Abstract
Purpose
To evaluate the role of positron emission tomography/computed tomography (PET/CT) in predicting pathologic complete response (pCR) and identify relevant prognostic factors from clinico-imaging-pathologic features of locally advanced esophageal squamous cell carcinoma (eSCC) patients undergoing trimodality therapy.
Methods
We evaluated 275 patients with eSCCs of T3-T4aN0M0 and T1-T4aN1-N3M0 who received trimodality therapy. We correlated volume-based PET/CT parameters before and after concurrent chemoradiation therapy with pCR after surgery, clinico-imaging-pathologic features, and patient survival.
Results
pCR occurred in 75 (27.3%) of 275 patients, of whom 61 (80.9%) showed 5-year survival. Pre-total lesion glycolysis (pre-TLG, OR = 0.318, 95% CI 0.169 to 0.600), post-metabolic tumor volume (post-MTV, OR = 0.572, 95% CI 0.327 to 0.999), and % decrease of average standardized uptake value (% SUVavg decrease, OR = 2.976, 95% CI = 1.608 to 5.507) were significant predictors for pCR. Among them, best predictor for pCR was pre-TLG with best cutoff value of 205.67 and with AUC value of 0.591.
Performance status (HR = 5.171, 95% CI 1.737 to 15.397), pathologic tumor size (HR = 1.645, 95% CI 1.351 to 2.002), pathologic N status (N1, HR = 1.572, 95% CI 1.010 to 2.446; N2, HR = 3.088, 95% CI 1.845 to 5.166), and post-metabolic tumor volume (HR = 1.506, 95% CI 1.033 to 2.195) were significant predictors of overall survival.
Conclusion
Pre-TLG, post-MTV, and % SUVavg decrease are predictive of pCR. Additionally, several clinico-imaging-pathologic factors are significant survival predictors in locally advanced eSCC patients undergoing trimodality therapy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In early-stage or resectable esophageal squamous cell carcinoma (eSCC), FDG PET/CT has been known to be effective for T staging; its efficacy has been comparable with that of endoscopic ultrasonography. The maximum standardized uptake values (SUVmax) of primary cancers help in differentiating various T stages and predicting patient survival [1,2,3].
Many patients with esophageal cancers present with a locally advanced disease that may be managed with multimodality therapy such as concurrent chemoradiation therapy (CCRT) before surgery [4]. The trimodality therapy (pre-surgical CCRT and surgery) for locally advanced esophageal cancers has been reported to improve survival [5, 6]. The eSCC has been known to be more sensitive to CCRT, demonstrating a higher complete response (CR) rate than esophageal adenocarcinoma [7]. With the CR to neoadjuvant concurrent CCRT, salvage esophagectomy may not be performed because the surgery is prone to postsurgical morbidity (up to 50%) and mortality (up to 10%) [8, 9], and because nonsurgical neoadjuvant CCRT alone based on clinical CR leads to a significant survival benefit compared to patients who did not undergo esophagectomy owing to other reasons [10]. In this context, the accuracy of 18F-fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) in predicting pCR of primary cancers and lymph node metastasis is important. However, there are conflicting data on the role of PET/CT in predicting pathologic complete response (pCR) [11,12,13].
In locally advanced eSCC, metabolic parameters at PET/CT [14,15,16], pCR, and other pathologic features [17,18,19,20,21], patients’ performance status [22], and surgical factors [18, 19] have been reported to be associated with survival. However, no study has used an integrated approach to survival analyses, including imaging and evaluations of pathologic and clinical factors, and included a large number of patients.
Thus, the purpose of this retrospective study was to evaluate the role of PET/CT in the prediction of pCR, and determine the most relevant prognostic factors, including PET/CT parameters and pathologic and clinical features in locally advanced eSCC patients undergoing trimodality therapy.
Methods
The Institutional Review Board (IRB) of the Samsung Medical Center, Seoul, South Korea, approved this study (IRB no. 2020–06–018). The need for informed consent for reviewing the patients’ electronic medical records was waived.
Patient enrollment
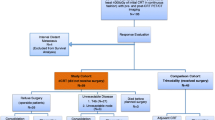
Using the Esophageal Cancer Surgery Registry in a tertiary referral hospital in Seoul in South Korea, 480 patients who received neoadjuvant CCRT followed by esophagectomy and lymph node dissection between January 2007 and December 2018 were identified. All the patients had eSCCs of clinical stages of T3-T4aN0M0 or T1-T4aN1-N3M0 according to the 7th version of the eSCC staging scheme. Among them, 310 patients who underwent PET/CT studies before and after the neoadjuvant CCRT were included. Of the 310 patients, 12 with cervical esophageal cancer (located proximally or 15 cm from the incisor teeth on endoscopy) or gastroesophageal junction cancer (located distally or 40 cm from the incisor teeth) were excluded. Additionally, 23 patients with difficulty-to-analyze volume-based PET/CT parameters due to small tumor size or disturbance from radiation esophagitis were excluded (Fig. 1).
PET/CT acquisition and analysis
FDG PET/CT was performed before (mean, 17 days; range, 1–95 days) and after (mean, 22 days; range, 1–47 days) neoadjuvant CCRT.
All the patients fasted for at least 6 h before PET examination. Blood glucose levels were measured before the injection of FDG; they were required to be < 200 mg/dL. Whole-body PET and unenhanced CT images were acquired using two types of PET/CT scanners (Discovery LS, GE Healthcare, Milwaukee, WI, USA; Discovery STe, GE Healthcare, Milwaukee, WI, USA) 60 min after FDG injection (5.5 MBq/kg). Attenuation-corrected PET images (4.3 × 4.3 × 3.9 mm) were reconstructed from the CT data using an ordered-subset expectation–maximization algorithm (28 subsets, 2 iterations). The standardized uptake value (SUV) was derived from the injected dose of FDG and the patient’s body weight [2].
One nuclear medicine physician (17 years of experience in PET/CT interpretation), who was unaware of the clinical results, visually and quantitatively analyzed the PET images. The physician placed an automatically delineated VOI over the primary eSCC lesion, after which the software was used to calculate the metabolic tumor volume (MTV), maximum SUV (SUVmax), and average SUV (SUVavg) within the entire primary cancer. MTV was defined as the total tumor volume segmented via the threshold SUV (2.5). In addition, we calculated the total lesion glycolysis (TLG), which is a composite parameter determined by multiplying MTV by SUVavg. [23] We measured four PET/CT parameters before and after neoadjuvant CCRT and analyzed a total of eight parameters as follows: pre- and post-SUVmax, pre- and post-SUVavg, pre- and post-MTV, pre- and post-TLG. In addition, % SUVmax decrease ([pre-post/pre] × 100), % SUVavg decrease, % MTV decrease, and % TLG decrease were analyzed and compared with postsurgical pathology results and survival data (PET/CT techniques in the Supplement).
As for nodal station, positive nodes were characterized by FDG uptake higher than that of mediastinal blood pool within nodes and without increase in nodal CT attenuation compared with mediastinal structure [24, 25]. Lymph nodes were classified into 15 nodal-station groups according to a modified lymph node mapping system for esophageal cancer [26]. A volume-based analysis was not performed for the positive node(s).
Neoadjuvant concurrent chemoradiation therapy and surgery
Thoracic irradiation was delivered to patients with a total dose of 44 Gy with 2.2 Gy/fraction over 5 weeks. The chemotherapy regimens consisted of weekly intravenous fluorouracil (4000 mg/m2) plus cisplatin (60 mg/m2) on weeks 1 and 3 in combination with ongoing radiotherapy. Within 3 or 4 weeks following the completion of neoadjuvant therapy, restaging procedures were performed with PET/CT. Unless the restaging workup showed evidence of progressive disease, surgical resection was performed 4–6 weeks following the completion of neoadjuvant therapy.
At our institution, transthoracic esophagectomy, gastric pull-up through posterior mediastinal route, and standard two-field LN dissection (Ivor-Lewis procedure) were adopted as routine surgical strategies for middle to lower thoracic tumors without cervical node involvement, whereas 3-field resection (McKeown procedure) was selectively performed for tumors within the upper thoracic location or suspected cervical lymph node involvement.
Pathologic evaluation
The resected specimen after neoadjuvant CCRT was evaluated by a dedicated pathologist, blinded to the PET/CT analyses results. Tumors were staged according to the American Joint Committee on Cancer staging system, 7th edition for eSCC [27]. The degree of pathological response to neoadjuvant therapy was scored using a tumor regression grade (TLG) classification according to the modified Mandard scoring system: TRG1 = no residual cancer cells; TRG2 = residual cancer cells scattered through the fibrosis; TRG3 = increased residual cancer cells with predominated fibrosis; TRG4 = residual cancer predominate fibrosis; and TRG5 = no regressive changes within the tumor [28]. Among them, TRG1 was used for the classification of pCR. Pathologic complete response (pCR, i.e., ypT0N0) was defined as the absence of viable tumor cells (0%) based on the review of the hematoxylin–eosin–stained slides after a complete evaluation of a resected specimen, including all the regional lymph nodes. Therapy-induced characteristics such as necrosis, fibrosis, and foamy histiocyte collections were considered to be signs of tumor regression after CCRT. The presence of tumor lymphovascular invasion (LVI) or perineural invasion (PNI) was also recorded.
Patient follow-up and calculation of disease-free survival and overall survival
After treatment completion, patients were followed-up at 3-month intervals, and chest CT was performed to detect recurrence after 1 year and at 6-month intervals during the second postsurgical year. When there was evidence of recurrence, additional imaging studies, including whole-body PET/CT, brain MRI, and bone scans, were performed. We diagnosed tumor recurrence based on imaging findings; recurrence was confirmed histologically when clinically needed. The date of recurrence was defined as the date of examination of patients whose diagnoses were based on clinicoradiologic findings or the date of histopathologic confirmation. Overall survival (OS) was calculated from the date of the surgery to that of death from any cause. Disease-free survival (DFS) was calculated from the date of surgery to that of tumor recurrence or the date of the last follow-up.
Statistical analysis
Continuous and categorical variables were summarized as mean (standardized deviation), median (inter-quartile range [IQR, 1st quartile–3rd quartile]), and frequency (percentage), respectively. Tumor size with skewed distribution was transformed by the natural log after adding 1 to all values because of zero values. To compare PET/CT parameters before and after neoadjuvant CCRT, paired T test was used. DFS and OS rates were estimated using the Kaplan–Meier method [29]. For univariable and multivariable analyses, Cox proportional hazards model or logistic regression model was used to identify independent factors for DFS, OS, or pCR. Prior to multivariable modeling, the variables which had P value < 0.1 from univariable analyses were assessed for multicollinearity using variance inflation factor (VIF). Variables with VIF < 3 were entered to a backward elimination process, allowing to build the multivariable model [30]. The proportional hazards assumption was verified for all variables by inspection of the plots of Schoenfeld residual for covariates [31]. Also, the linearity assumption of association between each continuous variable and DFS, OS, or pCR was checked with martingale residuals [32] or a plot of log odds ratios against the mid-point values for each quartile of a continuous variable. Continuous PET/CT parameters which did not fulfill the linearity assumption were converted to categorical variables after estimating cutoff values with the methods based on the maximally selected test statistic for outcomes [32,33,34]. Statistical significance was defined as two-tailed P < 0.05. Testing for multiple factors was corrected using Bonferroni’s method due to inflated type I error. Statistical analysis was executed using SAS version 9.4 (SAS Institute, Cary, NC) and R 4.0.1 (Vienna, Austria; http://www.R-project.org/).
Results
Patient characteristics
Of the 275 patients who received trimodality therapy, 265 had T1-4aN1-3M0 and 10 had T3-4N0M0. Of the 275 patients, 253 (92.0%) were male. The most common tumor location was the middle thoracic (114/275, 41.5%), followed by the lower thoracic (84/275, 30.5%) and upper thoracic (77/275, 28.0%) esophagus. Regarding pathologic stage, stage II was the most common (110/275, 40.0%), followed by stage III (70/275, 5.5%), stage I (14/275, 5.1%), and stage 0 (4/275, 1.5%). Seventy-five patients (27.3%) demonstrated pCR after neoadjuvant CCRT (Table 1).
PET/CT findings after neoadjuvant CCRT
All the values of PET/CT parameters for primary esophageal cancers decreased significantly after neoadjuvant CCRT. SUVmax values decreased significantly from 15.5 ± 5.9 (mean ± SD) to 5.4 ± 2.4 (P < 0.001); similarly, SUVavg, MTV, and TLG values decreased significantly (5.3 ± 1.5 to 3.0 ± 0.5; 39.2 ± 29.6 to 11.8 ± 15.4; 228.8 ± 193.4 to 37.3 ± 49.7, respectively) (Ps < 0.001). Median and range of relative % decrease of PET/CT parameters were as follows: % SUVmax decrease, 65.9 (50.8, 76.3); % SUVavg decrease, 42.6 (31.1, 53.0); % MTV decrease, 78.4 (54.4, 91.1); % TLG decrease, 87.2 (71.1, 95.3) (Table 2).
Prognostic factors related to pathologic complete remission
In the univariable analysis, pre-MTV, pre-TLG, post-SUVmax, post-SUVavg, post-MTV, post-TLG, % SUVmax decrease, and % SUVavg decrease were possible predictive factors for pCR (eTable 1 in the Supplement). Due to the correlation between post-SUVavg, post-MTV, and post-TLG, we analyzed two different models, once with the post-SUVavg and post-MTV and once with post-TLG. After checking multiple collinearity and performing backward selection, three variables were identically selected (pre-TLG, post-MTV, % SUVavg decrease), and multiple logistic regression analysis was performed with these variables.
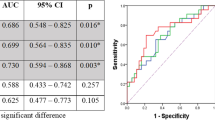
In multivariable analysis, a higher pre-TLG (> 205.67) was associated with a low probability of pCR (OR = 0.318, 95% CI = 0.169–0.600, P = 0.0318). A higher post-MTV (> 4.99) was associated with a low probability of pCR (OR = 0.572, 95% CI = 0.327–0.999, P = 0.0496). Additionally, a higher % SUVavg decrease (> 45.38) was associated with a high probability of pCR (OR = 2.976, 95% CI = 1.608–5.507, P = 0.0005) (Table 3). The cutoffs for each PET parameter related to pCR were as follows: pre-TLG, 205.67; post-MTV, 4.99; % SUVavg decrease, 45.38. The detailed process of cutoff estimation, including sensitivity and specificity, is described in eTable 3 and eFigure 1.
Patients’ survival
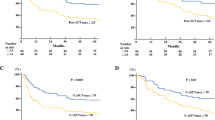
The mean follow-up period was 32 months (range, 6 days–126 months). By the last follow-up date, 114 of the 275 patients (41.5%) had recurrence (mean DFS; 24 months) and 122 (44.4%) died from any cause (mean OS; 32 months). The DFS rates of all the patients at 1, 3, and 5 years were 66.1%, 52.9%, and 51.1%, respectively; the OS rates were 80.3%, 55.2%, and 48.9%, respectively. For patients with pathologic stage II, the 5-year OS rate was 52.9%, which was higher than the 16.8% of stage III patients. The patients with pCR had the highest 5-year OS rate of 80.9% (61 of 75 patients). All the survival curves were significantly different based on the ypTs, ypNs, and pathologic stages (Ps < 0.0001) (Fig. 2). After surgery, the 30-day and 90-day mortality rates were 0.7% (2/275) and 4.0% (11/275), respectively.
Kaplan–Meier survival curves stratified according to pathologic T, N, and M stages. A Overall survival in pathologic T stages. B Overall survival in pathologic N stages. C Overall survival in various pathologic stages. D Disease-free survival in pathologic T stages. E Disease-free survival in pathologic N stages. F Disease-free survival in various pathologic stages
Prognostic factors related to patients’ survival
Various clinico-imaging-pathologic parameters were a possible predictor for patient survival (eTable 2). Owing to the correlation between post-SUVavg, post-MTV, and post-TLG, we analyzed two different models, once with the post-SUVavg and post-MTV and once with post-TLG.
Regarding the prediction of patients’ OS, post-MTV, ECOG score, pathologic tumor size, and pathologic N status were selected for multiple logistic regression analysis. The cutoff for post-MTV related to OS was 7.53 based on the maximally selected test statistic for outcome (eFigure 2). Post-MTV (> 7.53) had higher risk of OS compared to post-MTV (≤ 7.53) (HR = 1.506, 95% CI = 1.033–2.195). ECOG score 2 demonstrated higher risk of OS compared to ECOG score 0 (HR = 5.171, 95% CI = 1.737–15.397). Pathologic tumor size was also independent factor associated with OS (HR = 1.645, 95% CI = 1.351–2.002). And, higher pathologic N status had higher risk of death; the respective HRs (95% CI) were 1.572 (95% CI: 1.010–2.446) and 3.088 (95% CI: 1.845–5.166) for N1 and N2 versus N0 status (Table 3).
Regarding the prediction of patients’ DFS, age, pathologic T stage and pathologic N stage were selected for multiple logistic regression analysis. Age (HR = 0.975, 95% CI = 0.952–0.999) was an independent factor associated with DFS. Higher pathologic T status had a higher risk of recurrence; the respective HRs (95% CI) were 1.755 (95% CI: 0.956–3.219), 1.766 (95% CI: 1.048–2.975), 1.897 (95% CI: 1.105–3.257), and 4.976 (95% CI: 2.221–11.151) for T1, T2, T3, and T4 versus T0 status. And, higher pathologic N status also had a higher risk of recurrence; the respective HRs (95% CI) were 2.078 (95% CI: 1.319–3.274), 3.446 (95% CI: 2.021–5.875), and 4.195 (95% CI: 2.019–8.715) for N1, N2, and N3 versus N0 status (Table 3).
Kaplan–Meier survival curves illustrated the relationship between prognostic factors and the period of survival (Figs. 2 and 3).
Discussion
Trimodality therapy is currently considered the standard of care in locally advanced esophageal cancer, and randomized studies have shown significant survival benefits [6, 35,36,37]. However, two major randomized trials that compared definitive CCRT with trimodality therapy based on patient survival showed similar survival outcomes, even though surgery was associated with improved local control after trimodality therapy [38, 39]. Since surgical resection could result in high morbidity and mortality, if pCR can be predicted by clinic-imaging parameters before surgery, it may be avoided. This study assessed the values of preoperative clinical parameters and FDG PET/CT for predicting pCR in patients with locally advanced eSCC who underwent trimodality therapy; PET parameters such as pre-TLG, post-MTV, and % SUVavg decrease appeared to be independent predictive factors of pCR.
Valkema et al. reported that neither qualitative nor quantitative analysis of PET/CT can accurately detect tumor regression of grades 3–4 (> 10% of vital residual tumor) and discriminate substantial residual disease from benign inflammation-induced uptake after neoadjuvant CCRT [40]. In our study, however, the PET/CT parameters could help predict pCR instead. Smaller values of pre-TLG and post-MTV and higher value of % SUVavg decrease were associated with higher probabilities of pCR; and pre-TLG, which is an initial PET/CT parameter before CCRT, may have been more meaningful. The importance of pre-CCRT TLG value as a predictor of pCR occurs because PET/CT parameters after neoadjuvant CCRT may be affected by the inflammation caused by CCRT effects such as radiation esophagitis.
Additionally, in locally advanced eSCC, volume-based parameters may be more meaningful than simple SUVmax values. SUVmax is based on only a single pixel, not considering the entire tumor volume. For locally advanced eSCC, exhibiting a larger and more complex morphology than early esophageal cancers, volume-based parameters may reflect the metabolism of the entire tumor more precisely. To the best of our knowledge, this is the first study that found the initial volume-based PET/CT parameter can be used to predict pCR.
% SUVavg decrease was a significant predictor of pCR in our study, just as relative changes in PET parameters were predictors of pCR or survival in other studies [41, 42]. Pathologic tumor response is associated with prognosis after trimodality therapy [43, 44]. In cases of locally advanced eSCC, Hamai et al. recently reported that both post-SUVmax and % SUVmax decrease in preoperative PET/CT are significantly associated with ypT, ypN, and cancer recurrence after trimodality therapy. They stated that tumor response to neoadjuvant CCRT, rather than initial metabolic activity of the primary tumor, may be an important factor for predicting patient prognosis [41]. Similarly, in our study, post-MTV and % SUVavg decreases, which are affected by tumor response to neoadjuvant CCRT, were important factors in determining pCR. However, % SUVavg decrease was not a predictor of patient’s survival. This discrepancy may be partially explained by the difficulty in calculating the values of the PET/CT parameters after neoadjuvant CCRT. After neoadjuvant CCRT, small tumor lesions cannot be reliably distinguished from physiologic esophageal metabolism or post-radiation esophagitis. In this respect, the importance of pre-TLG as a predictor of pCR is further emphasized as described above.
There have been many studies to predict pCR using various modalities such as EUS, CT, PET/CT, and MRI [45]. Restaging after neoadjuvant CCRT and prediction of pCR is considered a major challenge because radiologic appearance of treated tumor and lymph nodes are difficult to interpret due to induced fibrosis and ulceration. Even if there are factors that can predict pCR with a high probability, there is a risk of unjustified omission of surgery without pCR. Gouw et al. reported a comparable risk of unjustified omission of surgery without ypCR based on CT (12% of all patients), PET-CT (21%), and MRI (13%). Currently, an organ-preserving strategy is under investigation by the ESOSTRATE and SANO trial [46, 47]. If the evidence for the omission of surgery is uncovered in these trials, our findings would be more meaningful.
Regarding survival, performance status, pathologic tumor size, and N status, and post-MTV were independent prognostic factors of OS, whereas age and pathologic T and N status were independent prognostic factors of DFS. These results are generally consistent with those of previous studies that also assessed the prognostic factors of patients treated with trimodality therapy [19, 20, 22, 48]. Hamai et al. found that the ypN stage and performance status are significantly associated with poor prognosis after trimodality therapy for eSCCs [22]. Guo et al. found that performance status and post-CCRT pathologic stage can be used to predict the survival of patients with locally advanced eSCCs after trimodality therapy [48]. Khan et al. stated that pCR, low nodal index, and margin-negative resection are predictors of DFS [19]. Similarly, in our study, clinical (performance status), pathologic (pathologic tumor size, N status), and metabolic imaging (post-MTV) factors were significant prognostic factors for predicting the survival of patients with locally advanced eSCCs who underwent trimodality therapy.
DFS and OS curves differed significantly for pathologic T, pathologic N, and pathologic stages; generally, higher grades resulted in worse prognoses. However, the survival curve of yp0 stage showed a sharp decrease in slope. In addition, there was a cross-over between the OS curves of yp1 and yp2 stages (Fig. 2C) and an overlap between the DFS curves of yp1 and yp2 stages (Fig. 2F). This phenomenon may be partly explained by the small number of cases involving yp0 (n = 4) and yp1 (n = 14) disease. The findings for the yp2, yp3 and yp4, and pCR stages, accounting for the majority of cases, demonstrated that the survival rates significantly worsened with increasing stage.
Our study has several limitations. First, this study was conducted respectively in a single institution. Therefore, selection bias could not be avoided. Second, there could be an issue with reproducibility in the quantitative measurement of the PET/CT parameters. However, the quantitative tumor SUV changes measured across multiple sites and readers show a high correlation [49]. Third, 23 patients who had difficult-to-analyze volume-based PET/CT parameters were excluded, which may have introduced bias. However, the statistical results for the 298 and 275 patients did not differ. Fourth, in one patient, the time interval between baseline PET/CT and CCRT was as long as 95 days. However, the time interval for the remaining patients was within 40 days. Fifth, there is a limitation of not measuring and analyzing SUVpeak, which is known to other established PET parameters used for response assessment. Analyzing and comparing SUVpeak will be an interesting topic in the future in addition to the PET parameters used in our study. Finally, accreditation of PET/CT scanners was not implemented, and that is a limitation. However, we tried to perform PET/CT with the same scanner before and after neoadjuvant CCRT. Of the 275 patients, 208 (76%) adopted the same scanner before and after neoadjuvant CCRT. Of the 208 patients, 187 (90%) used PET/CT scanner of Discovery STe, GE Healthcare, Milwaukee, WI, USA. Of the 275 patients, 67 (24%) patients used different scanners for follow-up imaging before and after neoadjuvant CCRT. Considering that harmonization method was not applied for compensation, this might have affected the measurement of PET parameters and consequent results.
In conclusion, the pre-TLG, post-MTV, and % decrease of SUVavg values determined by FDG-PET were significant predictors of pCR in patients with locally advanced eSCC who underwent trimodality therapy. Thus, preoperative FDG-PET findings may be used to predict the degree of tumor response; they should be considered when selecting further treatment strategies. Additionally, clinical and pathologic features and post-MTV were significant prognostic factors for predicting patient survival.
Data availability
Not applicable.
Code availability
Not applicable.
Abbreviations
- CCRT:
-
Concurrent chemoradiation therapy
- CI :
-
Confidence interval
- DFS :
-
Disease-free survival
- EUS:
-
Endoscopic ultrasonography
- MTV :
-
Metabolic tumor volume
- eSCC :
-
Esophageal squamous cell carcinoma
- LVI :
-
Lymphovascular invasion
- OR :
-
Odds ratio
- OS :
-
Overall survival
- PNI :
-
Perineural invasion
- pCR :
-
Pathologic complete response
- RNL:
-
Recurrent laryngeal nerve
- SD:
-
Standard deviation
- SUV:
-
Standardized uptake value
- SUVavg :
-
Average standardized uptake value
- SUVmax:
-
Maximum standardized uptake value
- TLG:
-
Total lesion glycolysis
References
Jeong DY, Kim MY, Lee KS, et al. Surgically resected T1- and T2-stage esophageal squamous cell carcinoma: T and N staging performance of EUS and PET/CT. Cancer Med. 2018;7(8):3561–70. https://doi.org/10.1002/cam4.1617.
Jeong DY, Lee KS, Choi JY, et al. Surgically resected esophageal squamous cell carcinoma: patient survival and clinicopathological prognostic factors. Sci Rep. 2020;10(1):5077. https://doi.org/10.1038/s41598-020-62028-5.
Huang YC, Lu HI, Huang SC, et al. FDG PET using SUVmax for preoperative T-staging of esophageal squamous cell carcinoma with and without neoadjuvant chemoradiotherapy. BMC Med Imaging. 2017;17(1):1. https://doi.org/10.1186/s12880-016-0171-7.
Chuang HH, Macapinlac HA. The evolving role of PET-CT in the management of esophageal cancer. Q J Nucl Med Mol Imaging. 2009;53(2):201–9.
Gebski V, Burmeister B, Smithers BM, et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol. 2007;8(3):226–34. https://doi.org/10.1016/S1470-2045(07)70039-6.
Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26(7):1086–92. https://doi.org/10.1200/JCO.2007.12.9593.
Bollschweiler E, Metzger R, Drebber U, et al. Histological type of esophageal cancer might affect response to neo-adjuvant radiochemotherapy and subsequent prognosis. Ann Oncol. 2009;20(2):231–8. https://doi.org/10.1093/annonc/mdn622.
Connors RC, Reuben BC, Neumayer LA, Bull DA. Comparing outcomes after transthoracic and transhiatal esophagectomy: a 5-year prospective cohort of 17,395 patients. J Am Coll Surg. 2007;205(6):735–40. https://doi.org/10.1016/j.jamcollsurg.2007.07.001.
Chang AC, Ji H, Birkmeyer NJ, Orringer MB, Birkmeyer JD. Outcomes after transhiatal and transthoracic esophagectomy for cancer. Ann Thorac Surg. 2008;85(2):424–9. https://doi.org/10.1016/j.athoracsur.2007.10.007.
Depypere L, Thomas M, Moons J, et al. Analysis of patients scheduled for neoadjuvant therapy followed by surgery for esophageal cancer, who never made it to esophagectomy. World J Surg Oncol. 2019;17(1):89. https://doi.org/10.1186/s12957-019-1630-8.
Arnett ALH, Merrell KW, Macintosh EM, et al. Utility of (18)F-FDG PET for predicting histopathologic response in esophageal carcinoma following chemoradiation. J Thorac Oncol. 2017;12(1):121–8. https://doi.org/10.1016/j.jtho.2016.08.136.
Elimova E, Wang X, Etchebehere E, et al. 18-fluorodeoxy-glucose positron emission computed tomography as predictive of response after chemoradiation in oesophageal cancer patients. Eur J Cancer. 2015;51(17):2545–52. https://doi.org/10.1016/j.ejca.2015.07.044.
Song SY, Kim JH, Ryu JS, et al. FDG-PET in the prediction of pathologic response after neoadjuvant chemoradiotherapy in locally advanced, resectable esophageal cancer. Int J Radiat Oncol Biol Phys. 2005;63(4):1053–9. https://doi.org/10.1016/j.ijrobp.2005.03.033.
Li Y, Zschaeck S, Lin Q, Chen S, Chen L, Wu H. Metabolic parameters of sequential 18F-FDG PET/CT predict overall survival of esophageal cancer patients treated with (chemo-) radiation. Radiat Oncol. 2019;14(1):35. https://doi.org/10.1186/s13014-019-1236-x.
Hyun SH, Ahn HK, Ahn MJ, et al. Volume-based assessment with 18F-FDG PET/CT improves outcome prediction for patients with stage IIIA-N2 non-small cell lung cancer. AJR Am J Roentgenol. 2015;205(3):623–8. https://doi.org/10.2214/ajr.14.13847.
Hamai Y, Hihara J, Emi M, et al. Ability of fluorine-18 fluorodeoxyglucose positron emission tomography to predict outcomes of neoadjuvant chemoradiotherapy followed by surgical treatment for esophageal squamous cell carcinoma. Ann Thorac Surg. 2016;102(4):1132–9. https://doi.org/10.1016/j.athoracsur.2016.04.011.
Blum Murphy M, Xiao L, Patel VR, et al. Pathological complete response in patients with esophageal cancer after the trimodality approach: the association with baseline variables and survival-The University of Texas MD Anderson Cancer Center experience. Cancer. 2017;123(21):4106–13. https://doi.org/10.1002/cncr.30953.
Kurokawa T, Hamai Y, Emi M, et al. Risk factors for recurrence in esophageal squamous cell carcinoma without pathological complete response after trimodal therapy. Anticancer Res. 2020;40(8):4387–94. https://doi.org/10.21873/anticanres.14442.
Khan M, Urooj N, Syed AA, et al. Prognostic factors for recurrence in esophageal cancer patients treated with neoadjuvant therapy and surgery: a single-institution analysis. Cureus. 2020;12(5): e8108. https://doi.org/10.7759/cureus.8108.
Tu CC, Hsu PK, Chien LI, et al. Prognostic histological factors in patients with esophageal squamous cell carcinoma after preoperative chemoradiation followed by surgery. BMC Cancer. 2017;17(1):62. https://doi.org/10.1186/s12885-017-3063-5.
Stahl M, Lehmann N, Walz MK, Stuschke M, Wilke H. Prediction of prognosis after trimodal therapy in patients with locally advanced squamous cell carcinoma of the oesophagus. Eur J Cancer. 2012;48(16):2977–82. https://doi.org/10.1016/j.ejca.2012.03.010.
Hamai Y, Hihara J, Emi M, et al. Evaluation of prognostic factors for esophageal squamous cell carcinoma treated with neoadjuvant chemoradiotherapy followed by surgery. World J Surg. 2018;42(5):1496–505. https://doi.org/10.1007/s00268-017-4283-1.
Lee HY, Hyun SH, Lee KS, et al. Volume-based parameter of 18)F-FDG PET/CT in malignant pleural mesothelioma: prediction of therapeutic response and prognostic implications. Ann Surg Oncol. 2010;17(10):2787–94. https://doi.org/10.1245/s10434-010-1107-z.
Shim SS, Lee KS, Kim BT, et al. Non-small cell lung cancer: prospective comparison of integrated FDG PET/CT and CT alone for preoperative staging. Radiology. 2005;236(3):1011–9. https://doi.org/10.1148/radiol.2363041310.
Lee JY, Kim YH, Park YJ, et al. Improved detection of metastatic lymph nodes in oesophageal squamous cell carcinoma by combined interpretation of fluorine-18-fluorodeoxyglucose positron-emission tomography/computed tomography. Cancer Imaging. 2019;19(1):40. https://doi.org/10.1186/s40644-019-0225-5.
Korst RJ, Rusch VW, Venkatraman E, et al. Proposed revision of the staging classification for esophageal cancer. J Thorac Cardiovasc Surg. 1998;115(3):660–669; discussion 669–670. https://doi.org/10.1016/s0022-5223(98)70332-0
Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17(7):1721–4. https://doi.org/10.1245/s10434-010-1024-1.
Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73(11):2680–6. https://doi.org/10.1002/1097-0142(19940601)73:11%3c2680::aid-cncr2820731105%3e3.0.co;2-c.
Kaplan ELMP. Nonparametric-estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–81. https://doi.org/10.1080/01621459.1958.10501452.
Kock NLGS. Lateral collinearity and misleading results in variance-based SEM: an illustration and recommendations. J Assoc Inf Syst. 2012;13(7):546–80.
Schoenfeld DA. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69:239–41.
Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80:557–72.
Miller RaS D. Maximally selected chi square statistics. Biometrics. 1982;38:1011–6.
Contal C, O’Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Comput Stat Data Anal. 1999;30:253–70.
Shapiro J, van Lanschot JJB, Hulshof M, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16(9):1090–8. https://doi.org/10.1016/s1470-2045(15)00040-6.
Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12(7):681–92. https://doi.org/10.1016/s1470-2045(11)70142-5.
van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–84. https://doi.org/10.1056/NEJMoa1112088.
Bedenne L, Michel P, Bouché O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25(10):1160–8. https://doi.org/10.1200/jco.2005.04.7118.
Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23(10):2310–7. https://doi.org/10.1200/jco.2005.00.034.
Valkema MJ, Noordman BJ, Wijnhoven BPL, et al. Accuracy of (18)F-FDG PET/CT in predicting residual disease after neoadjuvant chemoradiotherapy for esophageal cancer. J Nucl Med. 2019;60(11):1553–9. https://doi.org/10.2967/jnumed.118.224196.
Hamai Y, Emi M, Ibuki Y, et al. Predictions of pathological features and recurrence based on FDG-PET Findings of esophageal squamous cell carcinoma after trimodal therapy. Ann Surg Oncol. 2020. https://doi.org/10.1245/s10434-020-08609-0.
Borggreve AS, Goense L, van Rossum PSN, et al. Preoperative prediction of pathologic response to neoadjuvant chemoradiotherapy in patients with esophageal cancer using (18)F-FDG PET/CT and DW-MRI: a prospective multicenter study. Int J Radiat Oncol Biol Phys. 2020;106(5):998–1009. https://doi.org/10.1016/j.ijrobp.2019.12.038.
Alnaji RM, Du W, Gabriel E, et al. Pathologic complete response is an independent predictor of improved survival following neoadjuvant chemoradiation for esophageal adenocarcinoma. J Gastrointest Surg. 2016;20(9):1541–6. https://doi.org/10.1007/s11605-016-3177-0.
Berger AC, Farma J, Scott WJ, et al. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol. 2005;23(19):4330–7. https://doi.org/10.1200/jco.2005.05.017.
de Gouw D, Klarenbeek BR, Driessen M, et al. Detecting pathological complete response in esophageal cancer after neoadjuvant therapy based on imaging techniques: a diagnostic systematic review and meta-analysis. J Thorac Oncol. 2019;14(7):1156–71. https://doi.org/10.1016/j.jtho.2019.04.004.
Bedenne L. MC. Comparison of systematic surgery versus surveillance and rescue surgery in operable oesophageal cancer with a complete clinical response to radiochemotherapy (esostrate).https://clinicaltrials.gov/ct2/show/NCT02551458.
Noordman BJ, Wijnhoven BPL, Lagarde SM, et al. Neoadjuvant chemoradiotherapy plus surgery versus active surveillance for oesophageal cancer: a stepped-wedge cluster randomised trial. BMC Cancer. 2018;18(1):142. https://doi.org/10.1186/s12885-018-4034-1.
Guo JC, Huang TC, Lin CC, et al. Postchemoradiotherapy pathologic stage classified by the American Joint Committee on the Cancer Staging System predicts prognosis of patients with locally advanced esophageal squamous cell carcinoma. J Thorac Oncol. 2015;10(10):1481–9. https://doi.org/10.1097/jto.0000000000000651.
O JH, Jacene H, Luber B, et al. Quantitation of cancer treatment response by (18)F-FDG PET/CT: multicenter assessment of measurement variability. J Nucl Med. 2017;58(9):1429–1434. https://doi.org/10.2967/jnumed.117.189605
Acknowledgements
We are grateful for the librarians Myung-Ah Shim and Jaero Park for their dedicated support of manuscript formatting. Both librarians are working at the Samsung Medical Information & Media Services of Samsung Medical Center located in Seoul, South Korea.
Funding
This work was supported by the National R&D Program for Cancer Control, Ministry of Health & Welfare of Korea [1720180].
Author information
Authors and Affiliations
Contributions
Study conception and design: Yeonu Choi, Joon Young Choi, Hong Kwan Kim, Kyung Soo Lee. Data acquisition and analysis: Yeonu Choi, Joon Young Choi, Tae Hee Hong, Yoon-La Choi, Sook Young Woo, Kyung Soo Lee. Data interpretation and manuscript writing: Yeonu Choi, Joon Young Choi, Tae Hee Hong, Yoon-La Choi, Sook Young Woo, Kyung Soo Lee. Revision of manuscript and contribution of intellectual content: All authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yeonu Choi and Joon Young Choi contributed equally to this manuscript.
This article is part of the Topical Collection on Oncology - Digestive tract
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Choi, Y., Choi, J.Y., Hong, T.H. et al. Trimodality therapy for locally advanced esophageal squamous cell carcinoma: the role of volume-based PET/CT in patient management and prognostication. Eur J Nucl Med Mol Imaging 49, 751–762 (2022). https://doi.org/10.1007/s00259-021-05487-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-021-05487-w