Abstract
Purpose
Our purpose was to examine the prognostic value of post-CRT PET based on the presence or absence of FDG-avid metastatic lymph node(s) and metabolic response of the primary tumor in patients with clinically node-positive ESCC treated with definitive chemoradiotherapy (dCRT).
Methods
We identified 108 eligible patients treated by chemoradiotherapy (CRT) with or without resection from our prospectively collected database. Absence of FDG-avid metastatic lymph node with at least partial response of the primary tumor on PET scan after initial CRT was defined as the Post-CRT PET favorable group (yPET-F), and otherwise as unfavorable group (yPET-U). The Kaplan-Meier method and Cox regression were performed for survival analyses and multivariable analysis, respectively.
Results
The study cohort was comprised of 59 patients receiving dCRT. Forty-five patients receiving trimodality therapy (TMT) comprised the comparative group and four patients were excluded from further analyses for developing interval distant metastasis detected on post-CRT PET scan. The median follow-up for the study cohort was 41 months. On K-M analysis of the study cohort, yPET-F was found to have significantly better OS (2-year: 72.5% vs 13.7%, p < 0.01) and DMFS (2-year: 71.6% vs 36.6%, p = 0.01) than yPET-U. In multivariable analysis, yPET-F remained as a strong independent favorable prognosticator on both OS (HR 0.08, p < 0.01) and DMFS (HR 0.14, p = 0.02) for the dCRT cohort. Compared with TMT cohort, for yPET-U patients, TMT had better OS (p = 0.03) than dCRT-Operable and dCRT-Operable had superior OS (p = 0.04) than dCRT-Unresectable. For yPET-F patients, there was no difference in both OS (p > 0.99) and DMFS (p = 0.92) between these three groups.
Conclusions
Absence of FDG-avid metastatic lymph node with at least partial response of the primary tumor on PET scan after CRT (i.e., yPET-F status) prognosticate for excellent OS and DMFS in cN+ ESCC patients treated with dCRT, and might be comparable to TMT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Esophageal squamous cell carcinoma (ESCC) has been thought to be more chemoradiation-sensitive as compared to adenocarcinoma histology based on studies [1,2,3] showing a higher pathological response rate in ESCC after neoadjuvant chemoradiotherapy (nCRT). The prognostic value of metabolic response after chemoradiotherapy evaluated by PET scans has been studied in patients with locally advanced ESCC [4,5,6,7,8,9]. Most of the studies were done in patients who received trimodality therapy (nCRT followed by a planned surgery, TMT) and focused on SUV parameters of the primary tumor [4,5,6,7,8,9]. Recent studies had revealed the prognostic significance of SUVs of the metastatic lymph node(s) in patients with ESCC, on both initial staging PET scans [10, 11] and post-induction chemotherapy PET scans [12, 13]. However, the prognostic value of the SUVs of the metastatic lymph node(s) on post-chemoradiotherapy (post-CRT) PET scans in patients with ESCC treated with definitive chemoradiotherapy (dCRT) had not been studied. Definitive chemoradiotherapy is the standard treatment option for patients unfit for surgery and those who refuse to undergo surgery, and even stated as an alternative treatment option for patients with operable ESCC in the ESMO guideline [14], if close surveillance and prompt salvage surgery could be executed; Thus, finding a non-surgical and practical way to evaluate treatment response, to prognosticate and even to guide the next step of management is of great clinical value. In this study, we aimed to examine the prognostic value of post-CRT PET imaging based on the presence or absence of FDG-avid metastatic lymph node(s) and metabolic response of the primary tumor in patients with clinically node-positive (cN+) ESCC treated with dCRT.
Material and methods
Patient selection
The institutional review board of our hospital has approved this study. We evaluated all esophageal cancer patients treated by CRT with or without surgery between 2011 and 2015 from the prospectively assembled cohort in the cancer registry of our institution. The inclusion criteria were the following: biopsy proven ESCC, staging FDG-PET/CT was done, staged as cN+, total radiation dose >40 Gy delivered with a continuous schedule and with chemotherapy as the initial treatment, post-CRT FDG-PET/CT was done within 8 weeks after initial CRT, and if planned surgery was performed, it must be done within 12 weeks after initial CRT. Patients with a history of prior or synchronous malignancy, or any M1 disease were excluded. The pretreatment staging examinations included esophagogastroduodenoscopy (EGD) with biopsies, endoscopic ultrasound (EUS), chest and abdominal CT with contrast, and FDG-PET/CT. Clinical staging was based on the American Joint Committee on Cancer, 7th edition [ 15 ].
Treatment
All patients received a course of CRT as the initial treatment. The prescribed total radiation dose must be >40 Gy and was delivered with a continuous schedule to be categorized as “treatment with curative intent” according to the treatment guideline of our institution. The regimens of chemotherapy were comprised of cisplatin and 5-fluorouracil, paclitaxel and carboplatin, or paclitaxel and cisplatin. All patients underwent a treatment response and surgical evaluation at a multidisciplinary tumor conference with post-CRT exams including chest/abdominal CT with contrast, EGD/EUS with or without biopsy, and FDG-PET/CT. Radical esophagectomy with lymphadenectomy was advised if the patient was eligible (i.e., resectable disease, medically fit, and no interval distant metastasis detected on post-CRT imaging). Adjuvant CRT was suggested to the patients who had post-operative risk factors. For those who were not eligible for surgery or refused surgery, consolidation, CRT was suggested to reach definitive dosage adapting the protocol of FFCD 9102 phase III trial [16].
FDG-PET/CT
Pretreatment FDG-PET/CT scans were performed for staging purposes. Post-CRT FDG-PET/CT scans were performed within 8 weeks after initial CRT for treatment response evaluation. The standardized protocol of the FDG-PET/CT exam, and the model and specifications of the PET/CT scanners used in our institution were described in our previous published paper [10]. The volume of interest for maximum standardized uptake value (SUVmax) calculation was drawn by edge-finding techniques. Then the SUVmax was calculated based on body weight using the following formula: PET count at the most intense point × calibration factor (MBq/kg)/injection dose (MBq)/bodyweight (kg). The FDG uptake of the tumor was visible when SUVmax was greater than 2.5 of the background mediastinal blood pool. Thus, patients with SUVmax of the primary tumor and lymph nodes of 2.5 or above were determined as PET-positive tumor and PET-positive lymph nodes, respectively.
Metabolic response by FDG-PET/CT
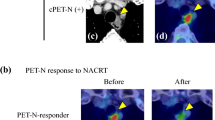
Metabolic tumor response was quantified using percentage reduction in SUVmax, and thresholds were defined by adapted PERCIST criteria (SUVmax) [17, 18]. Metabolic complete response of the primary tumor (T-mCR) was defined as complete resolution of F-18 FDG uptake within a measurable target lesion so that it is less than the mean liver activity and indistinguishable from surrounding background blood-pool levels. Primary tumors that showed a ≥ 30% reduction in SUVmax after CRT were defined as having a metabolic partial response (T-mPR) [ 17 ]. Progressive metabolic disease of the primary tumor (T-mPD) was defined as a ≥ 30% increase in SUVmax or presence of new FDG-avid lesion(s) in a pattern typical of cancer. Stable metabolic disease of the primary tumor (T-mSD) was disease other than T-mCR, T-mPR, or T-mPD. Absence of FDG-avidity in the metastatic lymph node(s) after CRT was classified as post-CRT PET-N negative [yPET-N(−)]. FDG-PET/CT images were interpreted by an experienced nuclear medicine specialist, and were peer-reviewed at the multidisciplinary tumor conference and correlated with computed tomography. Patients who achieved yPET-N(−) plus either T-mCR or T-mPR were categorized as “Post-CRT PET-Favorable Group” (yPET-F); otherwise they were categorized as “Post-CRT PET-Unfavorable Group” (yPET-U).
Comprehensive clinical response
Comprehensive clinical response to initial CRT was graded as complete response (cCR), partial response (cPR), stable disease (cSD) or progressive disease (cPD) based primarily on CT scan according to Response Evaluation Criteria in Solid Tumors [RECIST] criteria version 1.1 [19]. In addition, cCR must also fulfill the following criteria on endoscopic examination: (1) disappearance of the tumor, (2) disappearance of ulceration, (3) absence of stricture, and (4) biopsy was negative for cancer cells.
Post-therapy surveillance
According to the surveillance protocol of our institution, follow-up clinic appointments were arranged every 3 months during the first 2 years, every 4–6 months during the third and fourth years, and every 6–12 months thereafter; imaging was performed at a specific intervals complying the protocol: Chest X-ray every 3 months, CT scan every 3 to 6 months, and EGD every 3 to 6 months or when symptoms indicating recurrence occurred.
Statistical analysis
The median follow-up time was computed using the reverse Kaplan–Meier estimator [20]. Categorical variables were described by absolute frequency and percentage and numerical variables were described by median and interquartile range. The time to a specific endpoint in this study was calculated from the date of diagnosis. The actuarial survival data and curves were generated by the Kaplan-Meier (K-M) method and the p-values were determined by the Log-rank test. The independent influences of various prognostic factors were analyzed by Cox’s proportional hazards regression model. All tests were 2-sided, and a P-value <0.05 was considered statistically significant. We utilized IBM SPSS statistical software (version 23; SPSS, Inc., Chicago, IL, USA) for all statistical analyses.
Results
Patient and treatment characteristics
We identified 108 patients according to the preset inclusion and exclusion criteria. Four patients (3.7%) were found to have developed interval distant metastases by the post-CRT PET/CT and were excluded from further analyses. Fifty-nine patients were treated with dCRT (i.e., CRT without a planned surgery) due to unresectable disease (N = 30, 51%), patients’ refusal of surgery (N = 26, 44%), or death occurred before planned surgery date (N = 3, 5%). These 59 patients comprised the dCRT cohort. Patient and treatment characteristics of the dCRT cohort are summarized in Table 1. The other 45 eligible patients who were treated with TMT (CRT followed by a planned surgery) were used as a comparative group (Fig. 1).
Treatment response evaluation
The metabolic responses of the study cohort after the initial CRT are shown in Supplemental Table 1, in which T-mCR was achieved in 13 patients (22%), T-mPR in 41 patients (69.5%), T-mSD in four patients (6.8%), and T-mPD in one patient (1.7%). While yPET-N(−) status was achieved in 25 patients (42.4%) after the initial CRT. Out of these 25 patients, one patient (4%) had T-mSD and was categorized into the yPET-U group, and the rest (24 patients, 40.7% of the study cohort) were categorized into the yPET-F group. Ten (38.5%) out of 26 patients who refused a planned surgery (i.e., operable patients) and 14 (46.7%) out of 30 patients with unresectable diseases achieved yPET-F status, while all of the three patients who expired before planned surgery had a yPET-U status after the initial CRT. In regards to comprehensive clinical response evaluation, cCR was achieved in six patients (10.2%), cPR in 43 patients (72.9%), cSD in nine patients (15.3%), and cPD in one patient (1.7%) after the initial CRT.
Failure patterns and survival analyses
The median follow-up time for the study cohort was 41 months (95% Confidence Interval: 22.7–59.3 months). During the follow-up, 21 patients (35.6%) had no progression of disease, 17 patients (28.8%) had locoregional failure (LRF) alone, six patients (10.2%) had distant failure (DF) alone, 15 patients (25.4%) had LRF plus DF, and 44 patients (74.6%) died. In K-M analyses, the median overall survival (OS) for the study cohort was 15.4 months, and the 2-year OS and distant metastasis free survival (DMFS) were 37.1% and 55.8%, respectively.
Comprehensive clinical response significantly correlated with OS (cCR vs. cPR vs. cSD/cPD; 2-year OS: 83.3% vs. 37.3% vs. 0%; Median OS: 34.1 months vs. 15.4 months vs. 9.7 months; p = 0.01) but not with DMFS (Fig. 2a and b).The majority of patients (N = 43, 72.9%) was classified as cPR. Further stratifying the cPR patients by post-CRT PET prognostic groups, two groups of patients with significantly different OS (yPET-F vs. yPET-U; 2-year OS: 72.3% vs. 17.8%; Median OS: 31.7 months vs. 10.3 months; p < 0.01) and borderline different DMFS (2-year DMFS: 67% vs. 37%, p = 0.08) were identified (Fig. 2c and d). When applying the post-CRT PET prognostic grouping on the whole dCRT cohort, it distinguished the patients with yPET-F from those with yPET-U with greater statistical significance, for which yPET-F had significantly better OS (Median: 31.7 months vs. 9.9 months; 2-year: 72.5% vs. 13.7%, p < 0.01) and DMFS (2-year: 71.6% vs. 36.6%, p = 0.01) than yPET-U (Fig. 3).
Kaplan-Meier analyses on overall survival and distant-metastasis free survival in the dCRT cohort. a, b Whole dCRT cohort stratified by comprehensive clinical response (cCR, N = 6 vs. cPR, N = 43 vs. cSD/cPD, N = 10). c, d cPR patients stratified by post-CRT PET prognostic groups (yPET-F, N = 16 vs. yPET-U, N = 27)
Survival results of those who refused surgery (i.e., operable patients who received dCRT, dCRT-Operable) and those with unresectable disease (dCRT-Unresectable) were compared with that of the TMT cohort, which was treated during the same period of time and selected with same criteria. Twenty-two (48.9%) out of the 45 patients treated with TMT achieved yPET-F status after the initial CRT. For the yPET-F patients, there was no significant difference in both OS (p > 0.99) and DMFS (p = 0.92) between TMT, dCRT-Operable, and dCRT-Unresectable (Fig. 4a and b). The locoregional progression free survival (LRPFS) had no significant difference between TMT and dCRT-Operable (2-year LRPFS: 76.7% vs. 55.6%, respectively; p = 0.21). In contrast, for the yPET-U patients (Fig. 4c and d), there was a significant difference on OS between TMT, dCRT-Operable and dCRT-Unresectable (Median OS: 27.4 months vs. 11.7 months vs. 9.2 months; 2-year OS: 64.6% vs. 25% vs. 0%; pooled p < 0.01). With pairwise comparisons using the Log-Rank test, the TMT subgroup had superior OS than did the dCRT-Operable subgroup (p = 0.03), and the dCRT-Operable subgroup had better OS than the dCRT-Unresectable subgroup (p = 0.04). In terms of DMFS, there was a trend for difference between the three groups (2-year DMFS: TMT 55.1% vs. dCRT-Operable 46.4% vs. dCRT-Unresectable 0%, pooled p = 0.19).
Kaplan-Meier analyses on overall survival and distant-metastasis free survival, comparing patients who refused surgery (dCRT-Operable), patients with unresectable disease (dCRT-Unresectable) and patients treated with trimodality therapy (TMT). a, b Patients in yPET-F group: TMT (N = 22), dCRT-Operable (N = 10), and dCRT-Unresectable (N = 14). c, d Patients in yPET-U group: TMT (N = 23), dCRT-Operable (N = 16) and dCRT-Unresectable (N = 16)
Univariable and multivariable analyses
Results of the univariable analysis of the influence of various clinical factors on OS and DMFS in the dCRT cohort are shown in Supplemental Table 2. Relevant clinical factors as shown in Table 2 were entered into the subsequent multivariable Cox regression model. After adjusting for the potential confounders, yPET-F remained as a strong independent favorable prognostic factor for both OS (HR 0.08, p < 0.01) and DMFS (HR 0.14, p = 0.02); In addition, cT4 stage and grade 3 tumor histology were independent poor prognostic factors for OS; While younger age, higher initial tumor SUVmax and grade 3 tumor histology were independent poor prognostic factors for DMFS.
Discussion
Recent data has made evident that clinical stage groups did not match with the pathologic stage groups in terms of prognostic implications, with cTNM giving poorer survival for early stage patients and better survival for advanced stage ones [21]; and that survivals were different between patients who received neoadjuvant therapy and those who received esophagectomy alone [22]. Thus, for better prognostic accuracy, separate ypTNM stage groups were introduced in the Eighth edition AJCC cancer staging manual for esophageal cancer patients who received TMT [23]. However, there is a lack of practical and useful post-treatment prognostic grouping for esophageal cancer patients who received dCRT. In this study, we proposed a novel prognostic grouping (Favorable group vs. Unfavorable group) by post-CRT PET scan for cN+ ESCC patients treated with dCRT. The rationale for defining the post-CRT PET favorable group as “absence of FDG-avid metastatic lymph node with at least partial response of the primary tumor” was based on the results of previous studies [ 9 , 12 , 13 ]: Two Japanese studies examined the prognostic value of FDG uptake in the metastatic lymph node(s) after induction chemotherapy (ICT) in patients with ESCC treated with ICT followed by surgical resection found that absence of FDG-avid metastatic lymph node after ICT was associated with a less number of pathological positive lymph nodes, lower distant metastasis rate, higher relapse free survival and better OS [12, 13]. A study from MD Anderson Cancer Center found that a partial metabolic response of the primary tumor after CRT was associated with better OS in esophageal cancer patients treated with TMT [9].
The majority of patients (95%) of our study cohorts were stage III and translated into a median OS around 15.4 months and 2-year OS around 37.1%, which was comparable to the previous studies on ESCC patients treated with dCRT [24,25,26]. In the current study, patients could be stratified into groups with distinct survival prognoses according to the tumor responses evaluated by CT scan and EGD with biopsy. However, this method had allocated most of the patients (72.9% of the cohort) into a single group, cPR. When stratifying the cPR patients according the two post-CRT PET prognostic groups, we identified that these two groups of patients had significantly different OS, with the survival of yPET-F approaching that of cCR patients and the survival of yPET-U approaching that of cSD/cPD (Fig. 2c and d). Thus, prognosticating the heterogeneous cPR patients with an averaged OS is inappropriate. We proposed that it would be more appropriate to designate cN+ ESCC patients treated with dCRT into two groups (yPET-F vs. yPET-U) using the post-CRT PET prognostic grouping for prognostic implications (Fig. 3).
Esophageal cancer with squamous cell carcinoma histology is thought to be more chemoradiation-sensitive than those with adenocarcinoma histology. In fact, two prospective randomized trials evaluated the effect of adding surgery to CRT. Stahl et al. reported no OS difference between adding surgery and adding additional radiotherapy after induction chemotherapy plus CRT in patients with locally advanced ESCC [27]. Similarly, Bedenne et al. also reported that in ESCC patients who experienced a response (evaluated by esophagogram and improvement of dysphagia) to initial CRT, there was no survival benefit from adding surgery following initial CRT compared with dCRT with additional CRT [16]. However, both trials reported a higher locoregional relapse rate in the dCRT group compared to the TMT group. In the current study, for the yPET-F patients, there was no significant difference in OS and DMFS between the dCRT-Operable subgroup and the TMT subgroup. In addition, half of the yPET-F patients who refused surgery remained free from locoregional progression after dCRT by the time of last follow-up, and four out of the five locoregional progressions were successfully salvaged by surgery (two out of three patients who experienced a local progression on first failure underwent a salvage esophagectomy with lymphadenectomy and both patients who experienced an isolated nodal progression on first failure underwent a salvage lymphadenectomy alone). In fact, 70% of the yPET-F patients who refused surgery remained esophagectomy-free and local progression-free by the time of last follow-up. A large multicenter study reported that salvage surgery after dCRT can offer similar overall and disease-free survival compared with upfront TMT, and there was no significant difference in major complications and in-hospital mortality [28]. Thus, we hypothesize that dCRT with salvage surgery on demand may be a treatment option in operable cN+ ESCC patients who achieved yPET-F status after CRT. In addition, the current study found that there were no significant OS and DMFS differences between the operable patients and the unresectable patients treated with dCRT when yPET-F status was achieved after CRT, and a high rate (46.7%) of yPET-F status after the initial CRT was observed among the unresectable patients in our study cohort. In contrast, for operable patients with yPET-U status after CRT, adding an upfront surgery (TMT) may improve the OS. Thus, TMT should be the treatment of choice in this group of patients.
We acknowledge that this study suffers from several major limitations including: (1) the results of a retrospective study might suffer from methodological and analytical variability; (2) a small sample size of the study especially in subgroups analysis and (3) selection bias and confounders could not be fully eliminated in a retrospective study. The results of this study are hypothesis generating and should be validated by future prospective studies with a large sample size.
Conclusion
This study showed that absence of FDG-avid metastatic lymph node with at least partial response of the primary tumor on PET scan after CRT (i.e.,e yPET-F status) was a strong favorable prognosticator on OS and DMFS for patients with cN+ ESCC treated with dCRT. Our data suggested that patients treated with dCRT who achieved yPET-F status might have OS and DMFS rates comparable to trimodality therapy. In contrast, for operable patients with yPET-U status, trimodality therapy may provide better OS than dCRT. The results of this study have implications for the design of future clinical trials, and for a more appropriate selection of an individualized treatment approach.
References
Piessen G, Petyt G, Duhamel A, Mirabel X, Huglo D, Mariette C. Ineffectiveness of (1)(8)F-fluorodeoxyglucose positron emission tomography in the evaluation of tumor response after completion of neoadjuvant chemoradiation in esophageal cancer. Ann Surg. 2013;258:66–76. https://doi.org/10.1097/SLA.0b013e31828676c4.
Shapiro J, van Lanschot JJ, Hulshof MC, van Hagen P, van Berge Henegouwen MI, Wijnhoven BP, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–8. https://doi.org/10.1016/s1470-2045(15)00040-6.
van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84. https://doi.org/10.1056/NEJMoa1112088.
Kim MK, Ryu JS, Kim SB, Ahn JH, Kim SY, Park SI, et al. Value of complete metabolic response by (18)F-fluorodeoxyglucose-positron emission tomography in oesophageal cancer for prediction of pathologic response and survival after preoperative chemoradiotherapy. Eur J Cancer. 2007;43:1385–91. https://doi.org/10.1016/j.ejca.2007.04.001.
Dewan A, Sharma SK, Dewan AK, Khurana R, Gupta M, Pahuja A, et al. Impact on radiological and pathological response with neoadjuvant chemoradiation and its effect on survival in squamous cell carcinoma of thoracic esophagus. J Gastrointest Cancer. 2017;48:42–9. https://doi.org/10.1007/s12029-016-9870-0.
Sasaki K, Uchikado Y, Okumura H, Omoto I, Kita Y, Arigami T, et al. Role of 18F-FDG-PET/CT in esophageal squamous cell carcinoma after neoadjuvant chemoradiotherapy. Anticancer Res. 2017;37:859–64. 10.21873/anticanres.11390.
Brucher BL, Weber W, Bauer M, Fink U, Avril N, Stein HJ, et al. Neoadjuvant therapy of esophageal squamous cell carcinoma: response evaluation by positron emission tomography. Ann Surg. 2001;233:300–9.
Flamen P, Van Cutsem E, Lerut A, Cambier JP, Haustermans K, Bormans G, et al. Positron emission tomography for assessment of the response to induction radiochemotherapy in locally advanced oesophageal cancer. Ann Oncol. 2002;13:361–8.
Javeri H, Xiao L, Rohren E, Lee JH, Liao Z, Hofstetter W, et al. The higher the decrease in the standardized uptake value of positron emission tomography after chemoradiation, the better the survival of patients with gastroesophageal adenocarcinoma. Cancer. 2009;115:5184–92. https://doi.org/10.1002/cncr.24604.
Yap WK, Chang YC, Tseng CK, Hsieh CH, Chao YK, Su PJ, et al. Predictive value of nodal maximum standardized uptake value of pretreatment [18F]fluorodeoxyglucose positron emission tomography imaging in patients with esophageal cancer. Dis Esophagus. 2017;30:1–10. https://doi.org/10.1093/dote/dox021.
Yasuda T, Higuchi I, Yano M, Miyata H, Yamasaki M, Takiguchi S, et al. The impact of (1)(8)F-fluorodeoxyglucose positron emission tomography positive lymph nodes on postoperative recurrence and survival in resectable thoracic esophageal squamous cell carcinoma. Ann Surg Oncol. 2012;19:652–60. https://doi.org/10.1245/s10434-011-1928-4.
Miyata H, Yamasaki M, Takahashi T, Murakami K, Kurokawa Y, Nakajima K, et al. Relevance of [18F]fluorodeoxyglucose positron emission tomography-positive lymph nodes after neoadjuvant chemotherapy for squamous cell oesophageal cancer. Br J Surg. 2013;100:1490–7. https://doi.org/10.1002/bjs.9253.
Yasuda T, Yano M, Miyata H, Yamasaki M, Takiguchi S, Fujiwara Y, et al. Prognostic significance of (18)F-fluorodeoxyglucose positron emission tomography (FDG-PET)-positive lymph nodes following neoadjuvant chemotherapy and surgery for Resectable thoracic Esophageal Squamous cell carcinoma. Ann Surg Oncol. 2015;22:2599–607. https://doi.org/10.1245/s10434-014-4299-9.
Lordick F, Mariette C, Haustermans K, Obermannova R, Arnold D. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v50–v7. https://doi.org/10.1093/annonc/mdw329.
Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC cancer staging manual: esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17:1721–4. https://doi.org/10.1245/s10434-010-1024-1.
Bedenne L, Michel P, Bouche O, Milan C, Mariette C, Conroy T, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25:1160–8. https://doi.org/10.1200/jco.2005.04.7118.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122s–50s. https://doi.org/10.2967/jnumed.108.057307.
Findlay JM, Bradley KM, Wang LM, Franklin JM, Teoh EJ, Gleeson FV, et al. Predicting pathologic response of Esophageal cancer to Neoadjuvant chemotherapy: the implications of metabolic nodal response for personalized therapy. J Nucl Med. 2017;58:266–75. https://doi.org/10.2967/jnumed.116.176313.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. https://doi.org/10.1016/j.ejca.2008.10.026.
Clark TG, Bradburn MJ, Love SB, Altman DG. Survival analysis part I: basic concepts and first analyses. Br J Cancer. 2003;89:232–8. https://doi.org/10.1038/sj.bjc.6601118.
Rice TW, Apperson-Hansen C, DiPaola LM, Semple ME, Lerut TE, Orringer MB, et al. Worldwide Esophageal cancer collaboration: clinical staging data. Dis Esophagus. 2016;29:707–14. https://doi.org/10.1111/dote.12493.
Rice TW, Lerut TE, Orringer MB, Chen LQ, Hofstetter WL, Smithers BM, et al. Worldwide Esophageal cancer collaboration: neoadjuvant pathologic staging data. Dis Esophagus. 2016;29:715–23. https://doi.org/10.1111/dote.12513.
Rice TW, Ishwaran H, Kelsen DP, Hofstetter WL, Apperson-Hansen C, Blackstone EH. Recommendations for neoadjuvant pathologic staging (ypTNM) of cancer of the esophagus and esophagogastric junction for the 8th edition AJCC/UICC staging manuals. Dis Esophagus. 2016;29:906–12. https://doi.org/10.1111/dote.12538.
Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA Jr, Al-Sarraf M, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623–7.
Conroy T, Galais MP, Raoul JL, Bouche O, Gourgou-Bourgade S, Douillard JY, et al. Definitive chemoradiotherapy with FOLFOX versus fluorouracil and cisplatin in patients with oesophageal cancer (PRODIGE5/ACCORD17): final results of a randomised, phase 2/3 trial. Lancet Oncol. 2014;15:305–14. https://doi.org/10.1016/s1470-2045(14)70028-2.
Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, et al. INT 0123 (radiation therapy oncology group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167–74. https://doi.org/10.1200/jco.2002.20.5.1167.
Stahl M, Stuschke M, Lehmann N, Meyer HJ, Walz MK, Seeber S, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23:2310–7. https://doi.org/10.1200/jco.2005.00.034.
Markar S, Gronnier C, Duhamel A, Pasquer A, Thereaux J, du Rieu MC, et al. Salvage surgery after Chemoradiotherapy in the Management of Esophageal Cancer: is it a viable therapeutic option? J Clin Oncol. 2015;33:3866–73. https://doi.org/10.1200/jco.2014.59.9092.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Supplemental Table 1
(DOCX 47 kb)
Supplemental Table 2
(DOCX 83 kb)
Rights and permissions
About this article
Cite this article
Yap, WK., Chang, YC., Hsieh, CH. et al. Favorable versus unfavorable prognostic groups by post-chemoradiation FDG-PET imaging in node-positive esophageal squamous cell carcinoma patients treated with definitive chemoradiotherapy. Eur J Nucl Med Mol Imaging 45, 689–698 (2018). https://doi.org/10.1007/s00259-017-3901-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-017-3901-3