Abstract
Malignant carcinomatous change is a rare complication in an enteric duplication cyst, and papillary adenocarcinoma is especially unusual. We describe a papillary adenocarcinoma, arising from a duplication of the colon, seen as a cyst with an enhancing papillary projection nodule located adjacent to the wall of the ascending colon and cecum on computed tomography.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Duplications of the alimentary tract are unusual developmental anomalies that may occur anywhere in the gastrointestinal tract. They are usually located in, or adjacent to, the wall of part of the bowel; additionally, they have smooth muscle in their walls, and are lined by mucosa similar to that of the adjacent segment of the digestive tube [1, 2]. Such a developmental anomaly is most common in the ileum, followed by the esophagus and duodenum, but less frequent in the colon or rectum. Malignant change in an intestinal duplication is extremely rare, and occurs most often in the colon. Adenocarcinoma is the most common histological type of malignancy arising in an intestinal duplication. Papillary adenocarcinoma is extremely rare, and the only reported case was in the small bowel [3]. We present the first case of papillary adenocarcinoma arising in colon duplication.
Case report
A 40-year-old woman complained of a palpable abdominal mass that she first noticed 3 weeks before admission. On physical examination, there were no positive findings except for a palpable mass in the right periumbilical area. The laboratory data were normal, except for a high serum carcinoembryonic antigen (CEA) level of 10.59 ng/mL.
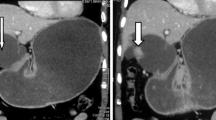
The abdomen supine film showed a round mass density in the right lower abdomen that displaced the ascending colon superiorly and the small bowel loops medially. Enhanced abdomen computed tomography (CT) showed a 11.0 × 8.3 × 10.0 cm, round, sharply marginated, thin-walled cystic mass in the right lower abdomen displacing the ascending colon superiorly and the distal ileal loop inferomedially, so that it was located just inferior to the proximal ascending colon (Fig. 1A, B). The cystic mass contained a 4.0 × 4.4 × 4.5 cm lobulated nodule. The fluid component was homogenous low density, while the nodules enhanced homogenously with contrast. An enlarged vascular structure was seen on the posterolateral side of the cystic mass. This was the ileocolic branch of the superior mesenteric artery, which supplied the lesion. There were a few regional lymph nodes, ranging from 0.5 to 1.0 cm in size, around the cystic mass. Normal looking ovaries, uterus, and appendix were seen in situ. No small or large bowel invasion, bowel obstruction, or vascular encasement was evident. Colonoscopy revealed a normal mucosal layer throughout the colon, but an intraluminal protruding mass near the proximal ascending colon and cecum.
A 40-year-old woman complained of an abdominal mass in the right lower abdomen. A, B Enhanced abdominal CT shows a 11.0 × 8.3 × 10.0 cm, round, sharply marginated, thin-walled cystic mass containing lobulated nodules extending inferiorly from the proximal ascending colon. The base of the cecum and part of the appendix are anteromedial to the cystic mass (arrow). An enlarged ileocolic branch of the superior mesenteric artery surrounding the posterior wall of the cystic mass is also seen in B (arrow), representing the artery supplying the tumor. C The gross pathologic features after evacuating the fluid from the cyst shows a cyst containing papillary configured nodules in the cecal area (arrow), with much debris from the polypoid mass in the cyst wall. D Photomicrograph (H&E stain ×100) shows well-differentiated adenocarcinoma with areas of intraluminal necrotic debris and complex papillary architecture lined by pseudo-stratified malignant cells (arrows). E, F On immunohistochemical staining (×100), CK20 (E) and CDX2 (F) stains strongly in the nucleus and cytoplasm of the malignant neoplastic cells, while CK7 did not stain (not shown). This is typical of adenocarcinoma arising from the colon, but not of metastasis from other organs.
Given the characteristics of the mass, including the pattern of enhancement and its location, the high serum CEA, and its proximity to the colon and mesentery, the differential diagnosis consisted of a complicated mesenteric cyst, a gastrointestinal stromal tumor (GIST), or an intestinal duplication cyst arising from the cecum or ascending colon with a high probability of malignancy.
A right hemicolectomy was done. At surgery, a huge, well-encapsulated, cystic mass was found abutting the normal cecum and mesentery (Fig. 1C). No ascites, adhesion, or seeding were evident. A few small lymph nodes 0.5–1.0 cm in size were seen in the mesentery around the mass. The cystic fluid was dark brown, but not sticky, representing hemorrhagic fluid. There was some sludge from the growing papillary mass within the cyst. Histologic examination of the surgical specimen revealed a papillary adenocarcinoma with micro-invasion of the muscle layer of the cecum arising within an intestinal duplication cyst of the cecum (Fig. 1D).
On immunohistochemical staining, CDX2 and CK20 stained strongly, while CK7 was negative in the papillary adenocarcinoma, which represented an adenocarcinoma arising from the colon (Fig. 1E, F). The cyst consisted of well-organized layers of smooth muscle, typical of the colon, and lined by benign epithelium of columnar cells. There were no metastatic lymph nodes, but reactive lymph node hyperplasia.
Discussion
Malignant carcinomatous change in a duplication of the gastrointestinal tract is rare in adults, but has been well documented in the English literature [4–10]. In our survey, most of the reported cases were located in the colon, followed by the small bowel, stomach, and intrathoracic cavity. Various malignancies have been reported in enteric duplications, including adenocarcinoma, squamous cell carcinoma, and carcinoid tumor. Papillary adenocarcinoma was extremely rare, with the only reported case in a small bowel duplication [3]. We described the first case of papillary adenocarcinoma in a duplication of the colon.
Primary papillary adenocarcinoma is a rare histologic subtype of adenocarcinoma of the colon, but it frequently occurs in the ovary, pancreas, and thyroid. The pathologist reported papillary adenocarcinoma when the soft tissue density nodule showed a papillary configuration consisting of fibrovascular cores. Given the rarity of primary papillary adenocarcinoma of the colon, the differential diagnosis should include metastatic carcinoma, such as papillary adenocarcinoma of the ovary, uterus, pancreas, and head and neck. In order to rule out these tumors, detection of an intramucosal component to the carcinoma and the normal appearance of these organs are important. Immunohistochemical staining using CK20, CDX2, and CK7 has been reported to be useful in distinguishing adenocarcinoma arising from colon mucosa from that in other organs [11–13]. We used CK20, CDX2, and CK7 to exclude metastatic papillary adenocarcinoma from other organs, because the CK20+/CDX2+/CK7− immunohistochemical staining pattern is usually retained in primary and metastatic adenocarcinoma of the colon. Our case of papillary adenocarcinoma in a colonic duplication cyst was CDX2+/CK20+/CK7−, which is typical of colonic cancer, not metastasis from other organs.
Radich et al. [3] described magnetic resonance findings in one case of papillary adenocarcinoma in a small bowel duplication, which consisted of a sharply marginated, thin-walled cystic mass with internal multiple dependent and nondependent lobulated nodules. The fluid component was hyperintense on T1- and hypointense on T2-weighted images, representing hemorrhage. The nodules were hypointense on T1- and increased in signal intensity on T2-weighted images, representing carcinoma.
Our case had similar CT findings. We described the CT findings as a sharply marginated, fluid-filled cyst with a lobulated, enhancing soft tissue nodule located adjacent to the wall of the colon. We did not see any calcifications (so-called psammoma bodies) in the mass; these can be seen in papillary adenocarcinoma arising from any body site. Histologically, our case did not contain mucin within the cyst, making it a non-mucin producing papillary adenocarcinoma. Initially, we did not diagnose the tumor as papillary adenocarcinoma, but only as a malignant cystic mass arising from a mesenteric cyst, duplication cyst, or unusual GIST, based on previous reports describing malignant cystic masses as soft-tissue-density nodules revealed within the cyst [4, 9]. Based on the high CEA level, we strongly suspected an adenocarcinoma arising within a duplication cyst of the colon.
In conclusion, if a large cystic mass is located in or adjacent to the alimentary tract, one should include enteric duplication cyst in the differential diagnosis. Other cystic masses are mesenteric and omental cysts, pancreatic pseudocysts, renal or splenic cysts, ovarian cysts, or loculated ascites. When a lobulated nodule is seen within the cyst and the serum CEA level is high, the possibility of papillary adenocarcinoma should be considered.
References
Berrocal T, Lamas M, Gutierrez J et al (1999) Congenital anomalies of the small intenstine, colon, and rectum. Radiographics 19:1219–1236
Teele RL, Henshke CI, Tpper D (1980) The radiographic and ultrasonographic evaluation of enteric duplication cysts. Pediatr Radiol 10:9–12
Radich GA, Altinok D, Adsay NV et al (2006) Papillary adenocarcinoma in a small-bowel duplication in a pregnant woman. AJR 186:895–897
Inoue Y, Nakamura H (1998) Adenocarcinoma arising in coloinc duplication cysts with calcification: CT findings of two cases. Abdom Imaging 23:135–137
Fletcher DJ, Goodfellow PB, Bardsley D (2002) Metastatic adenocarcinoma arising from a small bowel duplication cyst. Eur J Surg Oncol 28:93–94
Hata H, Hiraoka N, Ojima H et al (2006) Carcinoid tumor arising in a duplication cyst of the duodenum. Pathol Int 56:272–278
Coit DG, Mies C (1992) Adenocarcinoma arising within a gastric duplication cyst. J Surg Oncol 50:274–277
William FH, Joseph MC (1981) Squamous cell carcinoma arising in a duplication of the colon: case report and literature review of squamous cell carcinoma of the colon and of malignancy complicating colonic duplication. Cancer 47:602–609
Rice CA, Anderson TM, Sepahdari S (1986) Computed tomography and ultrasonography of carcinoma in duplication cysts. JCAT 10:233–235
Kusunoki N, Shimada Y, Fukumoto S et al (2003) Adenocarcinoma arising in a tubular duplication of the jejunum. J Gastroenterol 38:781–785
Sakamoto K, Watanabe M, Cruz C et al (2005) Primary invasive micropapillary carcinoma of the colon. Histopathology 47:479–484
Song JS, Khang SK, Huh J et al (2006) Sinonasal low-grade adenocarcinoma: report of three cases with the clinicopathologic and immunohistochemical findings. Korean J Pathol 40:235–240
Kennedy MT, Jordan RC, Berean KW et al (2004) Expression pattern of CK7, CK20, CDX-2, and villin in intestinal-type sinonasal adenocarcinoma. J Clin Pathol 57:932–937
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, J., Jeon, Y.H. & Lee, S. Papillary adenocarcinoma arising in a duplication of the cecum. Abdom Imaging 33, 601–603 (2008). https://doi.org/10.1007/s00261-007-9330-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-007-9330-1