3). Of note, the Health Physics Society (HPS) which specializes in radiation protection updated their position statement in 2016 to state that “below levels of about 100 mSv above background from all sources combined, the observed radiation effects in people are not statistically different from zero” [45]. Additionally, the HPS also states that “the LNT hypothesis cannot provide reliable projections of future cancer incidence from low-level radiation” [45]. The 2017 American Association of Physicists in Medicine (AAPM) updated policy states that “there is no convincing epidemiological evidence of increased cancer incidence or mortality from low radiation doses (< 100 mSv).” This dose is 15–30 times higher than the dose range for CTC. The AAPM also states that “the AAPM discourages describing potential risks associated with medical imaging using predictions of hypothetical cancer incidence and deaths. These predictions are contrary to directives of radiation protection organizations, are highly speculative and can lead to sensationalistic coverage in the public media, leading some patients to fear or refuse appropriate medical imaging” [46]. In a study by Berrington de González et al., the benefit to risk ratio of the possible lives saved using CTC to the possible deaths caused by radiation-induced cancers from CTC was calculated [47]. Any possible radiation risk from additional imaging of extracolonic findings identified on CTC was included in the calculation. All of the microsimulation models used identified a large benefit to risk ratio in favor of screening CTC, ranging from 24:1 to 35:1.
In 2010, major radiology organizations including the American College of Radiology (ACR), the Radiological Society of North America (RSNA), the American Society of Radiologic Technologists (ASRT), and the American Association of Physicists in Medicine (AAPM) developed a collaborative initiative called Image Wisely to encourage and aid in radiation safety in imaging, particularly for CT imaging [48]. This initiative parallels the successful Image Gently campaign which helps to raise awareness of the opportunities to lower radiation dose in imaging children [49].
How is radiation dose measured?
Many sites include measurements of radiation dose in CT reports which are mandated in some states. It is therefore important that referring providers understand the metrics employed to measure radiation dose and help to manage cumulative exposure. CT scanner dosimetry is currently performed using two main measurements: the volumetric CT dose index (CTDIvol) and the dose-length product (DLP). These measurements display CT scanner output and provide estimates of the amount of radiation that the patient is exposed to, but do not represent the actual amount of radiation absorbed by the patient. The CTDIvol is presented in units of milligray (mGy) and represents the dose within a scanned slice using a specific CT protocol performed on a phantom [50]. The DLP is presented in units of milligray-centimeter (mGy-cm) and is equivalent to the CTDIvol multiplied by the scan length. The DLP quantifies the amount of radiation exposure for the irradiated scan length [51].
CT colonography requires scanning in two opposing positions, typically in supine and prone. Alternatively, patients with large body mass index (BMI) ≥ 30 may be scanned in the right and left lateral decubitus positions. Therefore, the CTC dose report usually includes two series each with CTDIvol and DLP. Individual DLPs are added together and then converted to an effective dose presented in mSv. The total DLP is multiplied by a conversion factor (k) which is determined by the patient size and the part of the body scanned. Since CTC occurs in the abdomen and pelvis, a conversion factor of 0.015 is employed to obtain the effective dose (Fig. 4) [52]. Flicek et al. were able to reduce radiation dose for CTC by using an effective tube current of 50 mAs in the supine position and decreasing the effective tube current to 25 mAs in the prone position. This strategy results in a low CTC effective dose of about 3 mSv or less which is at least 50% less than previously reported CTC dosage [53]. An effective dose of 3 mSv is also equivalent to the annual background dose that each of us is typically exposed to.
Methods to decrease CT colonography radiation dose
There are multiple strategies currently available to assist in lowering CT radiation dose. Referring providers should assure that there is clear indication for performing CTC. The joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology include CT colonography as an indicated test for colorectal cancer screening every 5 years and is an exam that can detect both the malignancy and the precursor polyp [3]. According to the joint practice parameters from the American College of Radiology, the Society of Abdominal Radiology, and the Society of Computed Body Tomography and Magnetic Resonance, indications for CTC include colorectal cancer screening, surveillance, and diagnosis [54]. CTC may also be performed following incomplete colonoscopy and for characterization of colorectal lesions that are indeterminate on optical colonoscopy. CTC is indicated for patients who may be at increased risk for complications during optical colonoscopy (e.g., advanced age, anticoagulant therapy, sedation risk, and prior incomplete colonoscopy) and to follow up patients with a colonic stoma or after colectomy. CTC may also be performed prior to laparoscopic surgery for colorectal cancer in order to accurately localize the tumor or to search for synchronous lesions.
Communication between referring physicians and radiologists can help assure that as many of these strategies are employed as possible when sending patients to imaging centers performing CT colonography. Appropriate centering of the patient in the middle of the scanner gantry is relatively simple to implement to lower radiation dose. Decreasing tube current and/or voltage and use of automatic dose modulation can help lower radiation dose significantly. Iterative reconstruction techniques should be incorporated in CT colonography protocols whenever possible to maximize radiation dose reduction.
Positioning the patient close to the center of the CT gantry helps to optimize image quality and permits the best use of automatic dose modulation. If a patient is not centered correctly on the scanner table, this can result in an unnecessary increase in radiation dose. Habibzadeh et al. found that even a small amount of miscentering of 2.2 cm on a 64-slice CT scanner resulted in an increase of approximately 23% in patient dose with images that were slightly noisier [55]. Proper training of technologists in the use of laser guides is needed in order to help correctly position the patient in the isocenter of the scanner gantry.
Lowering tube current is one of the most common techniques employed to reduce radiation dose. There is a linear correlation between the tube milliampere-seconds (mAs) and radiation dose so that as the mAs is reduced, there is a concurrent proportionate decrease in radiation dose [56]. Typically, low-dose CTC protocols employ effective mAs of 25 to 50. However, image noise increases significantly as the mAs decreases. We are fortunate since detection of intraluminal masses depends on the high contrast between the soft tissue density of polyps and the gas density of carbon dioxide or room air in the lumen. Vogt et al. evaluated 115 patients with 150 colorectal lesions using an ultra-low-dose CTC protocol with 10 mAs [57]. Results showed that the sensitivity and the specificity for detection of polyps 5 mm or larger were 94% and 84%, respectively. For adenomatous lesions larger than 5 mm, sensitivity was 94%, and specificity was 92%. The calculated effective radiation dose was very low and ranged between 0.75 and 1.25 mSv. In a study by Iannaccone et al., 88 patients underwent ultra-low-dose CTC with 10 mAs and sensitivities for detection of polyps 6 mm in diameter or larger were comparable for CT colonography and initial colonoscopy at 86 and 84%, respectively [58]. Ginsburg et al. evaluated 96 patients using 15 or 30 mAs on CTC and found that radiation dose could be reduced by 40 and 70% for overweight and normal body mass index (BMI)-patients, respectively [59]. With large reductions in mAs, three-dimensional images may contain artifacts or minor wall irregularities which may be distracting although smoothing filters can be applied. The use of low mAs technique combined with iterative reconstruction has allowed for improved image quality with significant dose reduction. Readers of low-dose CTC scans will find that extracolonic findings may be more difficult to identify or characterize although this may be offset by using thicker slices or iterative reconstruction.
Additional dose reduction may be achieved with the use of automatic dose modulation. Automatic tube current modulation is a technique where the user determines the preferred image quality, and then the scanner automatically selects the mAs. The tube current is then adjusted during the scan according to the size and density of the part of the body examined also taking into consideration the scan plane. Transverse or longitudinal modulation is determined by referring to the scout images. Both types of modulation may be combined with an angular modulation into a three-dimensional automatic dose modulation which is also termed automatic exposure control (AEC) [60]. The majority of body CT scans is performed currently with the use of AEC. Noisier images are acceptable for many CTC readers given the focus on the high contrast soft tissue–gas interface allowing the ability for significantly higher dose savings than in most other CT exams.
There is increasing use of tube voltage reduction as an effective method to decrease radiation dose for CTC. An exponential correlation occurs between tube voltage and radiation dose so that as peak kilovoltage (kVp) decreases, there is an approximate dose reduction multiplied by a power of 2.6 [61]. Although lower kVp images are noisier, there is a relative increase in the attenuation of residual material tagged with oral contrast on CTC since imaging occurs closer to the K-edge of iodine. This means that readers are still able to identify soft tissue polyps located within tagged fluid pools on low kVp CTC images. Iterative reconstruction may be used in conjunction with low kVp images to decrease image noise allowing for additional dose reduction. Patients with a large body habitus may not be able to undergo low kVp CTC since there will be inadequate penetration by low-energy photons and suboptimal image quality [62]. Chang et al. studied 63 patients who underwent supine (120 kVp) and prone (100 kVp) CTC [63]. A reduction from 120 to 100 kVp resulted in a 20% decrease in CDI vol and a 16% decrease in DLP. Image noise increased by 32%, but there was only a slight decrease in three-dimensional image qualities. In a phantom study by Shin et al., an even lower tube voltage of 80 kVp was employed resulting in a very low effective radiation dose on only 0.166 mSv [64]. However, there was a significant increase in image noise and a decrease in polyp detection sensitivity. Excellent polyp detection sensitivity was achieved by using iterative reconstruction with the 80 kVp scans which were similar to 100 or 120 kVp scans. It is expected that there will be continued dose reduction for clinical CTC examinations to sub-mSv levels.
Until recently filtered back projection (FBP) was the primary reconstruction algorithm used for CT images. However, limitations of FBP include noise and artifacts, and with current increased computing power, iterative reconstruction is now used. Typically, iterative algorithms are employed in conjunction with FBP and allow decreased image noise on low-radiation dose CT images. All major CT manufacturers offer versions of iterative reconstruction and the latest CTC protocols typically employ a blend of FBP and iterative reconstruction [65]. Flicek et al. performed a study evaluating 18 patients undergoing low-radiation dose CTC using 50 mAs in the supine position and 25 mAs combined with 40% adaptive statistical iterative reconstruction (ASIR) in the prone position [53]. This pilot study showed that CTC radiation dose could be decreased by 50% below previously achieved levels without any significant compromise of image quality when ASIR is used. Similarly in a study of 30 patients by Fletcher et al., comparable image quality for colonic evaluation on CTC was achieved for full-dose and half-dose images reconstructed using iterative reconstruction [66]. A larger study by Nagata et al. evaluated 210 patients and found that supine CTC dose was 1.88 mSv compared to < 0.92 mSv for low-dose prone CTC with comparable image quality for supine FBP images and prone images using iterative reconstruction [67]. This study showed that dose could be lowered even more to up to about 75% without significant degradation of image quality. Other studies have corroborated the finding that significant dose reduction of greater than 50% can currently be achieved for CTC particularly when employing model-based iterative reconstruction (MBIR) without compromise of image quality. (Figs. 5, 6) [68,69,70]. MBIR is more computationally intensive and requires longer reconstruction times although it allows greater noise reduction than other iterative reconstruction algorithms.
Sessile polyp on low-radiation dose CT colonography easily identified on filtered back projection and iterative reconstruction images. Axial 2D (A–C) and 3D (D–F) images of a small sessile polyp (white arrow). Axial 2D and correlative 3D images using filtered back projection (A, D) 40% adaptive statistical iterative reconstruction (B, E), and model-based iterative reconstruction (C, F)
Flat lesion detectable on low-radiation dose CT colonography-axial 2D (A–C) and 3D (D–F) images of a small flat polyp (white arrow). Axial 2D and correlative 3D images using filtered back projection (A, D) 40% adaptive statistical iterative reconstruction (B, E), and model-based iterative reconstruction (C, F)
Conclusions
Although the vast majority of extracolonic findings in screening cohorts are benign and can be ignored, radiologists need to be responsible in reporting extracolonic findings so that the potential benefits of early detection of significant disease offset the additional costs incurred.
Referring providers must be knowledgeable about the theoretic risks and controversies regarding the use of ionizing radiation. As a screening test, CT colonography imparts a low level of radiation to patients. Using currently available techniques, CTC effective dose may be significantly minimized according to the As Low As Reasonable Achievable (ALARA) principle to a level that is equivalent to the annual background dose of 3 mSv or less.
References
Zalis ME, Barish MA, Choi JR, et al. (2005) CT colonography reporting and data system: a consensus proposal. Radiology 236:3–9
Pickhardt PJ, Choi JR, Hwng I, et al. (2003) Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med 349:2191–2200
Levin B, Lieberman DA, McFarland B, et al. (2008) Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 58:130–160
US Preventative Task Force (2008) Screening for colorectal cancer: US Preventative Services Task Force Recommendation Statement. Ann Intern Med 149:627–637
US Preventative Services Task Force (2016) Screening for colorectal cancer–US Preventative Services Task Force Recommendation Statement. JAMA 2016:2564–2575
Lin JS, Piper M, Perdue LA, et al. (2016) Screening for colorectal cancer: updated evidence report and systemic review for the US preventive services task force. JAMA 315:2576–2594
Hara AK, Johnson CD, MacCarty RL, Welch TJ (2000) Incidental extracolonic findings at CT colonography. Radiology 215:353–357
Gluecker TM, Johnson CD, Wilson LA, et al. (2003) Extracolonic findings at CT colonography: evaluation of prevalence and cost in a screening population. Gastroenterology 124:911–916
Chin M, Mendelson R, Edwards J, Foster N, Forbes G (2005) Computed tomographic colonography: prevalence, nature, and clinical significance of extracolonic findings in a community screening program. Am J Gastroenterol 100:2771–2776
Yee J, Kumar NN, Godara S, et al. (2005) Extracolonic abnormalities discovered incidentally at CT colonography in a male population. Radiology 236:519–526
Kim DH, Pickhardt PJ, Taylor AJ, et al. (2007) CT colonography versus colonoscopy for the detection of advanced neoplasia. N Engl J Med. 357(14):1403–1412
Flicker MS, Tsoukas AT, Hazra A, Dachman AH (2008) Economic impact of extracolonic findings at computed tomographic colonography. J Comput Assist Tomogr 32:497–503
Johnson CD, Chen MH, Toledano AY, et al. (2008) The national CT colonography trial: multicenter assessment of accuracy for detection of large adenomas and cancers. N Engl J Med 359:1207–1217
Pickhardt PJ, Hanson ME, Vanness DJ, et al. (2008) Unsuspected extracolonic findings at screening CT colonography: clinical and economic impact. Radiology. 249:151–159
Pickhardt PJ, Kim DH, Meiners RJ, et al. (2010) Colorectal and extracolonic cancers detected at screening CT colonography in 10,286 asymptomatic adults. Radiology 255(1):83–88
Veerappan GR, Ally MR, Choi JH, et al. (2010) Extracolonic findings on CT colonography increases yield of colorectal cancer screening. AJR 195:677–686
Kim DH, Pickhardt PJ, Hanson ME, Hinshaw JL (2010) CT colonography: performance and program outcome measures in an older screening population. Radiology. 254(2):493–500
Macari M, Nevsky G, Bonavita J, et al. (2011) CT colonography in senior versus nonsenior patients: extracolonic findings, recommendations for additional imaging, and polyp prevalence. Radiology 259(3):767–774
Zalis ME, Blake MA, Cai W, et al. (2012) Diagnostic accuracy of laxative-free computed tomographic colonography for detection of adenomatous polyps in asymptomatic adults: a prospective evaluation. Ann Intern Med 156:692–702
Cash BD, Riddle M, et al. (2012) Observed outcomes with computerized tomographic colonography in a Medicare-aged screening population: an analysis of over 1,400 patients. AJR 199:W27–W34
Stoop EM, de Haan MC, de Wijkerslooth TR, et al. (2012) Participation and yield of colonoscopy vs non-cathartic CT colonography in population-based screening for colorectal cancer: a randomized controlled trial. Lancet Oncol 13:55–64
Pooler BD, Kim DH, Pickhardt PJ (2016) Potentially important extracolonic findings at screening CT colonography: incidence and outcomes data from a clinical screening program. AJR 206:313–318
Pooler BD, Kim DH, Pickhardt PJ (2016) Indeterminate but likely unimportant extracolonic findings at screening CT colonography (C-RADS category E3): incidence and outcomes data from a clinical screening program. AJR 207:996–1001
Khan KY, Xiong T, McCafferty I, et al. (2007) Frequency and impact of extracolonic findings detected at computed tomographic colonography in a symptomatic patient. Br J Radiol 94:355–361
Spreng A, Netzer P, Mattich J, et al. (2005) Importance of extracolonic findings at IV contrast medium-enhanced CT colonography versus those at non-enhanced CT colonography. Eur Radiol 15:2088–2095
Hellstrom M, Svensson MH, Lasson A (2004) Extracolonic and incidental findings on CT colonography (virtual colonoscopy). AJR 182:631–638
Edwards JT, Wood CJ, Mendelson RM, Forbes GM (2001) Extracolonic findings at virtual colonoscopy: implications for screening programs. Am J Gastroenterol 96:3009–3012
Wonhainen A, Lundkvist J, Bergqvist D, Bjorck M (2005) Cost-effectiveness of different screening strategies for abdominal aortic aneurysms. J Vasc Surg 41:741–751
Wilmink AB, Quick CR, Hubbard CS, Day NE (2003) Effectiveness and cost of screening for abdominal aortic aneurysm-results of a population screening program. J Vasc Surg 38:72–77
Hassan C, Pickhardt PJ, Laghi A, et al. (2008) Computed tomographic colonography to screen for colorectal cancer, extracolonic cancer, and aortic aneurysm: model simulation with cost-effectiveness analysis. Arch Intern Med. 168(7):696–705
Fletcher RH, Pignone M (2008) Extracolonic findings with computed tomographic colonography, asset or liability? Arch Intern Med 168:685–686
Harris RP, Helfand M, Woolf SH, et al. (2001) Methods Working Group; third US Preventive Services Task Force. Current methods for the US Preventative Services Task Force-a review of the process. Am J Prev Med 20:21–35
Cochrane AL, Holland WW (1971) Validation of screening procedures. Br Med Bull 27:3–8
Pooler DB, Kim DH, Pickhardt PJ (2017) Extracolonic findings at screening CT colonography: prevalence, benefits, challenges and opportunities. AJR 209:1–9
Chin J, Syrek Jensen T, Ashby L, et al. (2015) Screening for lung cancer with low dose CT-translating science into Medicare coverage policy. NEJM 372:2083–2085
Berland LL, Silverman SG, Gore RM, et al. (2010) Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol 7:754–773
Patel MD, Ascher SM, Paspulati RM, et al. (2013) Managing incidental findings on abdominal and pelvic CT and MRI, part 1: white paper of the ACR incidental findings committee II on adnexal findings. J Am Coll Radiol 10:675–681
Heller MT, Harisinghani M, Neitlich JD, Yeghiayan P, Berland LL (2013) Managing incidental findings on abdominal and pelvic CT and MRI, part 3: white paper of the ACR incidental findings committee II on splenic and nodal findings. J Am Coll Radiol 10:833–839
Sebastian S, Araujo C, Neitlich JD, Berland LL (2013) Managing incidental findings on abdominal and pelvic CT and MRI, part 4: white paper of the ACR incidental findings committee II on gallbladder and biliary findings. J Am Coll Radiol 10:953–956
Doshi AM, Kiritsy M, Rosenkrantz AB (2015) Strategies for avoiding recommendations for additional imaging through a comprehensive comparison with prior studies. J Am Coll Radiol 12:657–663
https://www.acr.org/Quality-Safety/National-Radiology-Data-Registry/CT-Colonography/Registry
Radiation Control: Health Facilities and Clinics, SB1237, February 19, 2010. http://www.leginfo.ca.gov/pub/09-10/bill/sen/sb_12011250/sb1237bill_20100929chaptered.html.
Radiation Dose in X-Ray and CT Exams. Effective radiation dose in adults. https://www.radiologyinfo.org/en/info.cfm?pg=safety-xray.
Health Physics Society (2016) Radiation risk in perspective. Position Statement of the Health Physics Society. http://hps.org/documents/risk_ps010-3.pdf.
American Association of Physicists in Medicine (2017) Position statement on radiation risks from medical imaging procedures. http://www.aapm.org/org/policies/details.asp?id=318&type=PP¤t=true.
Berrington de González A, Kim KP, Knudsen AB, et al. (2011) Radiation-related cancer risks from CT colonography screening: a risk-benefit analysis. AJR Am J Roentgenol 196:816–823
Brink JA, Amis ES (2010) Image wisely: a campaign to increase awareness about adult radiation protection. Radiology 257:601–602
Applegate K, Frush DP (2017) Image gently: a decade of international collaborations to promote appropriate imaging for children. J Am Coll Radiol 14:956–957
McCollough CH, Leng S, Yu L, et al. (2011) CT dose index and patient dose: they are not the same thing. Radiology 259:311–316
Thakur Y, McLaughlin PD, Mayo JR (2013) Strategies for radiation dose optimization. Curr Radiol Rep 1:1–10
AAPM Report No. 96: the measurement, reporting, and management of radiation dose in CT. (2008). https://www.aapm.org/pubs/reports/RPT_96.pdf.
Flicek KT, Hara AK, Silva AC, et al. (2010) Reducing the radiation dose for CT colonography using adaptive statistical iterative reconstruction: a pilot study. AJR 195:126–131
ACR-SAR-SCBT-MR practice parameter for the performance of computed tomography (CT) colonography in adults. https://www.acr.org/~/media/ACR/Documents/PGTS/guidelines/CT_Colonography.pdf.
Habibzadeh MA, Ay MR, Asl AR, Ghadiri H, Zaidi H (2012) Impact of miscentering on patient dose and image noise in x-ray CT imaging: phantom and clinical studies. Phys Med 28:191–199
Chang KJ, Yee J (2013) Dose reduction methods for CT colonography. Abdom Imaging 38:224–232
Vogt C, Cohnen M, Beck A, et al. (2004) Detection of colorectal polyps by multislice CT colonography with ultra-low-dose technique: comparison with high-resolution videocolonoscopy. Gastrointest Endosc 60:201–209
Iannaccone R, Catalano C, Mangiapane F, et al. (2005) Colorectal polyps: detection with low-dose multidetector row helical CT colonography versus two sequential colonoscopies. Radiology 237:927–937
Ginsburg M, Obara P, Wise L, et al. (2013) BMI-based radiation dose reduction in CT colonography. Acad Radiol 20:486–492
McMillan K, Bostani M, Cagnon CH, et al. (2017) Estimating patient dose from CT exams that use automatic exposure control: development and validation of methods to accurately estimate tube current values. Med Phys 44:4262–4275
Elojeimy S, Tipnis S, Huda W (2010) Relationship between radiographic techniques (kilovolt and milliampere-second) and CTDI(VOL). Radiat Prot Dosimetry 141(1):43–49
Seyal AR, Arslanoglu A, Abboud SF, et al. (2015) CT of the abdomen with reduced tube voltage in adults: a practical approach. Radiographics 35:1922–1939
Chang KJ, Caovan DB, Grand DJ, et al. (2013) Reducing radiation dose at CT colonography: decreasing tube voltage to 100 kVp. Radiology 266:791–800
Shin CI, Kim SH, Lee ES, et al. (2014) Ultra-low peak voltage CT colonography: effect of iterative reconstruction algorithms on performance of radiologists who use anthropomorphic colonic phantoms. Radiology 273:759–771
Beister M, Kolditz D, Kalender WA (2012) Iterative reconstruction methods in X-ray CT. Phys Med 28:94–108
Fletcher JG, Grant KL, Fidler JL, et al. (2012) Validation of dual-source single-tube reconstruction as a method to obtain half-dose images to evaluate radiation dose and noise reduction: phantom and human assessment using CT colonography and sinogram-affirmed iterative reconstruction (SAFIRE). J Comput Assist Tomogr 36:560–569
Nagata K, Fujiwara M, Kanazawa H, et al. (2015) Evaluation of dose reduction and image quality in CT colonography: comparison of low-dose CT with iterative reconstruction and routine-dose CT with filtered back projection. Eur Radiol 25:221–229
Millerd PJ, Paden RG, Lund JT, et al. (2015) Reducing the radiation dose for computed tomography colonography using model-based iterative reconstruction. Abdom Imaging 40:1183–1189
Lambert L, Ourednicek P, Jahoda J, et al. (1048) Model-based vs hybrid iterative reconstruction technique in ultralow-dose submillisievert CT colonography. Br J Radiol 2015(88):20140667
Lubner MG, Pooler BD, Kitchin DR, et al. (2015) Sub-milliSievert (sub-mSv) CT colonography: a prospective comparison of image quality and polyp conspicuity at reduced-dose versus standard-dose imaging. Eur Radiol 25:2089–2102
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was provided for this manuscript.
Conflict of interest
Judy Yee, MD, FACR has received research Grants from Echopixel and Philips. Elizabeth McFarland, MD is a member of the Medical Advisory Board for Vital Images.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Yee, J., McFarland, E. Extracolonic findings and radiation at CT colonography: what the referring provider needs to know. Abdom Radiol 43, 554–565 (2018). https://doi.org/10.1007/s00261-018-1461-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-018-1461-z