Abstract
Purpose
To investigate whether multiphasic MDCT enhancement can help differentiate type 1 papillary renal cell carcinoma (RCC) from type 2 papillary RCC.
Methods
With IRB approval for this HIPAA-compliant retrospective study, we derived a cohort of 36 type 1 papillary RCCs and 33 type 2 papillary RCCs with preoperative multiphasic MDCT with up to four phases (unenhanced, corticomedullary, nephrographic, and excretory) from 2000 to 2013. Following segmentation, a computer-assisted detection (CAD) algorithm selected a 0.5 cm-diameter region of maximal attenuation within each lesion in each phase; a 0.5 cm-diameter region of interest was manually placed on uninvolved renal cortex in each phase. The relative attenuation of each lesion was calculated as [(Lesion attenuation−cortex attenuation)/cortex attenuation] × 100. Absolute and relative attenuation values were compared using Mann–Whitney tests with Bonferroni correction for multiple comparisons.
Results
Relative excretory phase attenuation of type 2 papillary RCCs was significantly greater than that of type 1 papillary RCCs (2.0 vs. −18.3, p = 0.005). Relative excretory phase attenuation differentiated type 1 papillary RCCs from type 2 papillary RCCs with an accuracy of 73% (36/49), sensitivity of 87% (26/30), positive predictive value of 74% (26/35), and negative predictive value of 71% (10/14).
Conclusion
Multiphasic MDCT enhancement may assist in differentiating type 1 papillary RCCs from type 2 papillary RCCs, if prospectively validated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Within the Vancouver classification system, the most common subtype of renal cell carcinoma (RCC) is clear cell, accounting for 70%–80% of cases of RCC [1, 2]. Papillary RCC is the second most common subtype, accounting for 14%–17% of RCCs, followed by chromophobe RCC, which accounts for 4%–8% of RCCs [1, 2]. Each subtype portends a differing prognosis. Clear cell RCC is the most lethal with a 5-year survival rate of 44%–69% [3–7]. Chromophobe RCC has a 5-year survival rate of 78%–87%, and papillary RCC has a 5-year survival rate of 82%–92% [3–7]. However, among papillary RCCs, there are two distinct histopathologic entities, each with characteristic genetic abnormalities and distinct patterns of behavior [8]. Type 1 papillary RCCs are characterized histologically by papillae containing small cells with basophilic cytoplasm and uniform, small, oval-shaped nuclei [8]. On the other hand, type 2 papillary RCCs are characterized histologically by papillae containing large cells with eosinophilic cytoplasm, large, spherical-shaped nuclei, and prominent nucleoli [8]. While type 1 papillary RCCs have a 5-year survival rate of 95%, type 2 papillary RCCs are more aggressive with a 5-year survival rate of 66% that is more comparable to clear cell RCC [9].
Several studies have shown that enhancement on multiphasic multidetector CT (MDCT) can help discriminate papillary RCC from other RCC subtypes (clear cell and chromophobe) and oncocytoma (a benign mimic of RCC) [10–16]. After the administration of intravenous contrast, the majority of papillary RCCs peak enhance in the nephrographic phase, while clear cell RCCs, chromophobe RCCs, and oncocytomas typically exhibit peak enhancement in the corticomedullary phase [11]. While a few studies have attempted to identify imaging features to predict the histopathologic subtype of papillary RCCs [17–19], there are currently no published robust imaging biomarkers to differentiate type 1 papillary RCCs from type 2 papillary RCCs. Because of their differing prognoses, a simple, non-invasive means of differentiating type 1 papillary RCCs from type 2 papillary RCCs can help guide further management. The goal of this study was to investigate whether multiphasic MDCT enhancement can help differentiate type 1 papillary RCCs from type 2 papillary RCCs.
Materials and methods
Patients
With Institutional Review Board approval for this HIPAA-compliant study and a waiver of informed consent, we queried our institution’s pathology database to derive all histologically proven cases of type 1 and type 2 papillary RCC during a 13-year period, from January 2000 to May 2013. Approximately 700 histologically proven cases of type 1 and type 2 papillary RCC were identified. Of these cases, all cases of type 1 and type 2 papillary RCC with preoperative multiphasic MDCT (consisting of 2-phase, 3-phase, and 4-phase studies) were included. Approximately 630 cases were excluded for lacking preoperative multiphasic MDCT. This resulted in a cohort of 36 type 1 and 33 type 2 papillary RCCs. The total cohort was composed of 69 consecutive lesions, representing 64 unique patients. Of the 64 patients in our cohort, five patients had two lesions each. One of these five patients had one unique type 1 papillary RCC and one unique type 2 papillary RCC. Two patients had two type 1 papillary RCCs; two patients had two type 2 papillary RCCs. The mean time from MDCT imaging to nephrectomy or biopsy was 67 days. The mean age at the time of diagnosis was 64 years; 84% of the patients were men, and 16% were women (Table 1). The T stage of each lesion was obtained by reviewing pathology reports for the resected specimens.
CT examination
All CT examinations were performed at our institution using multidetector row CT [mainly 64 (2005–present) or 16-detector (2003–2008) row helical scanners (Sensation 64 or Sensation 16, Siemens Medical Solutions, Erlangen, Germany); or 4-detector (2000–2003) scanner (Lightspeed Qx/i, GE Medical Systems, Milwaukee, WI)]. Images were obtained with the patient breath holding with the following parameters: 120 kVP, variable mA, section thickness interval of 3–5 mm depending on the protocol used. Protocols differed depending upon the type of examination ordered. During 4-phase studies, patients underwent an unenhanced scan of the abdomen from the top of the diaphragm to the iliac crest. Then, following the injection of 100–150 ml of non-ionic intravenous iohexol (Omnipaque 350, GE Healthcare, Milwaukee, WI) based upon patient weight with a power injector at 3 ml per second, a bolus tracking algorithm (CareBolus, Siemens Medical Solutions; SmartPrep, GE Medical Systems) was utilized to determine the onset of imaging of the corticomedullary, nephrographic, and excretory phases. A region of interest was placed in the thoracoabdominal aorta junction with a trigger set to begin at 150 HU. Corticomedullary, nephrographic, and excretory phase imaging occurred 40 s, 90 s, and 8 min after the threshold level of 150 HU was reached. For 2-phase and 3-phase studies, in addition to an unenhanced scan, time delay images were obtained at varied combinations of corticomedullary, nephrographic, and excretory phases.
CT image analyses
Based upon the appearance of normal kidney parenchyma, images were classified as unenhanced if contrast was absent, corticomedullary if the renal cortex (but not the medulla) enhanced in a ribbon-like pattern, nephrographic if the cortex and medulla enhanced in a uniform manner, or excretory if the concentrated contrast material was excreted in the renal pelvis and ureters. Images were sent to our picture archiving and communications system (Centricity, GE Medical Systems, Milwaukee, WI) for interpretation.
Each lesion was segmented in its entirety on axial images in each phase using proprietary non-commercial Food and Drug Administration (FDA) 510(k) cleared software. Lesion contours were drawn slightly internal to the lesion’s outer margin to avoid partial volume averaging artifact [20]. Each contour was reviewed by two abdominal fellowship-trained genitourinary radiologists with 9 and 17 years of experience, who were blinded to clinical and histopathologic findings. If there was any discordance between the two readers regarding the appropriate placement of a contour, the case was discussed by both readers until a consensus was reached. Using the resultant whole lesion contours, a computer-assisted detection (CAD) algorithm then selected a 0.5 cm-diameter circular region of maximal attenuation within each lesion in each phase, excluding pixels with attenuation greater than 300 Hounsfield units (HU) to exclude calcifications. Each CAD-selected region of maximal attenuation was reviewed by each reader for appropriate placement. A small region of maximal attenuation was selected because it has been shown that small regions of interest are best for renal lesion characterization [20]. For each lesion, a 0.5 cm-diameter region of interest was manually placed on uninvolved renal cortex in each phase; these regions of interest were also reviewed by each reader for appropriate placement. In each phase, the relative attenuation (compared to uninvolved cortex) of each lesion was calculated as [(Lesion attenuation–cortex attenuation)/cortex attenuation] × 100. Lesion attenuation refers to the mean attenuation of the 0.5 cm circular region of maximal lesion attenuation. Cortex attenuation refers to the mean attenuation of the 0.5 cm circular region of interest manually placed on uninvolved renal cortex. A similar method of quantifying absolute attenuation and relative attenuation (compared to uninvolved cortex) has been utilized in prior papers examining multiphasic MDCT enhancement as a means of identifying prognostically significant cytogenetic abnormalities in clear cell RCC (the gain of chromosome 20, the gain of chromosome 12, and the loss of the Y chromosome) and examining multiphasic MR enhancement as a means of differentiating clear cell RCC from papillary RCC, chromophobe RCC, and oncocytoma [21–24]. Lesion size was assessed by identifying the maximal lesion diameter on axial images.
Statistical analyses
Absolute and relative attenuation in each phase between type 1 and type 2 papillary RCCs were compared using Mann–Whitney tests with Bonferroni correction for multiple comparisons. Differences with p-values less than 0.017 were considered to be statistically significant. We also performed Mann–Whitney tests to compare the attenuation of uninvolved renal cortex in each phase between the two groups. Receiver operating characteristic (ROC) analysis was used to identify optimal thresholds to differentiate type 1 from type 2 papillary RCCs. Analyses were performed using SPSS 23 for Windows (IBM Corp, Armonk, NY). To account for potential clustering effects (5 of 64 patients in our cohort had two lesion each), the data were analyzed while including only one lesion per histologic subtype per patient. For patients with multiple lesions, the lesion included was selected at random.
Results
Lesions
Of the 69 papillary RCCs included in this study, 36 (52%) were type 1 papillary RCCs and 33 (48%) were type 2 papillary RCCs. Baseline characteristics for both groups are presented in Table 1. The mean lesion size for the type 1 and type 2 papillary RCC subcohorts was 2.9 and 3.8 cm, respectively, although this difference was not statistically significant. 65% of the lesions had 4-phase MDCT studies. 12% had 3-phase studies, and 23% had 2-phase studies. In the type 1 papillary RCC subcohort, 34 of 36 tumors had a stage of T1a or T1b. In the type 2 papillary RCC subcohort, 25 of 33 tumors had a stage of T1a or T1b.
Magnitude of enhancement
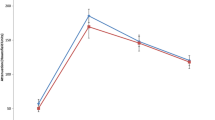
Absolute and relative attenuation values for type 1 and type 2 papillary RCCs are shown in Table 2 and Figs. 1, 2. Both type 1 and type 2 papillary RCCs had similar unenhanced attenuation and similar progressively increasing attenuation in the corticomedullary phase, with peak enhancement in the nephrographic phase. The subcohort of type 2 papillary RCCs consistently had a greater degree of enhancement than the subcohort of type 1 papillary RCCs in the corticomedullary and nephrographic phases, although these differences were not statistically significant. In the excretory phase, type 2 papillary RCCs had greater attenuation than type 1 papillary RCCs with a trend toward significance after Bonferroni correction for multiple comparisons (103 HU vs. 87 HU, p = 0.036). The relative attenuation of type 2 papillary RCCs was significantly greater than that of type 1 papillary RCCs in the excretory phase (2.0 vs. −18.3, p = 0.005). The relative corticomedullary attenuation of type 2 papillary RCCs was greater than that of type 1 papillary RCCs with a trend toward significance (−24.9 vs. −38.2, p = 0.054). Typical lesions in the papillary type 1 and type 2 subcohorts are presented in Figs. 3, 4.
Multiphasic enhancement of a type 1 papillary RCC on axial MDCT in a 48-year-old man. Representative CAD-selected regions of maximal attenuation are shown in blue. (A) Maximal unenhanced attenuation was 58 HU. (B) Maximal corticomedullary phase attenuation was 74 HU. (C) Maximal nephrographic phase attenuation was 91 HU. (D) Maximal excretory phase attenuation was 81 HU
Multiphasic enhancement of a type 2 papillary RCC on axial MDCT in a 77-year-old man. Representative CAD-selected regions of maximal attenuation are shown in blue. (A) Maximal unenhanced attenuation was 49 HU. (B) Maximal corticomedullary phase attenuation was 105 HU. (C) Maximal nephrographic phase attenuation was 114 HU. (D) Maximal excretory phase attenuation was 106 HU
To account for potential clustering effects, the data were analyzed while including only one lesion per histologic subtype per patient. The results were similar (Table 3).
Relative attenuation threshold levels
ROC analysis was performed to determine the optimal relative attenuation thresholds in the corticomedullary and excretory phases to differentiate type 1 papillary RCCs from type 2 papillary RCCs. Relative corticomedullary attenuation differentiated type 1 from type 2 papillary RCCs with an area under the curve (AUC) of 0.64 (standard error = 0.07, p = 0.06). Relative excretory attenuation differentiated type 1 from type 2 papillary RCCs with an AUC of 0.70 (standard error = 0.08, p = 0.02). The performance parameters for monophasic and multiphasic thresholds are presented in Table 4. Individual monophasic thresholds helped to differentiate type 1 from type 2 papillary RCCs with accuracies ranging from 67% (41/61) to 73% (36/49). However, the use of multiphasic relative attenuation threshold levels of −23.5 in the corticomedullary phase and −7.5 in the excretory phase improved predictive performance, helping to differentiate type 1 papillary RCCs from type 2 papillary RCCs with an accuracy of 74% (34/46) and sensitivity of 93% (28/30).
Discussion
In this study, both type 1 and type 2 papillary RCCs adhered to previously described enhancement patterns [10, 11, 15, 16]. Both type 1 and type 2 papillary RCCs exhibited peak enhancement in the nephrographic phase. Although type 2 papillary RCCs enhanced more avidly than type 1 papillary RCCs in all contrast-enhanced phases, these differences were not statistically significant after Bonferroni correction for multiple comparisons. We also demonstrated for the first time that the relative attenuation (compared to uninvolved renal cortex) of type 2 papillary RCCs was significantly greater than that of type 1 papillary RCCs in the excretory phase and with a trend toward significance in the corticomedullary phase. Multiphasic relative attenuation thresholds helped to differentiate type 1 from type 2 papillary RCCs with an accuracy of 74% (34/46) and sensitivity of 93% (28/30). Recent studies have shown that type 1 and type 2 papillary RCCs are biologically distinct entities, each characterized by distinct molecular abnormalities which result in distinct patterns of clinical behavior [8]. The difference in MDCT enhancement patterns between type 1 and type 2 papillary RCCs is likely related to differences in the expression of genes that modulate angiogenesis. Type 2 papillary RCCs are characterized by increased expression of the NRF2-antioxidant response pathway, a signal transduction pathway that promotes angiogenesis [8, 25, 26]. Type 2 papillary RCCs are also characterized by mutation or promoter hypermethylation of CDKN2A, a gene that encodes two proteins that modulate angiogenesis, p16 and p14ARF [8, 27, 28]. Further work correlating the expression of specific genes will help elucidate the mechanistic relationship between papillary RCC subtypes and MDCT enhancement.
In contrast to type 1 papillary RCCs which have a 5-year survival rate of 95%, type 2 papillary RCCs have a more aggressive clinical behavior with a 5-year survival rate of 66% that is more comparable to clear cell RCCs [9]. Because of the prognostic significance, a simple, non-invasive means of differentiating type 1 papillary RCC from type 2 papillary RCC may be helpful clinically to counsel patients and potentially guide further management. For example, a lesion with an enhancement profile more consistent with a type 2 papillary RCC may be managed with surgery, while a lesion with an enhancement profile more consistent with a type 1 papillary RCC may undergo active surveillance or thermal ablation.
Both Egbert et al. and Yamada et al. attempted to predict the histopathologic subtype of papillary RCCs using imaging features, but both found that MDCT enhancement could not accomplish this task [17, 18]. This may be due to several key differences between our study and their studies. First, we used a larger cohort of papillary RCCs (69 total papillary RCCs: 36 type 1 and 33 type 2 lesions). Egbert et al. used a cohort of 56 papillary RCCs (43 type 1 and 13 type 2 lesions), and Yamada et al. used a cohort of 20 papillary RCCs (12 type 1 and 8 type 2 lesions). Second, most of the lesions in our cohort were assessed using a 4-phase MDCT protocol, composed of unenhanced scanning, followed by scanning in the corticomedullary (40 s), nephrographic (90 s), and true excretory phases (8 min after contrast administration). Most of the lesions in the Egbert et al. cohort were assessed using unenhanced scans, followed by either nephrographic phase (120 s) or delayed nephrographic phase (150 s after contrast administration) scans. Most of the lesions in the Yamada et al. cohort were assessed using unenhanced scans, followed by scanning in the early corticomedullary (30 s), nephrographic (90 s), and delayed nephrographic phases (180 s after contrast administration). Thus, the study by Egbert et al. lacked true corticomedullary and excretory phase scans; the study by Yamada et al. had an early corticomedullary phase scan and lacked a true excretory phase scan. Finally, neither Egbert et al. nor Yamada et al. reported on relative attenuation (compared to uninvolved renal cortex). Doshi et al. evaluated 21 type 1 and 17 type 2 papillary RCCs using magnetic resonance imaging [19]. They qualitatively assessed tumor signal intensity in comparison to renal cortex in the nephrographic phase and did not find a significant difference between type 1 and type 2 lesions. However, they did not attempt to quantitatively assess the degree of enhancement in the corticomedullary, nephrographic, and excretory phases, as we did in this study.
Our study has several potential limitations. First, due to the retrospective nature of this study, all lesions were not scanned on the same scanner with the same 4-phase MDCT protocol. However, the majority of renal masses included in our study were evaluated with a 4-phase renal mass protocol; our protocols were designed to standardize scanning parameters as much as possible. Furthermore, there were no significant differences in uninvolved renal cortex attenuation between the groups in any phase. Second, this was a preliminary, discovery-phase study evaluating the relationship between MDCT enhancement and papillary RCC histopathology. Our findings should be validated in an independent testing cohort. Third, even though the relative excretory phase attenuation of type 2 papillary RCCs was significantly greater than that of type 1 papillary RCCs, there is overlap of the 95% confidence intervals (Table 2; Fig. 2). However, despite this overlap, in our cohort, multiphasic MDCT still helped to differentiate type 1 papillary RCCs from type 2 papillary RCCs with an accuracy of 74% (34/46) and sensitivity of 93% (28/30). Although this level of predictive performance is not yet adequate to definitively guide further treatment, our findings suggest that multiphasic MDCT may have the potential to assist in differentiating between type 1 and type 2 papillary RCC, if validated prospectively. Fourth, at our institution, genitourinary pathologists can classify papillary RCCs as type 1 or type 2 in most cases. However, there are a minority of cases in which differentiating between type 1 and type 2 papillary RCCs is not possible histologically. Our study cohort included only papillary RCCs that were classified as type 1 or type 2 based upon histology.
To our knowledge, our study is the first proof of concept study to demonstrate that multiphasic MDCT enhancement may potentially assist in differentiating type 1 papillary RCCs from type 2 papillary RCCs, if validated in a large prospective trial. Because type 1 papillary RCCs have a more favorable prognosis in comparison to type 2 papillary RCCs, a non-invasive means of identifying type 1 papillary RCCs preoperatively may help guide clinical decision making. In addition, with a baseline MDCT scan prior to surgery or ablation, new lesions can be non-invasively evaluated to determine if their enhancement profiles are similar to previously treated lesions.
References
Kovacs G, Akhtar M, Beckwith BJ, et al. (1997) The Heidelberg classification of renal cell tumours. J Pathol 183:131–133
Truong LD, Shen SS (2011) Immunohistochemical diagnosis of renal neoplasms. Arch Path Lab Med 135:92–109
Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML (2003) Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol 27:612–624
Moch H, Gasser T, Amin MB, et al. (2000) Prognostic utility of the recently recommended histologic classification and revised TNM staging system of renal cell carcinoma: a Swiss experience with 588 tumors. Cancer 89:604–614
Hoffmann NE, Gillett MD, Cheville JC, et al. (2008) Differences in organ system of distant metastasis by renal cell carcinoma subtype. J Urol 179:474–477
Beck SD, Patel MI, Snyder ME, et al. (2004) Effect of papillary and chromophobe cell type on disease-free survival after nephrectomy for renal cell carcinoma. Ann Surg Oncol 11:71–77
Amin MB, Corless CL, Renshaw AA, et al. (1997) Papillary (chromophil) renal cell carcinoma: histomorphologic characteristics and evaluation of conventional pathologic parameters in 62 cases. Am J Surg Pathol 21:621–635
Cancer Genome Atlas Research Network, Linehan WM, Spellman PT (2016) Comprehensive molecular characterization of papillary renal-cell carcinoma. N Engl J Med 374:135–145
Mejean A, Hopirtean V, Bazin JP, et al. (2003) Prognostic factors for the survival of patients with papillary renal cell carcinoma: meaning of histological typing and multifocality. J Urol 170:764–767
Young JR, Margolis D, Sauk S, et al. (2013) Clear cell renal cell carcinoma: discrimination from other renal cell carcinoma subtypes and oncocytoma at multiphasic multidetector CT. Radiology 267:444–453
Lee-Felker SA, Felker ER, Tan N, et al. (2014) Qualitative and quantitative MDCT features for differentiating clear cell renal cell carcinoma from other solid renal cortical masses. AJR 203:W516–W524
Zhang J, Lefkowitz RA, Ishill NM, et al. (2007) Solid renal cortical tumors: differentiation with CT. Radiology 244:494–504
Sheir KZ, El-Azab M, Mosbah A, El-Baz M, Shaaban AA (2005) Differentiation of renal cell carcinoma subtypes by multislice computerized tomography. J Urol 174:451–455 (discussion 5)
Kim JK, Kim TK, Ahn HJ, et al. (2002) Differentiation of subtypes of renal cell carcinoma on helical CT scans. AJR 178:1499–1506
Jinzaki M, Tanimoto A, Mukai M, et al. (2000) Double-phase helical CT of small renal parenchymal neoplasms: correlation with pathologic findings and tumor angiogenesis. J Comput Assist Tomogr 24:835–842
Ruppert-Kohlmayr AJ, Uggowitzer M, Meissnitzer T, Ruppert G (2004) Differentiation of renal clear cell carcinoma and renal papillary carcinoma using quantitative CT enhancement parameters. AJR 183:1387–1391
Egbert ND, Caoili EM, Cohan RH, et al. (2013) Differentiation of papillary renal cell carcinoma subtypes on CT and MRI. AJR 201:347–355
Yamada T, Endo M, Tsuboi M, et al. (2008) Differentiation of pathologic subtypes of papillary renal cell carcinoma on CT. AJR 191:1559–1563
Doshi AM, Ream JM, Kierans AS, et al. (2016) Use of MRI in differentiation of papillary renal cell carcinoma subtypes: qualitative and quantitative analysis. AJR 206:566–572
Rosenkrantz AB, Matza BW, Portnoy E, et al. (2014) Impact of size of region-of-interest on differentiation of renal cell carcinoma and renal cysts on multi-phase CT: preliminary findings. Eur J Radiol 83:239–244
Young JR, Young JA, Margolis D, et al. (2016) Clear cell renal cell carcinoma: identifying the gain of chromosome 20 on multiphasic MDCT. Abdom Radiol 41:2175–2181
Young JR, Coy H, Douek M, et al. (2017) Clear cell renal cell carcinoma: identifying the gain of chromosome 12 on multiphasic MDCT. Abdom Radiol 42:236–241
Young, JR, Coy H., Douek, M. et al. (2017) Clear cell renal cell carcinoma: identifying the loss of the Y chromosome on multiphasic MDCT. Accepted for publication in AJR Am J Roentgenol on January 6, 2017.
Young JR, Coy H, Kim HJ, et al. (2017) Performance of relative enhancement on multiphasic MR imaging for differentiation of clear cell renal cell carcinoma from papillary and chromophobe RCC subtypes and oncocytoma. AJR Am J Roentgenol . doi:10.2214/AJR.16.17152
Florczyk U, Jazwa A, Maleszewska M, et al. (2014) Nrf2 regulates angiogenesis: effect on endothelial cells, bone marrow-derived proangiogenic cells and hind limb ischemia. Antioxid Redox Signal 20:1693–1708
Valcarcel-Ares MN, Gautam T, Warrington JP, et al. (2012) Disruption of Nrf2 signaling impairs angiogenic capacity of endothelial cells: implications for microvascular aging. J Gerontol A Biol Sci Med Sci 67:821–829
Chapman EJ, Harnden P, Chambers P, Johnston C, Knowles MA (2005) Comprehensive analysis of CDKN2A status in microdissected urothelial cell carcinoma reveals potential haploinsufficiency, a high frequency of homozygous co-deletion and associations with clinical phenotype. Clin Cancer Res 11:5740–5747
Harada H, Nakagawa K, Iwata S, et al. (1999) Restoration of wild-type p16 down-regulates vascular endothelial growth factor expression and inhibits angiogenesis in human gliomas. Cancer Res 59:3783–3789
Acknowledgements
We thank the UCLA Computer Vision and Imaging Biomarker (CVIB) group, including Dr. Jonathan Goldin, Dr. Matthew Brown, Moe Moe Ko, and War War Ko, for their assistance with this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
The Institutional Review Board waived the requirement of informed consent for this retrospective study.
Rights and permissions
About this article
Cite this article
Young, J.R., Coy, H., Douek, M. et al. Type 1 papillary renal cell carcinoma: differentiation from Type 2 papillary RCC on multiphasic MDCT. Abdom Radiol 42, 1911–1918 (2017). https://doi.org/10.1007/s00261-017-1091-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-017-1091-x