Abstract
Background
The degree of metabolic activity in tumor cells can be determined by 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET). Associations between FDG uptake by primary tumors of locally advanced esophageal squamous cell carcinoma (ESCC) under trimodal therapy and the pathological features of such tumors have not been fully investigated.
Patients and Methods
We evaluated relationships between the maximal standardized uptake (SUVmax) in primary tumors on preoperative PET images and pathological features as well as cancer recurrence in 143 patients with ESCC who underwent neoadjuvant chemoradiotherapy (NCRT) followed by surgery.
Results
The post-SUVmax significantly differed after NCRT for ypT and ypN status, lymphatic invasion (LI), venous invasion (VI), and recurrence. Furthermore, the %ΔSUVmax (rate of decrease between before and after NCRT) for LI, VI, and recurrence significantly differed. Univariate and multivariate analyses selected post-SUVmax and %ΔSUVmax as independent preoperative predictors of recurrence-free survival [hazard ratio (HR) 1.46; 95% confidence interval (CI) 1.24–1.72 and HR 0.97; 95% CI 0.96–0.99, respectively; p < 0.001 for both]. Recurrence-free and overall survival were significantly stratified according to optimal SUVmax cutoffs for predicting recurrence (post- and %ΔSUVmax: 2.8 and 70, respectively).
Conclusions
The post- and %ΔSUVmax of primary tumors were significantly associated with the pathological features and recurrence of ESCC under trimodal therapy. Therefore, FDG-PET can preoperatively predict the degree of aggressive tumor behavior in ESCC under trimodal therapy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Neoadjuvant chemoradiotherapy (NCRT) followed by surgery (trimodal therapy) is frequently needed for local control and to improve the survival of patients with locally advanced esophageal cancer.1,2,3,–4 Although the survival of patients with esophageal cancer has been improved by multimodal therapy, some patients still develop recurrence and die of cancer even after undergoing surgical procedures with curative intent and intensive NCRT. Prognoses after trimodal therapy correlate with the pathological features of tumor depth,5 lymph node (LN) metastasis,6,7,–8 lymphovascular invasion,8,9 and pathological tumor response.7,10
The degree of metabolic activity in tumor cells can be determined by 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET), and thus tumor staging can be improved in patients with esophageal cancer.11 Some studies, including ours, have shown that FDG-PET could differentiate pathological responders from nonresponders to neoadjuvant therapy.11,12,–13 However, associations between other pathological features and FDG uptake (maximal standardized uptake value: SUVmax) by primary locally advanced esophageal squamous cell carcinoma (ESCC) have not been fully investigated as far as we can ascertain. If malignant aggressive behavior could be predicted using preoperative FDG-PET, then treatment strategies could be optimized, and the prognosis of esophageal cancer might also become predictable. The present study aims to identify relationships between FDG uptake in primary ESCC tumors on preoperative PET images and the pathological features of such tumors, as well as recurrence among patients treated with NCRT followed by surgery.
Patients and Methods
Patients
Patients with performance status 0 or 1 according to the Eastern Cooperative Oncology Group (ECOG) criteria underwent NCRT and surgery if cancer of the esophagus or gastroesophageal junction was resectable and if a tumor was more deeply invasive than cT2, positive for LN metastasis (cN+), or resectable supraclavicular LN metastasis (cM1 LYM). Two patients with cT4 primary tumors that had been reduced and thus rendered potentially resectable after NCRT underwent esophagectomy.
We started preoperative evaluations of patients with esophageal cancer using FDG-PET/computed tomography (CT) during October 2006. We reviewed 143 consecutive patients with ESCC who were evaluated by FDG-PET/CT before and after NCRT induction and treated by esophagectomy with R0 resection between October 2006 and August 2019. Table 1 presents the characteristics of the patients. The clinicopathological profiles of the tumors and the definition of R0 resection as neither microscopic nor macroscopic residual tumors after surgery were based on the TNM Classification of Malignant Tumors, 7th edition.14
Recurrence and survival outcomes were evaluated in 126 patients who had been surgically treated up to September 2017 and followed up for at least 2 years. The Institutional Review Board at Hiroshima University approved this study.
Neoadjuvant Chemoradiotherapy and Surgery
Neoadjuvant chemoradiotherapy and surgery comprised concurrent radiotherapy (40 Gy in 20 fractions) and chemotherapy with 5-fluorouracil and either docetaxel or cisplatin or a combination of both, as described.7,10,12,15,16,17,–18 The chemotherapy regimens were docetaxel/5-fluorouracil, cisplatin/5-fluorouracil, docetaxel/cisplatin/5-fluorouracil, or nedaplatin/5-fluorouracil in 10 (7.0%), 110 (76.9%), 18 (12.6%), and 5 (3.5%) patients, respectively.
External beam radiotherapy with 10-MV x-rays was concurrently applied at five fractions per week for 4 weeks (total dose, 40 Gy). Three-dimensional treatment was planned using a CT simulator. The primary tumor was included with a craniocaudal margin of 2–3 cm. The irradiation field for cervical tumors included the region from the supraclavicular, cervical, and upper mediastinal lymph nodes. The irradiation field for upper thoracic tumors included the regions from the supraclavicular, cervical, and mediastinal lymph nodes to the carina. The field for midthoracic or lower thoracic tumors included the cervical, mediastinal, and perigastric lymph nodes, and the supraclavicular fossa was included if the cervical nodes tested positive. The field for esophagogastric junction tumors included the mediastinal (lower than subcarinal), perigastric, and celiac lymph nodes.15,16,18
Surgery was scheduled for all patients at 4–8 weeks after completing NCRT. All patients underwent open transthoracic or thoracoscopic esophagectomy and at least two-field (thoracic and abdominal fields) LN dissection. Esophageal cancer in the upper and middle third of the thoracic esophagus and LN metastasis in the superior mediastinum were essentially treated by cervical lymphadenectomy. A gastric tube was subsequently lifted via the posterior mediastinal or retrosternal route for cervical anastomosis with the esophagus.
FDG-PET/CT Imaging
Tumors in all patients were clinically staged based on systematic FDG-PET/CT imaging findings before and after NCRT. Patients were assessed by FDG-PET/CT after NCRT at a mean ± standard deviation (SD) of 35 ± 15 days after completing radiation therapy.
Patients fasted for at least 4 h before receiving an intravenous injection of 3.7 MBq/kg FDG and then rested for 1–1.5 h before images were acquired. Blood glucose levels were determined before tracer injection to confirm a < 150 mg/dL level. Patients with blood glucose levels ≥ 150 mg/dL were excluded from imaging. All images were acquired using a GE Discovery ST16 integrated PET/CT scanner (GE Healthcare, Little Chalfont, UK) or a Siemens Biograph mCT (Siemens Healthcare GmbH, Erlangen, Germany) scanner. Low-dose nonenhanced CT images of 2- to 4-mm-thick sections were obtained from the head to the pelvic floor of each patient for attenuation correction, and the localization of lesions identified using PET according to a standard protocol. Immediately after CT, PET covered the identical axial field of view for 2–4 min per table position depending on the condition of the patient and scanner performance. The SUVmax for each patient was established by drawing regions of interest (ROI) around the primary tumor on attenuation-corrected FDG-PET images and calculated using the software integrated within the PET/CT scanner based on the formula: SUVmax = (C [mCi/mL]/ID [mCi])/w, where C represents the activity at a pixel within the tissue identified by the ROI and ID represents the injected dose/kg of body weight (w). The SUVmax was measured by qualified radiologists with at least 10 years of experience in FDG-PET diagnosis. If a primary tumor was unidentified due to clinically complete response in FDG-PET after NCRT, ROI was set in the same area as those of FDG-PET before NCRT. Interdevice variations in SUV were minimized using an NEMA NU2-2001 anthropomorphic body phantom (Data Spectrum Corp., Hillsborough, NC) as follows: A calibration factor was assessed by dividing the actual SUV by the gauged mean SUV in the phantom background to decrease interdevice variations in SUV; The final SUV used in this study is referred to as the revised SUVmax because this has the advantage of reproducibility compared with mean SUV;19 The adjusted interdevice variations in SUV decreased the range from 0.93 to 0.98.
We analyzed relationships between pathological features and the SUVmax of the primary tumor before and after NCRT (pre- and post-SUVmax, respectively) and the rate of decrease in the SUVmax (%ΔSUVmax) after NCRT, where %ΔSUVmax = (pre-SUVmax – post-SUVmax)/pre-SUVmax × 100.
Clinical Responses and Pathological Assessment
Clinical tumor responses between pre-NCRT and restaging examinations before surgery were assessed according to the Response Evaluation Criteria in Solid Tumors criteria.20 Overall responses were determined using CT and gastrointestinal endoscopy from a combination of primary tumor and metastatic LN responses and the presence or absence of new lesions. When a measurable lesion was not evident on CT images and when only objectively nonmeasurable lesions such as primary tumors without LN metastases were identified, the overall response was determined by gastrointestinal endoscopy of the primary tumor.
Resected esophageal and LN specimens were fixed in formalin immediately after surgery. All areas that were thought to be primary tumors before treatment were cut into 5-mm sections, embedded in paraffin, and stained with hematoxylin–eosin. Residual tumors and tumor depth were pathologically assessed. Specific immunostaining (D2-40) and elastica van Gieson stain were routinely applied along with standard hematoxylin and eosin staining to evaluate lymphatic and venous invasion, respectively. All LN were cut along the longest axis and stained with hematoxylin–eosin; then metastasis was evaluated.
Follow-Up Protocol
All patients underwent postoperative medical and blood examinations and CT imaging every 3–4 months for at least 2 years after surgery and every 6 months from 3 years thereafter and endoscopy annually. More detailed examinations proceeded if any symptoms were reported. Almost all survivors attended an outpatient clinic for annual health checks after 5 years. Recurrence was diagnosed by radiology and, when possible, by cytology or histology.
Statistical Analysis
We compared SUVmax with each pathological factor and recurrence using unpaired t-tests. Optimal SUVmax cutoffs for significant factors in these analyses were determined from receiver operator characteristics (ROC) curves.
The effects of various clinical parameters on recurrence-free survival (RFS) were evaluated using univariate analysis and multivariate Cox proportional hazards analysis. Factors with p < 0.1 on the univariate analysis were included in the multivariate analysis. Survival data were analyzed using the Kaplan–Meier method and compared using log-rank tests. RFS was defined as the elapsed time from the date of surgery until esophageal cancer recurrence or the most recent follow-up. Overall survival (OS) was defined as the elapsed time from the date of surgery until death from any cause or the most recent follow-up. All data were statistically analyzed using SPSS software version 20.0 (IBM Corporation, Armonk, NY).
Results
SUVmax of Primary Tumor According to Pathological Factors and Recurrence
Table 2 presents the relationships between the pre-, post-, and %ΔSUVmax of the primary tumor and pathological factors as well as cancer recurrence. Pre-SUVmax values were not associated with either pathological features or recurrence.
Post-SUVmax values for ypT (0 versus 2: p = 0.01, 0 versus 3/4: p < 0.001), ypN (0 versus 2: p = 0.03, 0 versus 3: p = 0.01), lymphatic invasion (LI, − vs. +: p = 0.02), and venous invasion (VI, − vs. +: p = 0.02) significantly differed. Furthermore, the post-SUVmax was significantly higher in patients with than without recurrence (p = 0.01). The %ΔSUVmax values for ypT (0 versus 3/4: p = 0.005), LI (− vs. +: p = 0.02), and VI (− vs. +: p = 0.02) significantly differed. Furthermore, the %ΔSUVmax was significantly higher among patients with than without recurrence (p = 0.02).
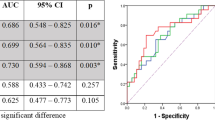
Optimal SUVmax Cutoffs According to ROC Curve Analyses to Predict Pathological Factors and Recurrence
The optimal SUVmax cutoffs for significant factors in the above analyses were determined from ROC curves (Table 3). Optimal post-SUVmax cutoffs that were significant for predicting aggressive pathological features ranged from 2.8 to 3.7 (3.0 for ypT 0/1 versus 2/3/4; 3.0 for ypN 0 versus 1/2/3; 3.7 for ypN 0/1 versus 2/3; 3.0 for LI − versus + and 3.5 for VI − versus +). Furthermore, the optimal %ΔSUVmax cutoffs for predicting aggressive pathologic features ranged from 60 to 75% (75 for LI − versus +, 60 for VI − versus +).
The optimal post-SUVmax cutoff for predicting recurrence was 2.8, with diagnostic sensitivity and specificity of 61.5% and 62.2%, respectively (p = 0.03). The optimal %ΔSUVmax cutoff for predicting recurrence was 70, with diagnostic sensitivity and specificity of 63.5% and 55.8%, respectively (p = 0.02).
Univariate and Multivariate Analyses of Preoperative Factors for Recurrence-Free Survival
Various preoperative clinical factors, including post- and %ΔSUVmax with the cutoffs described above, were evaluated as prognostic indicators in Cox regression models (Table 4). Univariate analysis showed that cN (p = 0.03), cM (p = 0.01), post-SUVmax (p < 0.001), and %ΔSUVmax (p = 0.01) for RFS were statistically significant.
Subsequently, factors with p < 0.1 on univariate analysis (gender, cN, cM, and clinical response) and post- or %ΔSUVmax were simultaneously entered into separate multivariate analyses that included either post- or %ΔSUVmax along with the other factors to avoid confounding. Among these variables, post-SUVmax was an independent covariate for RFS [multivariate analysis 1: hazard ratio (HR) 1.46; 95% confidence interval (CI) 1.24–1.72; p < 0.001]. Furthermore, %ΔSUVmax was also an independent covariate for RFS (multivariate analysis 2: HR 0.97; 95% CI 0.96–0.99; p < 0.001).
Survival According to Optimal SUVmax Cutoffs for Recurrence
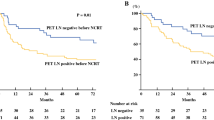
The 5-year RFS and OS rates for patients with post-SUVmax ≥ 2.8 and < 2.8 were 34.6% and 59.0% (p = 0.001; Fig. 1a) and 39.1% and 61.9%, respectively (p = 0.01; Fig. 1b). The 5-year RFS and OS rates for patients with %ΔSUVmax < 70 and ≥ 70 were 37.2% and 55.5% (p = 0.003; Fig. 1c) and 39.7% and 60.8%, respectively (p = 0.01; Fig. 1d).
Survival according to optimal SUVmax cutoffs for recurrence: a recurrence-free and b overall survival rates in patients with post-SUVmax ≥ 2.8 and < 2.8: p = 0.001 and p = 0.01, respectively; c recurrence-free and d overall survival rates in patients with %ΔSUVmax ≥ 70 and < 70: p = 0.003 and p = 0.01, respectively
Discussion
Patients with locally advanced esophageal cancer often undergo trimodal therapy comprising NCRT followed by surgery.1,2,3,–4 The present study assesses the value of preoperative FDG-PET for predicting various pathological features and recurrence in a uniform cohort of patients with locally advanced ESCC who underwent NCRT and subsequent curative surgery with adequate LN dissection. We found that both post- and %ΔSUVmax evaluated by preoperative FDG-PET were significantly associated with ypT and ypN status, LI and VI, and cancer recurrence after trimodal therapy.
The status of ypT after trimodal therapy is an important prognostic factor for esophageal cancer.5 Tumor depth is frequently assessed by endoscopic ultrasonography (EUS). However, EUS has some disadvantages in terms of evaluating tumor depth, especially in advanced esophageal cancer treated with NCRT. EUS frequently does not pass the part of esophageal stenosis due to advance esophageal cancer. Furthermore, measuring tumor depth after NCRT using EUS is reportedly inaccurate. The ability of EUS to differentiate between inflammatory changes due to NCRT and residual disease at primary sites is also limited, and its overall reported accuracy ranges between 27 and 50%.21,22 Post-SUVmax in the present study significantly distinguished between ypT 0/1 and ypT 2/3/4. Therefore, FDG-PET can contribute to more accurate preoperative predictions of advanced tumor depth of ESCC treated with NCRT.
Pathological LN metastasis is an important prognostic factor for esophageal cancer treated with trimodal therapy.6,7,–8 The present study indicates that the post-SUVmax of a primary tumor after NCRT is a significant predictor of pathological LN metastasis, especially for patients with ypN 2/3. The high residual SUVmax of primary tumors even after NCRT represents malignant tumor activity and a low tumor response, and this might also be associated with LN metastasis. In fact, tumors in three of our patients were diagnosed by CT and FDG-PET as cN0 before treatment but were ypN2 or 3 after trimodal therapy. Their post-SUVmax were 3.8, 5.1, and 7.8, and all values were > 3.7, which is the cutoff for predicting ypN2/3. If enlarged LN with high FDG uptake was not indicated by preoperative CT and FDG-PET, the possibility of occult LN metastasis should be considered when the SUVmax of a primary tumor is high after NCRT.
The invasion of lymphatic ducts and vessels by tumor cells indicates aggressive malignant behavior. Actually, LI and VI are independent prognostic factors for survival after initial surgical ESCC resection23,24,–25 and after neoadjuvant therapy followed by surgery.8,9 Therefore, the preoperative diagnosis of LI and VI is very important for predicting the prognosis of esophageal cancer treated by not only surgery but also neoadjuvant therapy before surgery. Although imaging modalities cannot preoperatively diagnose LI and VI, the present findings suggest that LI and VI can be preoperatively predicted from the post- and %ΔSUVmax of primary tumors in patients with ESCC after NCRT.
Although pre-SUVmax was not entirely associated with the pathological features and prognosis, both post- and Δ%SUVmax were significantly associated with aggressive malignant behaviors, such as advanced tumor depth, LN metastasis, LI, and VI in the present study. Thus, post- and Δ%SUVmax were also significantly associated with cancer recurrence. The SUVmax of a primary tumor might correlate with the degree of tumor metabolic activity, which would indicate the malignant potential of ESCC. However, the tumor response to NCRT was also associated with malignant behavior of ESCC treated with trimodal therapy. Therefore, both post- and %ΔSUVmax, which are influenced by the tumor response to NCRT, rather than pre-SUVmax, were important factors in determining the malignant pathological features and predicting the prognosis of patients with ESCC who underwent trimodal therapy.
The present univariate and multivariate analyses of RFS show that risk of recurrence significantly increases together with a higher post-SUV and a lower %ΔSUVmax. These findings indicate that patients with a very high post-SUV or low %ΔSUVmax after NCRT are at high risk of recurrence even after highly invasive esophageal surgery. Therefore, these two values might be able to serve as important indicators of appropriate strategies after NCRT for ESCC. If, for example, the risk of recurrence is considered high among surgical patients with severe comorbidities or patients who are reluctant to undergo surgery, highly invasive surgery should probably be omitted after NCRT, and additional CRT might also be a useful treatment option for such patients. Other treatment strategies for patients with a high post-SUV or low %ΔSUVmax after NCRT and a high risk of recurrence even after curative esophagectomy could include more intensive NCRT, additional neoadjuvant chemotherapy, and/or postoperative adjuvant therapy.
The present study has some limitations, one of which is its retrospective design. Others are that the chemotherapy regimens varied at different times during the study period, and the intervals between completing NCRT and assessment by FDG-PET somewhat differed among the patients. Furthermore, the reproducibility of the SUV measurements and the interobserver variability of evaluating primary tumors on FDG-PET images before and after NCRT might be problematic. However, quantitative changes in tumor SUV closely correlate in assessments of interobserver variability among FDG PET parameters when assessing responses to cancer treatment across multiple sites and evaluators.26 The present study also included a relatively large cohort of uniform patients with locally advanced ESCC all of whom underwent NCRT with 40 Gy of radiation followed by surgically adequate LN dissection.
In conclusion, the preoperative SUVmax determined by FDG-PET was significantly associated with the pathological features (ypT, ypN, LI, and VI) and recurrence of ESCC treated with trimodal therapy. Thus, SUVmax after NCRT and the ratios of changes during NCRT should help to predict the degree of aggressive tumor behavior and recurrence among patients with ESCC and should be considered when selecting treatment strategies.
References
Sjoquist KM, Burmeister BH, Smithers BM, et al; Australasian Gastro-Intestinal Trials Group. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011; 12: 681–692.
Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet. 2017; 390: 2383–96.
van Hagen P, Hulshof MC, van Lanschot JJ, et al. CROSS Group: Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012; 366: 2074–84.
Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodal therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008; 26: 1086–92.
Sepesi B, Schmidt HE, Lada M, et al. Survival in patients with esophageal adenocarcinoma undergoing trimodality therapy is independent of regional lymph node location. Ann Thorac Surg. 2016; 101:1075–80.
Akutsu Y, Shuto K, Kono T, et al. The number of pathologic lymph nodes involved is still a significant prognostic factor even after neoadjuvant chemoradiotherapy in esophageal squamous cell carcinoma. J Surg Oncol. 2012; 105: 756–60.
Hamai Y, Hihara J, Emi M, et al. Evaluation of prognostic factors for esophageal squamous cell carcinoma treated with neoadjuvant chemoradiotherapy followed by surgery. World J Surg. 2018; 42: 1496–1505.
Hamai Y, Emi M, Ibuki Y, et al. Early recurrence and cancer death after trimodal therapy for esophageal squamous cell carcinoma. Anticancer Res. 2019; 39: 1433–1440.
Chen WH, Huang YL, Chao YK, et al. Prognostic significance of lymphovascular invasion in patients with esophageal squamous cell carcinoma treated with neoadjuvant chemoradiotherapy. Ann Surg Oncol. 2015; 22: 338–43.
Hamai Y, Hihara J, Taomoto J, Yamakita I, Ibuki Y, Okada M. Effects of neoadjuvant chemoradiotherapy on postoperative morbidity and mortality associated with esophageal cancer. Dis Esophagus. 2015; 28: 358–64.
Schmidt T, Lordick F, Herrmann K, Ott K. Value of functional imaging by PET in esophageal cancer. J Natl Compr Canc Netw. 2015; 13: 239–47.
Hamai Y, Hihara J, Emi M, et al. Ability of fluorine-18 fluorodeoxyglucose positron emission tomography to predict outcomes of neoadjuvant chemoradiotherapy followed by surgical treatment for esophageal squamous cell carcinoma. Ann Thorac Surg. 2016; 102: 1132–39.
Cong L, Wang S, Gao T, Hu L. The predictive value of 18F-FDG PET for pathological response of primary tumor in patients with esophageal cancer during or after neoadjuvant chemoradiotherapy: a meta-analysis. Jpn J Clin Oncol. 2016; 46:1118–26.
Sobin L, Gospodarowicz M, Wittekind C, eds. International Union Against Cancer (UICC): TNM classification of malignant tumours (7th edition). Wiley: New York, 2009.
Hamai Y, Hihara J, Emi M, et al. Results of neoadjuvant chemoradiotherapy with docetaxel and 5-fluorouracil followed by esophagectomy to treat locally advanced esophageal cancer. Ann Thorac Surg. 2015; 99: 1887–93.
Emi M, Hihara J, Hamai Y, et al. Neoadjuvant chemoradiotherapy with docetaxel, cisplatin, and 5-fluorouracil for esophageal cancer. Cancer Chemother Pharmacol. 2012; 69: 1499–505.
Hamai Y, Hihara J, Emi M, et al. Effects of neoadjuvant chemoradiotherapy on pathological TNM Stage and their prognostic significance for surgically-treated esophageal squamous cell carcinoma. Anticancer Res. 2017; 37: 5639–46.
Murakami Y, Hamai Y, Emi M, et al. Long-term results of neoadjuvant chemoradiotherapy using cisplatin and 5-fluorouracil followed by esophagectomy for resectable, locally advanced esophageal squamous cell carcinoma. J Radiat Res. 2018; 59: 616–24.
Lee JR, Madsen MT, Bushnel D, Menda Y. A threshold method to improve standardized uptake value reproducibility. Nucl Med Commun. 2000; 21: 685–90.
Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000; 92: 205–16.
Beseth BD, Bedford R, Isacoff WH, Holmes EC, Cameron RB. Endoscopic ultrasound does not accurately assess pathologic stage of esophageal cancer after neoadjuvant chemoradiotherapy. Am Surg. 2000; 66: 827–31.
Schneider PM, Metzger R, Schaefer H, et al. Response evaluation by endoscopy, rebiopsy, and endoscopic ultrasound does not accurately predict histopathologic regression after neoadjuvant chemoradiation for esophageal cancer. Ann Surg. 2008; 248: 902–8.
Brücher BL, Stein HJ, Werner M, Siewert JR. Lymphatic vessel invasion is an independent prognostic factor in patients with a primary resected tumor with esophageal squamous cell carcinoma. Cancer. 2001; 92: 2228–33.
Zhu CM, Ling YH, Xi SY, et al. Prognostic significance of the pN classification supplemented by vascular invasion for esophageal squamous cell carcinoma. PLoS One. 2014; 9: e96129.
Jeon JH, Lee JM, Moon DH, et al. Prognostic significance of venous invasion and maximum standardized uptake value of 18F-FDG PET/CT in surgically resected T1N0 esophageal squamous cell carcinoma. Eur J Surg Oncol. 2017; 43: 471–7.
O JH, Jacene H, Luber B, Wang H, et al. Quantitation of cancer treatment response by 18F-FDG PET/CT: Multicenter assessment of measurement variability. J Nucl Med. 2017; 58: 1429–1434.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hamai, Y., Emi, M., Ibuki, Y. et al. Predictions of Pathological Features and Recurrence Based on FDG-PET Findings of Esophageal Squamous Cell Carcinoma after Trimodal Therapy. Ann Surg Oncol 27, 4422–4430 (2020). https://doi.org/10.1245/s10434-020-08609-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-020-08609-0