Abstract
A 59-year-old man with anorexia who had a history of cholecystectomy was referred to our hospital. Imaging examinations revealed a contrast-enhanced tumor in the residual cystic duct and a part of the common bile duct. Endoscopic retrograde cholangiopancreatography and peroral-cholangioscopy showed a papillary tumor with movement and a change in the shape. Under a diagnosis of primary cystic duct cancer, subtotal stomach-preserving pancreaticoduodenectomy was performed. The microscopic examination of a resected specimen revealed intracholecystic papillary-tubular neoplasm located in the residual cystic duct, forming a polypoid protrusion to the common bile duct and extensive intraepithelial progress in the common bile duct.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intraductal papillary neoplasm of the bile duct (IPNB) is characterized by dilated intrahepatic and extrahepatic bile ducts filled with a papillary biliary neoplasm covering thin fibrovascular stalks [1]. The same type of tumor occurring in the gallbladder is commonly called as intracholecystic papillary-tubular neoplasm (ICPN) of the gallbladder [2]. Both of them are considered counterparts of intraductal papillary mucinous neoplasms (IPMN). As a precursor lesion of cholangiocarcinoma, IPNB was newly included in the World Health Organization (WHO) Classification of Bile Duct Tumors in 2010 and General Rules for Clinical and Pathological Studies on Cancer of the Biliary Tract The 6th Edition in 2013 [3], and an understanding of IPNB has gradually spread. However, morphological characteristics and progress of growth are not known well because ICPN is still new. We herein report a case of ICPN which originated in the cystic duct with intraepithelial spread in the common bile duct.
Case report
A 59-year-old man complaining of anorexia and a feeling of fatigue was referred to our hospital because of liver dysfunction and dilation of the bile duct determined using imaging evaluation. He was diagnosed with acute cholecystitis due to gallbladder stones, and laparoscopic cholecystectomy was performed in a different hospital 8 months prior. At the time, no malignancy was found in the surgically resected specimen of the gallbladder.
The laboratory data on admission were as follows: total bilirubin, 1.0 mg/dL; aspartate aminotransferase, 69 IU/L; alanine transaminase, 158 IU/L; alkaline phosphatase, 303 IU/L; gamma glutamyl transpeptidase, 338 IU/L. Tumor markers, such as serum carbohydrate antigen 19-9 (CA19-9) and carcinoembryonic antigen (CEA) levels, were within normal ranges.
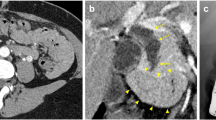
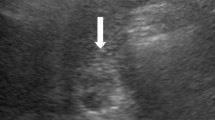
In a computed tomography (CT) image, there was a 33 × 23 mm contrast-enhanced tumor in the residual cystic duct and a part of the common bile duct with dilation of the upper stream bile duct (Fig. 1). Neither nodal metastasis nor distant metastasis was identified. In magnetic resonance images (MRIs), the tumor showed a low signal in the T1-weighted image, an iso-high signal in the T2-weighted image, and a high signal in the diffusion-weighted image. Magnetic resonance cholangiopancreatography (MRCP) showed a tumor-like defect in the bile duct with dilatation of the upstream bile duct (Fig. 2). Endoscopic ultrasonography (EUS) showed a hypoechoic mass occupying the residual cystic duct and extensive bile ducts from the perihilar to the distal bile duct. EUS with perflubutane enhanced the mass only in the confluence of the cystic duct with a normal exterior high-echo layer of the cystic duct (Fig. 3). Endoscopic retrograde cholangiopancreatography (ERCP) revealed a papillary-shaped defect in the bile duct. The defect showed slight movement and a change in the shape during ERCP. Using peroral cholangioscopy (POCS) [SpyGlass DS Direct Visualization System (SpyDS): Boston Scientific Co., Natick, MA, USA], a papillary tumor was found in the bile duct with no tumor extension in the confluence of the right and left hepatic ducts and the upper stream bile duct (Fig. 4). Mucin was not observed using duodenoscopy and POCS.
a Magnetic resonance (MR) images showing a low-intensity mass in the residual cystic duct and common bile duct in the T1 weighted image (arrow). b An iso-high intensity mass (arrow) in the T2 weighted image. c A positive signal intensity in the diffusion-weighted image was detected consistent with the site of the tumor (arrow). d MR cholangiopancreatography (MRCP) image indicating bile duct dilation with a tumor-like defect (arrow) in the common bile duct
Endoscopic ultrasonography (EUS) images showing the hypoechoic mass (arrows) occupying the residual cystic duct and the extensive bile duct from perihilar bile duct to distal bile duct. Contrast-enhanced EUS image with perflubutane (right images of a and b) showed encasement of the mass a only in the confluence of the bile duct (arrowhead) and b not in the other part, such as in the perihilar bile duct
a, b Endoscopic retrograde cholangiopancreatography (ERCP) images showing bile duct dilatation and a papillary-shaped defect (arrows) in the bile duct with slight movement and a change in the shape during ERCP. Peroral cholangioscopy (POCS) showed papillary tumor c located in the bile duct, and d there was no finding of the tumor progression in the bifurcation of the hepatic duct and the upper stream
A transpapillary biopsy of the tumor revealed adenocarcinoma. The B4 confluence, the confluence of the right anterior and right posterior segmental ducts, and the confluence of the right and left hepatic ducts were biopsied to investigate the cancer extension. Only the specimen from the confluence of the right and left hepatic ducts showed adenocarcinoma. The tumor was thought to begin in the cystic duct and to extend to the confluence of the right and left hepatic ducts and the distal bile duct because of the main occupying region and the mobility and deformability of the tumor in the common bile duct. The tumor was diagnosed as primary cystic duct cancer preoperatively, and ICPN was thought to be possible because of the finding from POCS. Subtotal stomach-preserving pancreaticoduodenectomy was performed, and pathological diagnosis during surgery revealed atypical epithelial cells in the resection stumps of the bilateral hepatic ducts.
Macroscopic examination of the resected specimen revealed a papillary tumor located in the residual cystic duct, forming a polypoid protrusion to the common bile duct (Fig. 5). Histologically, the papillary tumor in the cystic duct showed papillary atypical epithelial proliferation and had a thin fibrous vascular stalk (Fig. 6). The tumor was diagnosed as a papillary adenocarcinoma equivalent to intracholecystic papillary-tubular neoplasm (ICPN) of the gallbladder with no invasion on the basis of the WHO classification. The tumor had extensive intraepithelial progress with low-grade tubular adenocarcinoma from the lower end of the distal bile duct to the resection stumps of the bilateral hepatic ducts (Fig. 7). The volume of the cancer and the cellular atypia were greater in the cystic duct than in the bile duct, and the bases of the fibrous vascular stalks were confirmed to be in the cystic duct. Therefore, the tumor was diagnosed as primary cystic duct cancer. The results of immunostaining of the tumor in the cystic duct (Ki67 labeling index [Ki67LI] 40–80%, p53[−], MUC1[diffusely+], MUC2[−], MUC5AC[focally+], MUC6[−], CDX2[focally+]) suggested that the tumor was a pancreatobiliary-type ICPN (Fig. 8). In addition, the results of immunostaining of the tumor in the common bile duct (Ki67LI 30–60%, p53[−], MUC1[−], MUC2[focally+], MUC5AC[diffusely++], MUC6[diffusely +], CDX2[diffusely+]) suggested that the tumor was a gastric-type (high-grade dysplasia) ICPN (Fig. 9). In AB-PAS staining, a small amount of mucin in both the cystic duct and the common bile duct was observed. The results of immunostaining are summarized in Table 1, and the areas of pancreatobiliary-type and gastric-type ICPN are shown in Fig. 7. The final diagnosis was non-invasive gallbladder cancer, CBdBp, paptub, 72 × 15 × 10 mm, pTis(M), INF(−), ly0, v0, ne0, pN0, pDM0, pHM1, pEM0, pPV0, pA0, R1cis; fStage0 [3], with an papillary-expanding growth pattern.
Pathological findings from intracholecystic papillary-tubular neoplasm. a The papillary tumor, forming a polypoid protrusion to the common bile duct, in the cystic duct showed papillary atypical epithelial proliferation and had a thin fibrous vascular stalk (hematoxylin and eosin [H&E] staining, × 2.5). b A high-powered view of the papillary tumor showed cellular atypia and papillary form (hematoxylin and eosin [H&E] staining, × 25). c The tumor showed complex fern-leaves growing in the aperture of the cystic duct. (H&E staining, × 2.5). d The bases of the fibrous vascular stalks were confirmed to be in the cystic duct (arrow) (H&E staining, × 2.5). e The tumor had extensive intraepithelial progress with low-grade tubular adenocarcinoma in the common bile duct (H&E staining, × 25)
Histological mapping. A papillary tumor was located in the residual cystic duct, forming a polypoid protrusion to the common bile duct, with extensive intraepithelial progress in the bile duct. The tumor was detected in the cystic duct with pancreatobiliary-type (blue line) and extensive bile duct with gastric-type (red line)
2 months after surgery, postoperative chemotherapy (Tegafur/Gimeracil/Oteracil 120 mg/day on consecutive 28 days, repeated every 42 days) was started since the resection stumps of the bilateral hepatic ducts showed a positive margin.
Discussion
Primary carcinoma of the cystic duct was defined by Farrar in 1951 as the following: (1) growth restricted to the cystic duct, (2) absence of neoplasia in the gallbladder, hepatic ducts, or common bile duct, and (3) histological confirmation of carcinoma cells in the mass [4]. However, the definition is only applied to relatively early cystic duct cancer. In advanced tumors that have extensively invaded the surrounding structures, the site of origin is not obvious at first glance, but histological examination of serial sections of the resected specimens reveals the center of the tumor, thus allowing a diagnosis of cystic duct carcinoma. Ozden et al. have proposed that the tumor of which the main region is located in the cystic duct should be regarded as a primary cystic cancer even if it invades the surrounding structures, such as the gallbladder and the common bile duct [5]. Using this definition, the number of reports of primary cystic duct carcinoma have been increasing. The present case is not consistent with the Farrar definition because of extensive intraepithelial spread in the common bile duct. The volume of the cancer and the cellular atypia were larger in the residual cystic duct than they were in the bile duct. In addition, the base of the tumor stalk was in the cystic duct. Therefore, the tumor was diagnosed as a primary cystic duct cancer. Both cystic duct cancer and ICPN are relatively rare, and primary cystic duct cancers with ICPN features have not been reported on the basis of a Pubmed search with the key phrases “ICPN”, “cystic duct” and “gallbladder cancer”.
Zen et al. [1] proposed the concept of IPNB in 2006, and Adsay et al. [2] defined mass-forming pre-invasive polypoid neoplasms of the gallbladder as ICPN in 2012. Both are considered counterparts of IPMN, and more cases have gradually been reported [6]. However, the definition of IPNB/ICPN has not been standardized until now. The concept of IPNB/ICPN is being established, and recently, the Japan–Korea IPNB study group sponsored by the Japan Biliary Association and Korea Association of Hepato-Biliary and Pancreas Surgery have proposed that IPNB be classified into two types: Type1 IPNB (classical IPNB) and Type2 IPNB (formerly papillary cholangiocarcinoma) [7].
Adsay et al. have proposed that the term “ICPN” includes papillary or polypoid intramucosal gallbladder masses ≥ 1.0 cm in diameter and are composed of preinvasive neoplastic cells forming a compact lesion distinct from the neighboring mucosa with papillary or tubular histological characteristics [2]. In 123 cases of ICPN investigated by Adsay et al., 68 cases were invasive cancers, but the overall survival after diagnosis having an ICPN was good. Patients with noninvasive ICPNs had 3- and 5-year survival rates of 90%, and 78%, versus 60% and 60% for those with invasive ICPNs, respectively. Even cases of associated invasive ICPNs appear to have a significantly better prognosis than normal gallbladder cancer with median survivals of 35 versus 9 months does. The histological subtypes of ICPNs are classified into five types on the basis of the IPMN of the pancreas: pancreaticobiliary, intestinal, gastric foveolar, gastric pyloric and oncocytic. Although the gastric type is the major subtype (49%) of IPMNs of the pancreas [8], the pancreaticobiliary type is the major subtype (50%) of ICPNs (gastric pyloric type (20%), gastric foveolar type (16%), intestinal type (8%), and oncocytic type (6%) of ICPN). ICPNs have been reported to have more complicated histological forms than those of IPMNs and to have various subtypes in the same lesion [2]. In a previous study, it has been reported that 59.3% of ICPNs contain two or more histological subtypes of tumor cells [9]. In the present case, the tumor had two subtypes, including pancreatobiliary type in the cystic duct and gastric type in the bile duct. Although we considered the possibility of collision cancers because of the difference in the subtype in the cystic and bile ducts, consecutive histological images did not strongly support the presence of collision tumors.
Similar to IPNBs, ICPNs have been reported to be less invasive than other neoplasms are. In addition, both ICPNs and IPNBs are considered to be relatively slow-growing tumors and have characteristic formality of progression, such as lateral and superficial spread [2, 10]. Cases of IPNB/ICPN have been reported to have relatively good prognosis, whereas some previous studies have shown that the presence of at least dysplasia in the surgical margin is associated with poor survival rates [11, 12]. Correct diagnosis of the surface progression range before surgery is necessary to achieve R0 resection. Although it was possible to detect clearly a papillary tumor protruding to bile duct using CT, MRI and EUS in this case, these images could not be used to evaluate correctly the surficial progression range. Intraductal ultrasonography (IDUS) and biopsy of the bile duct using roentgenoscopy have mainly been used to evaluate the surface progression range previously [13]. However, these methods have problems in the diagnosis of the intraepithelial progress of tumors with low height and accuracy of sniper biopsy. Currently, observation using POCS and cholangioscopic-guided mapping biopsy are regarded as the most useful methods for determining the extent of the resection for bile duct cancer [14]. On the other hand, the usefulness of POCS for studying intraluminal papillary tumors have been reported, and POCS show surface progression as salmon roe-like and granular mucosa spreading around the main lesion [15]. Using POCS, no suspicious findings of surficial progression on the B4 confluence, the confluence of the right anterior and right posterior segmental ducts, and the confluence of the right and left hepatic ducts were obtained in this case. However, microscopic examination using biopsy revealed adenocarcinoma in the confluence of the right and left hepatic ducts, and from pathological diagnosis during surgery, there were atypical epithelial cells in the resection stumps of the bilateral hepatic ducts. The accuracy of using only POCS observation to evaluate the mucosal cancerous extension of bile duct cancer has been reported to be in the range of 75–95%, and that of a combination of POCS observation and cholangioscopic-guided mapping biopsy has been reported to be in the range of 87–100% [14, 16,17,18]. SpyDS has better maneuverability than electronic POCS (CHF-B260, CHF-BP260: Olympus Co., Tokyo, Japan) and peroral direct cholangioscopes (GIF-XP260N, Prototype PDCS: Olympus) do. In addition, SpyDS has better ability toward sniper biopsy [14]. However, SpyDS images are lower in quality than those from POCS and PDCS, meaning that diagnostic performance with only endoscopic images would be worse using SpyDS. In other words, all types of POCS and PDCS with biopsy can lead to the misdiagnosis of the range of surface progression for several reasons, such as inflammation, etc. We must determine the limitations of each examination method and evaluate the surface progression range of bile duct tumor.
We herein described a rare case of ICPN of the gallbladder originating in the cystic duct without invasion despite extensive intraepithelial progress in the common bile duct.
References
Zen Y, Fujii T, Itatsu K, et al. Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology. 2006;44:1333–43.
Adsay V, Jang KT, Roa JC, et al. Intracholecystic papillary-tubular neoplasms (ICPN) of the gallbladder (neoplastic polyps, adenomas, and papillary neoplasms that are ≥ 1.0 cm): clinicopathologic and immunohistochemical analysis of 123 cases. Am J Surg Pathol. 2012;36:1279–301.
Japanese society of hepato-biliary-pancreatic surgery. General rules for clinical and pathological studies on cancer of the biliary tract, 6th ed. Kanehara Shuppan; 2013.
Farrar DA. Carcinoma of the cystic duct. Br J Surg. 1951;39:183–5.
Ozden I, Kamiya J, Nagino M, et al. Cystic duct carcinoma: a proposal for a new “working definition”. Langenbecks Arch Surg. 2003;387:337–42.
Hashimoto S, Horaguchi J, Fujita N, et al. Intracholecystic papillary-tubular neoplasm of the gallbladder presenting with jaundice. Intern Med. 2014;53:2313–7.
Nakanuma Y, Kubota K. Subclassification of intraductal papillary neoplasm of bile duct (IPNB)—proposal by Japan–Korea Study Group on IPNB from pathological stand points of view. Biliary Tract. 2017;31:390.
Furukawa T, Hatori T, Fujita I, et al. Prognostic relevance of morphological types of intraductal papillary mucinous neoplasms of the pancreas. Gut. 2011;60:509–16.
Wan X, Shi J, Whang A, et al. Gallbladder papillary neoplasms share pathological features with intraductal papillary neoplasm of the bile duct. Oncotarget. 2017;8:31532–9.
Sakamoto E, Hayakawa N, Kamiya J, et al. Treatment strategy for mucinous-producing intrahepatic cholangiocarcinoma: Value of percutaneous transhepatic biliary drainage and cholangioscopy. World J Surg. 1999;23:1038–44.
Yeh TS, Tseng JH, Chiu CT, et al. Cholangiographic spectrum of intraductal papillary mucinous neoplasm of the bile ducts. Ann Surg. 2006;244:248–53.
Jung G, Park KM, Lee SS, et al. Long-term clinical outcome of the surgically resected intraductal papillary neoplasm of the bile duct. J Hepatol. 2012;57:787–93.
Noda Y, Fujita N, Kobayashi G, et al. Intraductal ultrasonography before biliary drainage and transpapillary biopsy in assessment of the longitudinal extent of bile duct cancer. Dig Endosc. 2008;20:73–8.
Ogawa T, Ito K, Koshita S, et al. Usefulness of cholangioscopic-guided mapping biopsy using SpyGlass DS for preoperative evaluation of extrahepatic cholangiocarcinoma: a pilot study. Endosc Int Open. 2018;6:E199–204.
Sakai Y, Tsuyuguchi T, Ishihara T, et al. Usefulness of peroral cholangioscopy in preoperative diagnosis of intraductal papillary neoplasm of the bile duct. Hepatogastroenterology. 2010;57:691–3.
Osanai M, Itoi T, Igarashi Y, et al. Peroral video cholangioscopy to evaluate indeterminate bile duct lesions and preoperative mucosal cancerous extension: a prospective multicenter study. Endoscopy. 2013;45:635–42.
Nishikasa T, Tsuyuguchi T, Sakai Y, et al. Preoperative assessment of longitudinal extension of cholangiocarcinoma with peroral video-cholangioscopy: a prospective study. Dig Endosc. 2014;26:450–7.
Kawakami H, Kuwatani M, Etoh K, et al. Endoscopic retrograde cholangiography versus peroral cholangioscopy to evaluate intraepithelial tumor spread in biliary cancer. Endoscopy. 2009;41:959–64.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human rights
All procedures followed have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from all patients for being included in the study
Rights and permissions
About this article
Cite this article
Fujii, Y., Noda, Y., Koshita, S. et al. Intracholecystic papillary-tubular neoplasm of the gallbladder originating in the cystic duct with extensive intraepithelial progress in the common bile duct. Clin J Gastroenterol 12, 197–204 (2019). https://doi.org/10.1007/s12328-018-0927-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-018-0927-4