Abstract
Purpose
The aim of the study was to evaluate extrastriatal dopaminergic and serotonergic pathways in patients with Parkinson’s disease (PD) and dementia with Lewy bodies (DLB) using 123I-FP-CIT SPECT imaging.
Methods
The study groups comprised 56 PD patients without dementia, 41 DLB patients and 54 controls. Each patient underwent a standardized neurological examination and 123I-FP-CIT SPECT. Binding in nigrostriatal and extrastriatal regions of interest was calculated in each patient from spatially normalized images. The occipital-adjusted specific to nondisplaceable binding ratio (SBR) in the different regions was compared among the PD patients, DLB patients and controls adjusting for the effects of age, sex, disease duration and serotonergic/dopaminergic treatment. Covariance analysis was used to determine the correlates of local and long-distance regions with extrastriatal 123I-FP-CIT deficits.
Results
Both PD and DLB patients showed lower 123I-FP-CIT SPECT SBR in several regions beyond the nigrostriatal system, especially the insula, cingulate and thalamus. DLB patients showed significantly lower 123I-FP-CIT SBR in the thalamus than controls and PD patients. Thalamic and cingulate 123I-FP-CIT SBR deficits were correlated, respectively, with limbic serotonergic and widespread cortical monoaminergic projections only in DLB patients but exhibited only local correlations in PD patients and controls.
Conclusion
PD and DLB patients both showed insular dopamine deficits, whereas impairment of thalamic serotonergic pathways was specifically associated with DLB. Longitudinal studies are necessary to determine the clinical value of the assessment of extrastriatal 123I-FP-CIT SPECT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nigrostriatal dopaminergic depletion is the hallmark of Parkinson’s disease (PD) [1] and dementia with Lewy bodies (DLB) [2]. These two conditions share several pathophysiological characteristics [3, 4] despite presenting with clinical phenotypes reflecting a different degree and timing of subcortical and cortical neuropathology [5, 6]. Differences between PD and DLB patients in terms of clinical presentation and disease course probably also reflect the involvement of different neurotransmitters. Indeed, neuropathological studies and studies of small groups using molecular imaging indicate that the patterns of neurodegeneration in the dopaminergic and serotonergic systems differ between PD and DLB patients [5, 7].
123I-FP-CIT [123I-N-ω-fluoropropyl-2β-carbomethoxy-3β-(4-iodophenyl) nortropane] SPECT enables the visualization in vivo of the striatal density of the dopamine transporter (DAT), which is typically reduced in neurodegenerative parkinsonism. Besides its high affinity for DAT (KI 3.5 nM), 123I-FP-CIT also shows high affinity for the serotonin transporter SERT (KI 9.73), with a DAT/SERT selectivity of 2.8 [7, 8]. Notably, DAT and SERT display a non-overlapping distribution in the brain: DAT levels are highest in the basal ganglia, whereas SERT is highly expressed in the thalamus and midbrain [7,8,9]. Thus, 123I-FP-CIT SPECT holds the potential for assessing the involvement of both serotonergic and dopaminergic transmission (nigrostriatal and extrastriatal projections) in vivo.
A few studies have investigated DAT and SERT in PD and DLB using 123I-FP-CIT imaging, and have shown lower putamen binding in PD, although not consistently [10, 11]. Findings in extrastriatal regions range from no differences between the two conditions to lower midbrain SERT binding in DLB [7, 9]. These discrepancies may have been due to sample selection, different methods of image analysis and choice of reference region [7, 9].
In the present study, we applied a newly developed Statistical Parametric Mapping (SPM) method for single-subject evaluation of 123I-FP-CIT SPECT data in patients with different forms of Lewy body disorders, focusing on both striatal and extrastriatal regions of interest using different normalization methods. The aim of this study was to evaluate shared and divergent deficits within the extrastriatal dopaminergic and serotonergic pathways in PD and DLB patients, paving the way for novel prognostic and diagnostic measures.
Materials and methods
Recruitment of patients and clinical assessment
Consecutive patients with a clinical diagnosis of PD [1] or DLB [12], supported by at least 2 years of follow-up, were recruited from the Neurology Unit, University of Brescia, Italy. All patients underwent structural imaging (brain MRI or CT scan) and a standardized neurological examination, including the Unified Parkinson’s Disease Rating Scale (UPDRS-III) in ON state and a global cognition evaluation using the Mini Mental State Examination (MMSE) 1 to 4 weeks prior to SPECT imaging. The levodopa equivalent daily dose (LEDD) was calculated according to the standard conversion method as previously described [13].
The following exclusion criteria were applied: (1) atypical parkinsonism such as corticobasal syndrome, progressive supranuclear palsy and multiple system atrophy; (2) prominent cortical or subcortical infarcts; (3) other neurological disorders or medical conditions potentially associated with cognitive deficits; (4) deep brain stimulation; (5) bipolar disorder, schizophrenia, history of drug or alcohol abuse or impulse control disorder [14]; (6) PD with hallucinations or dementia according to current criteria [15]; and (7) use of antipsychotics. A control group was included comprising 54 subjects with a confirmed clinical diagnosis of isolated action or rest tremor syndromes [16] over a 4-year follow-up period and with normal 123I-FP-CIT imaging (assessed visually and quantitatively by BRASS analysis).
The research protocol was approved by the Ethics Committee of the Brescia Hospital, Brescia, Italy (NP 1471). Written informed consent was obtained from all participants.
SPECT imaging
123I-FP-CIT (110–185 MBq) was administered intravenously 30 min after thyroid blockade (800 mg of KClO4) in all subjects, as previously described [14]. Antidepressant therapy (in those receiving it) was withdrawn 3 weeks before the SPECT assessment to minimize possible iatrogenic effects on nigrostriatal and extrastriatal 123I-FP-CIT binding. Brain SPECT was performed 3 h after 123I-FP-CIT administration using a dual-head gamma camera (Infinia Hawkeye; GE Healthcare) as previously described [14]. 123I-FP-CIT data from individual patients were spatially normalized to a normal template in Montreal Neurological Institute space [14] and smoothed (3D gaussian filter with 10 mm full-width at half-maximum) [14, 17].
Two regions of interest (ROIs) over the right and left occipital lobe provided the individual background uptake area and the mean uptake value of these ROIs was used to obtain the ratios for nigrostriatal and extrastriatal binding. Putamen and caudate binding, asymmetry index and caudate/putamen ratio were calculated according to the standard formula described by Walker et al. [10].
123I-FP-CIT specific to nondisplaceable binding ratios (SBR) in the frontal, parietal, temporal, cingulate cortices, the insula, thalamus and midbrain were evaluated using the Statistical Parametric Mapping (SPM12, https://www.fil.ion.ucl.ac.uk/spm/) WFU PickAtlas toolbox (http://fmri.wfubmc.edu/software/pickatlas, Wake Forest University Health Sciences Medical Center Blvd. Winston-Salem, NC) based on the Talairach Daemon database [18]. Briefly, a 3D anatomical ROI for each of the aforementioned regions was created, obtaining the mean binding in each patient. A ratio with the mean occipital binding in individual subjects was calculated according to the standard guidelines also used in semiquantitative tools [19, 20]. Additional analyses were performed using uptake in the cerebellar crus II region as the reference as recently suggested as an alternative reference region for extrastriatal regions by Joling et al. [9].
Statistical analyses
Differences in demographic and clinical variables between the groups were assessed by ANOVA or the Mann-Whitney U test for three-group comparisons (PD patients vs. DLB patients vs. controls) and two-group comparisons (PD vs. DLB patients), respectively. Differences in categorical variables were assessed using the chi-squared test. The significance level was set at p < 0.05. Nigrostriatal and extrastriatal 123I-FP-CIT SBR values, asymmetry indexes (normalized to the more affected side) and caudate/putamen ratios were compared among the three groups using ANOVA with Bonferroni post-hoc comparisons adjusted for the effects of age and sex. The multiple comparison-adjusted significance threshold was set at p = 0.0125 for striatal regions (p = 0.05/four tests) and p = 0.007 for extrastriatal regions (p = 0.05/seven tests). The post-hoc comparisons between DLB and PD patients were additionally evaluated in univariate analysis adjusted for the effects of age, sex, disease duration, selective serotonin reuptake inhibitor (SSRI) use and LEDD.
Discriminant analyses and patterns of covariance
ROIs significant by ANOVA were further analysed to assess their power to differentiate between PD patients, DLB patients and controls by means of ROC curve analysis (area under the curve, AUC), considering the clinical diagnosis as the diagnostic reference. ROI patterns of covariance were further obtained from significant comparisons. This approach relies on the assumption that functionally correlated brain regions display greater binding concordance as a result of structural and/or functional correlations [14, 21]. The regions of interest obtained from previous analyses (comparison among PD patients, DLB patients and controls) were used as “seed” regions, exploring the pattern of covariance between 123I-FP-CIT binding in each region and 123I-FP-CIT binding throughout the whole brain for each group (PD patients, DLB patients and controls). The pattern of covariance was thus separately obtained in PD patients, DLB patients and controls using a linear regression model in SPM12 considering age and sex as nuisance variables and mean occipital binding as global regressor. The significance threshold was set at p = 0.05 with adjustment for family-wise error. Only clusters containing more than 150 voxels were considered as significant. Data were analysed using SPSS 24.0 (IBM Corp., Armonk, NY).
Results
Recruitment of patients and clinical assessment
Of consecutively enrolled PD and DLB patients (69 PD, 51 DLB) who underwent 123I-FP-CIT imaging, 56 PD patients and 41 DLB patients were included in the final analysis. The remaining 23 patients were excluded because of: (1) subcortical infarcts on MRI (three PD, one DLB), (2) methodological issues (i.e. SPET image artefacts; two PD, two DLB), (3) diagnosis of progressive supranuclear palsy on follow-up (three PD), (4) PD with hallucinations (three), (5) use of antipsychotics (four DLB), (6) refusal to undergo the complete clinical assessment (two PD, three DLB; Supplementary Fig. 1).
Table 1 shows the demographic and clinical characteristics of the three groups. All DLB patients fulfilled probable criteria and presented with parkinsonism and abnormal nigrostriatal dopaminergic imaging. They differed in terms of age and sex distribution; thus, these were considered as covariates in the 123I-FP-CIT ANOVA. DLB patients showed lower total MMSE scores than PD patients (p < 0.001; 22.8 ± 4.0 vs. 28.5 ± 1.6, respectively). PD and DLB patients did not differ in terms of disease duration, total LEDD, antidepressant therapy use or mean UPDRS-III score.
123I-FP-CIT binding in PD and DLB patients
Concerning striatal binding, lower putamen and caudate SBRs were found in PD and DLB patients than in controls (Table 2). The post hoc analysis did not reveal any difference in nigrostriatal SBR, asymmetry index or putamen/caudate ratios between PD and DLB patients (Table 2). Comparable results were found when patients were stratified according to their asymmetric (left or right) motor impairment.
Extrastriatal SBR showed significant differences between the groups in the cingulate cortex, insula and thalamus. In the post-hoc analysis, DLB patients showed lower SBRs in all these three extrastriatal ROIs compared with controls, whereas PD patients showed lower SBRs only in the insula and thalamus compared with controls (Table 2). DLB patients showed significantly lower thalamic 123I-FP-CIT SBR compared with PD patients, even after adjusting for the effects of age, sex, disease duration, SSRI use in the previous months and LEDD. The same results were obtained after excluding patients receiving SSRI treatment (n = 13). Using crus II as the reference region, DLB patients showed significantly lower SBR in the right and left caudate (p = 0.02 and p = 0.03, respectively), parietal and frontal lobes (p = 0.01 for both), cingulate (p = 0.05), thalamus (p = 0.04) and insula (p = 0.001) compared with PD patients (Supplementary Table 1).
ROC analyses
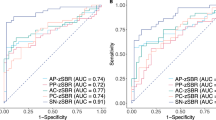
The sensitivity, specificity and AUC for those ROIs found to be significant in the above analyses were calculated to assess their power to differentiate between PD patients, DLB patients and controls. The ROIs with the best AUC for differentiating PD patients from controls were the putamen (AUC 0.94, 95% CI 0.90–1.00) and caudate (AUC 0.91, 95% CI 0.85–0.97), followed by the insula (AUC 0.88, 95% CI 0.81–0.94) and thalamus (AUC 0.74, 95% CI 0.64–0.83; Fig. 1a). The ROIs with the best AUC for differentiating DLB patients from controls were the caudate (AUC 0.96, 95% CI 0.92–1.00), putamen (AUC 0.96, 95% CI 0.93–1.00), insula (AUC 0.91. 95% CI 0.85–0.97) and thalamus (AUC 0.90, 95% CI 0.83–0.96), followed by the cingulate cortex (AUC 0.73, 95% CI 0.62–0.83; Fig. 1b). The ROIs with the best AUC for differentiating DLB from PD patients were the thalamus (AUC 0.73, 95% CI 0.63–0.83) and cingulate cortex (AUC 0.63, 95% CI 0.52–0.74; Fig. 1c).
Covariance patterns
The covariance pattern analysis of cingulate 123I-FP-CIT binding showed a cingulate-to-cingulate correlation in controls (local autocorrelation pattern). In PD patients, the cingulate cortex binding was also correlated with the inferior frontal gyrus and insula binding, whereas in DLB patients cingulate cortex binding was correlated with binding in a larger cingulate cluster and widespread cortical areas including the frontal, parietal and temporal lobes (Table 3, Fig. 2). Thalamic 123I-FP-CIT binding showed a local thalamus-to-thalamus correlation (autocorrelation pattern) in controls and PD patients. In DLB patients, 123I-FP-CIT binding was correlated with binding in a larger thalamic cluster, the right parahippocampus and the right precentral area (Table 3, Fig. 2).
Covariance patterns of 123I-FP-CIT SBR in the cingulate (a) and thalamus (b) in the three groups. Cingulate 123I-FP-CIT SBR showed a local autocorrelation in controls, a correlation with the cingulate/frontal regions in PD patients and with widespread regions (including the frontal parietal and temporal lobes) in DLB patients. Thalamic SBR showed a local autocorrelation pattern in PD patients and controls and was correlated with the frontal and parahippocampal 123I-FP-CIT SBR in DLB patients (b). The results are superimposed on a 2D standardized T1 brain template (p = 0.001, family-wise error-corrected, clusters 80 voxels). The Talairach x coordinates used were as follows: controls, cingulate, x = 79, 84, 98, 106; PD patients, cingulate, x = 75, 84, 98, 106; DLB patients, cingulate, x = 98, 60, 119, 128; controls, thalamus, x = 73, 80, 94, 100; PD patients, thalamus, x = 73, 78, 98, 102; DLB patients, thalamus, x = 51, 78, 103, 121.
Discussion
In the present study, we explored the involvement of extrastriatal dopaminergic and serotonergic pathways in PD and DLB patients using a newly developed SPM ROI-based approach. Both PD and DLB patients exhibited similar impairment in several extrastriatal regions, especially in the insula and cingulate cortex, with DLB patients also exhibiting specific thalamic involvement. The extensive 123I-FP-CIT extrastriatal impairment in Lewy body disorders is in line with the findings in several neuropathology series and molecular imaging group analyses [5, 22, 23]. Besides the expected deficits in nigrostriatal dopamine projections, we found a significantly lower insular 123I-FP-CIT SBR in patients, and this is the first time this finding has been reported with this tracer in the Lewy body disease spectrum. These findings are consistent with alpha-synuclein pathology in the insular and limbic systems and with the high prevalence of autonomic and affective dysfunctions in PD and DLB patients from the early stages [5, 24,25,26,27,28,29]. In the discriminant analysis of 123I-FP-CIT SBR impairment, the insular was the best extrastriatal region for differentiation between controls and both PD and DLB patients, exhibiting diagnostic accuracies of 88% and 91%, respectively, in the ROC analysis. Thus, assessment of insular 123I-FP-CIT SBR might increase the diagnostic accuracy of classical nigrostriatal evaluations in both PD and DLB patients [30].
Beyond sharing common dopaminergic nigrostriatal and insular deficits, PD and DLB patients showed divergent involvement of the monoaminergic cingulate and thalamic projections. Specifically, only DLB patients showed significantly lower 123I-FP-CIT binding in the cingulate areas than controls.
To evaluate the local and long-distance effects of these deficits, we analysed of patterns of covariance enabling the identification of those regions functionally related to specific deficits within a specific selected area of the brain [21]. This technique confirmed that cingulate dopaminergic deficits had long-distance widespread cortical effects specifically in DLB patients, reflecting the diffuse cortical pathology in DLB [2, 5] involving the cingulate projection areas [31, 32]. Notably, dopamine deficits in the prefrontal and anterior cingulate have been found in DLB patients by Marquie et al. [22] using 11C-altropane PET, a ligand known to be highly specific for dopamine in contrast to 123I-FP-CIT. Thus, the ability of 123I-FP-CIT to bind both DAT and SERT was used to evaluate serotonergic projections in the midbrain and thalamus – regions with higher levels of SERT than DAT [7,8,9]. DLB patients showed significantly lower thalamic 123I-FP-CIT SBR than controls and PD patients, who showed only a slight but significant reduction in thalamic SBR.
The analysis of patterns of covariance in thalamic 123I-FP-CIT SBR showed only a local autocorrelation in controls and PD patients but a wide correlation with binding in the frontal and parahippocampal projections in DLB patients, suggesting a possible long-distance effect of thalamic deficits on the limbic system. The thalamus is an important hub for subcortical–cortical projections [33] and modulates several cognitive activities, in particular attentional processes [34], and several studies have suggested that thalamic macrostructural and microstructural changes are associated with the typical cognitive fluctuations seen in DLB patients [35, 36]. Furthermore, neuropathology and imaging studies have shown thalamic dysfunction in DLB [35, 37, 38], especially in the pulvinar [39]. It is of interest that thalamic impairment has recently been identified in a subset of patients with idiopathic REM sleep behavioural disorder [40], which has been claimed to be common in the prodromal phase of Lewy body disorders [41]. This is in line with minor monoaminergic thalamic deficits reported in early PD [23, 42,43,44], and it is also possible that more relevant thalamic impairment could be useful to identify from the prodromal phase those subjects specifically at risk of conversion to DLB. Indeed, the discriminant analysis indicated that thalamic binding was more accurate in the early differentiation between PD and DLB patients than several nigrostriatal measures. These findings are at variance with those of a recent study by Joling et al. excluding differences in SERT 123I-FP-CIT binding between PD and DLB patients [9]. Their study, however, did not compare PD and DLB patients with controls and included a large number of patients with dementia in the PD group – who would most likely have had patterns of dysfunction similar to those in DLB patients [37, 45]. It should be underlined that the present study included only patients with probable DLB, all presenting with parkinsonian features. Thus, evaluation of 123I-FP-CIT extrastriatal binding needs to be extended to DLB patients without parkinsonism or with normal dopaminergic imaging in further studies.
We acknowledge that in our study we could not adjust for the effect of brain atrophy using structural coregistration and we thus decided to use large regions of interest [11]. The study included control patients with non-parkinsonian tremor who might have shown subtle serotonergic deficits, and a larger validation study including neurologically healthy controls is thus necessary. Another potential limitation was the availability of a single 123I-FP-CIT SPECT scan per subject (acquired in the standard window for DAT 3 h after injection [19]), as the optimal time for SERT assessment is around 2 h after injection [46,47,48]. Nevertheless, SERT and DAT binding data were consistent and non-overlapping, as confirmed by the autocorrelation pattern of both the thalamic and cingulate regions. The main strengths of the present study were the relatively large sample size and the accurate inclusion of additional covariates including disease duration, SSRI treatment and LEDD that are all potential modulators of 123I-FP-CIT binding [46] and have not been considered in previous studies. Another strength was the application of different normalization methods that showed consistent differences between PD and DLB patients using both occipital and crus II reference regions with some interesting divergencies. Indeed, the use of crus II amplified the differences in the caudate, frontal/parietal and insular SBR between PD and DLB patients, thus suggesting that this normalization region is even more sensitive for the assessment of extrastriatal 123I-FP-CIT binding.
Conclusion
Striatal dopaminergic deficits are pivotal in the diagnosis of PD and of DLB, and predict conversion from the prodromal phases of the diseases [49, 50]. Beyond the dopaminergic nigrostriatal system, PD and DLB patients exhibit several alterations in extrastriatal dopamine projections and divergent alterations in the thalamic serotonergic system. Despite the need for validation in a larger cohort of patients, the application of extrastriatal 123I-FP-CIT imaging might allow a better understanding of complex monoaminergic dysfunction in neurodegenerative diseases.
References
Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30:1591–601.
McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor J-P, Weintraub D, et al. Diagnosis and management of dementia with Lewy bodies. Neurology. 2017;89(1):88–100. https://doi.org/10.1212/WNL.0000000000004058.
Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. Abolishing the 1-year rule: how much evidence will be enough? Mov Disord. 2016;31(11):1623–7. https://doi.org/10.1002/mds.26796.
Maetzler W, Pilotto A, Apel A, Deuschle C, Kuebart G, Heinzel S, et al. In vivo markers of Parkinson’s disease and dementia with Lewy bodies: current value of the 5G4 α-synuclein antibody. Acta Neuropathol. 2014;128(6):893–5.
Halliday GM, Holton JL, Revesz T, Dickson DW. Neuropathology underlying clinical variability in patients with synucleinopathies. Acta Neuropathol. 2011;122:187–204. https://doi.org/10.1007/s00401-011-0852-9.
Goedert M, Jakes R, Grazia M, Neurosciences C, Building CA. The synucleinopathies: twenty years on. J Parkinsons Dis. 2017;7(s1):S51–69. https://doi.org/10.3233/JPD-179005.
Roselli F, Pisciotta NM, Pennelli M, Aniello MS, Gigante A, De Caro MF, et al. Midbrain SERT in degenerative parkinsonisms: a 123I-FP-CIT SPECT study. Mov Disord. 2010;25:1853–9.
Koch W, Unterrainer M, Xiong G, Bartenstein P, Diemling M, Varrone A, et al. Extrastriatal binding of [123I]FP-CIT in the thalamus and pons: gender and age dependencies assessed in a European multicentre database of healthy controls. Eur J Nucl Med Mol Imaging. 2014;41(10):1938–46. https://doi.org/10.1007/s00259-014-2785-8.
Joling M, Vriend C, van der Zande JJ, Lemstra AW, van den Heuvel OA, Booij J, et al. Lower 123I-FP-CIT binding to the striatal dopamine transporter, but not to the extrastriatal serotonin transporter, in Parkinson’s disease compared with dementia with Lewy bodies. Neuroimage Clin. 2018;19:130–6. https://doi.org/10.1016/j.nicl.2018.04.009.
Walker Z, Costa DC, Walker RWH, Lee L, Livingston G, Jaros E, et al. Striatal dopamine transporter in dementia with Lewy bodies and Parkinson disease: a comparison. Neurology. 2004;62(9):1568–72.
Colloby SJ, O’Brien JT, Fenwick JD, Firbank MJ, Burn DJ, McKeith IG, et al. The application of statistical parametric mapping to 123I-FP-CIT SPECT in dementia with Lewy bodies, Alzheimer’s disease and Parkinson’s disease. Neuroimage. 2004;23:956–66.
McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB consortium. Neurology. 2005;65:1863–72. https://doi.org/10.1212/01.wnl.0000187889.17253.b1.
Pilotto A, Turrone R, Liepelt-Scarfone I, Bianchi M, Poli L, Borroni B, et al. Vascular risk factors and cognition in Parkinson’s disease. J Alzheimers Dis. 2016;51:563–70. https://doi.org/10.3233/JAD-150610.
Premi E, Pilotto A, Garibotto V, Bigni B, Turrone R, Alberici A, et al. Impulse control disorder in PD: a lateralized monoaminergic frontostriatal disconnection syndrome? Parkinsonism Relat Disord. 2016;30:62–6. https://doi.org/10.1016/j.parkreldis.2016.05.028.
Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord. 2007;22:1689–707. https://doi.org/10.1002/mds.21507.
Bhatia KP, Bain P, Bajaj N, Elble RJ, Hallett M, Louis ED, et al. Consensus statement on the classification of tremors. From the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord. 2018;33:75–87. https://doi.org/10.1002/mds.27121.
Premi E, Calhoun VD, Garibotto V, Turrone R, Alberici A, Cottini E, et al. Source-based morphometry multivariate approach to analyze [123I]FP-CIT SPECT imaging. Mol Imaging Biol. 2017;19(5):772–8.
Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–31.
Darcourt J, Booij J, Tatsch K, Varrone A, Vander Borght T, Kapucu ÖL, et al. EANM procedure guidelines for brain neurotransmission SPECT using 123I-labelled dopamine transporter ligands, version 2. Eur J Nucl Med Mol Imaging. 2010;37:443–50.
Calvini P, Rodriguez G, Inguglia F, Mignone A, Guerra UP, Nobili F. The basal ganglia matching tools package for striatal uptake semi-quantification: description and validation. Eur J Nucl Med Mol Imaging. 2007;34:1240–53.
Paternicó D, Premi E, Alberici A, Archetti S, Bonomi E, Gualeni V, et al. Dyslexia susceptibility genes influence brain atrophy in frontotemporal dementia. Neurol Genet. 2015;1:e24. https://doi.org/10.1212/NXG.0000000000000024.
Marquie M, Locascio JJ, Rentz DM, Becker JA, Hedden T, Johnson KA, et al. Striatal and extrastriatal dopamine transporter levels relate to cognition in Lewy body diseases: an 11C altropane positron emission tomography study. Alzheimers Res Ther. 2014;6:52. https://doi.org/10.1186/s13195-014-0052-7.
Politis M, Wu K, Loane C, Kiferle L, Molloy S, Brooks DJ, et al. Staging of serotonergic dysfunction in Parkinson’s disease: an in vivo 11C-DASB PET study. Neurobiol Dis. 2010;40:216–21. https://doi.org/10.1016/j.nbd.2010.05.028.
Lewis DA, Melchitzky DS, Sesack SR, Whitehead RE, Auh S, Sampson A. Dopamine transporter immunoreactivity in monkey cerebral cortex: regional, laminar, and ultrastructural localization. J Comp Neurol. 2001;136:119–36.
Papapetropoulos S, Mash DC. Insular pathology in Parkinson’s disease patients with orthostatic hypotension. Parkinsonism Relat Disord. 2007;13:308–11.
Christopher L, Koshimori Y, Lang AE, Criaud M, Strafella AP. Uncovering the role of the insula in non-motor symptoms of Parkinson’s disease. Brain. 2014;137:2143–54.
Fathy YY, Jonker AJ, Oudejans E, de Jong FJJ, van Dam AW, Rozemuller AJM, et al. Differential insular cortex subregional vulnerability to α-synuclein pathology in Parkinson’s disease and dementia with Lewy bodies. Neuropathol Appl Neurobiol. 2019;45:262–7. https://doi.org/10.1111/nan.12501.
Roquet D, Noblet V, Anthony P, Philippi N, Cretin B, Martin-Hunyadi C, et al. Insular atrophy at the prodromal stage of dementia with Lewy bodies: a VBM DARTEL study. Sci Rep. 2017;7:9437. https://doi.org/10.1038/s41598-017-08667-7.
Liepelt-Scarfone I, Pilotto A, Müller K, Bormann C, Gauss K, Wurster I, et al. Autonomic dysfunction in subjects at high risk for Parkinson’s disease. J Neurol. 2015;262(12):2643–52.
Coon EA, Cutsforth-Gregory JK, Benarroch EE. Neuropathology of autonomic dysfunction in synucleinopathies. Mov Disord. 2018;33(3):349–58. https://doi.org/10.1002/mds.27186.
Shennhav A, Botvinick M, Cohen JD. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron. 2013;79:217–40.
Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137:12–32.
Haber SN, Calzavara R. The cortico-basal ganglia integrative network: the role of the thalamus. Brain Res Bull. 2009;78(2-3):69–74.
Mitchell AS, Sherman SM, Sommer MA, Mair RG, Vertes RP, Chudasama Y. Advances in understanding mechanisms of thalamic relays in cognition and behavior. J Neurosci. 2014;34:15340–6. https://doi.org/10.1523/JNEUROSCI.3289-14.2014.
Delli Pizzi S, Franciotti R, Taylor JP, Thomas A, Tartaro A, Onofrj M, et al. Thalamic involvement in fluctuating cognition in dementia with Lewy bodies: magnetic resonance evidences. Cereb Cortex. 2015;25:3682–9.
Watson R, Colloby SJ, Blamire AM, Wesnes KA, Wood J, O’Brien JT. Does attentional dysfunction and thalamic atrophy predict decline in dementia with Lewy bodies? Parkinsonism Relat Disord. 2017;45:69–74. https://doi.org/10.1016/j.parkreldis.2017.10.006.
Gazzina S, Premi E, Turrone R, Acosta-Cabronero J, Rizzetti MC, Cotelli MS, et al. Subcortical matter in the α-synucleinopathies spectrum: an MRI pilot study. J Neurol. 2016;263:1575–82. https://doi.org/10.1007/s00415-016-8173-5.
Jellinger KA, Attems J. Does striatal pathology distinguish Parkinson disease with dementia and dementia with Lewy bodies? Acta Neuropathol. 2006;112:253–60.
Erskine D, Ding J, Thomas AJ, Kaganovich A, Khundakar AA, Hanson PS, et al. Molecular changes in the absence of severe pathology in the pulvinar in dementia with Lewy bodies. Mov Disord. 2018;33:982–91.
Stokholm MG, Iranzo A, Østergaard K, Serradell M, Otto M, Bacher Svendsen K, et al. Extrastriatal monoaminergic dysfunction and enhanced microglial activation in idiopathic rapid eye movement sleep behaviour disorder. Neurobiol Dis. 2018;115:9–16. https://doi.org/10.1016/j.nbd.2018.02.017.
Pilotto A, Heinzel S, Suenkel U, Lerche S, Brockmann K, Roeben B, et al. Application of the Movement Disorder Society prodromal Parkinson’s disease research criteria in 2 independent prospective cohorts. Mov Disord. 2017;2:1025–34. https://doi.org/10.1002/mds.27035.
Moore RY, Whone AL, Brooks DJ. Extrastriatal monoamine neuron function in Parkinson’s disease: an 18F-dopa PET study. Neurobiol Dis. 2008;29:381–90.
Pavese N, Rivero-Bosch M, Lewis SJ, Whone AL, Brooks DJ. Progression of monoaminergic dysfunction in Parkinson’s disease: a longitudinal 18F-dopa PET study. Neuroimage. 2011;56:1463–8.
Albin RL, Koeppe RA, Bohnen NI, Wernette K, Kilbourn MA, Frey KA. Spared caudal brainstem SERT binding in early Parkinson’s disease. J Cereb Blood Flow Metab. 2008;28:441–4.
Pilotto A, Premi E, Paola Caminiti S, Presotto L, Turrone R, Alberici A, et al. Single-subject SPM FDG-PET patterns predict risk of dementia progression in Parkinson disease. Neurology. 2018;90(12):e1029–37. https://doi.org/10.1212/WNL.0000000000005161.
Booij J, de Jong J, de Bruin K, Knol R, de Win MM, van Eck-Smit BL. Quantification of striatal dopamine transporters with 123I-FP-CIT SPECT is influenced by the selective serotonin reuptake inhibitor paroxetine: a double-blind, placebo-controlled, crossover study in healthy control subjects. J Nucl Med. 2007;48:359–66.
Koopman KE, la Fleur SE, Fliers E, Serlie MJ, Booij J. Assessing the optimal time point for the measurement of extrastriatal serotonin transporter binding with 123I-FP-CIT SPECT in healthy, male subjects. J Nucl Med. 2012;53:1087–90. https://doi.org/10.2967/jnumed.111.102277.
Matsuoka K, Yasuno F, Shinkai T, Miyasaka T, Takahashi M, Kiuchi K, et al. Test-retest reproducibility of extrastriatal binding with 7#I-FP-CIT SPECT in healthy male subjects. Psychiatry Res Neuroimaging. 2016;258:10–5. https://doi.org/10.1016/j.pscychresns.2016.10.007.
Walker Z, Moreno E, Thomas A, Inglis F, Tabet N, Stevens T, et al. Evolution of clinical features in possible DLB depending on FP-CIT SPECT result. Neurology. 2016;87:1045–51.
Iranzo A, Valldeoriola F, Lomeña F, Molinuevo JL, Serradell M, Salamero M, et al. Serial dopamine transporter imaging of nigrostriatal function in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study. Lancet Neurol. 2011;10:797–805. https://doi.org/10.1016/S1474-4422(11)70152-1.
Author information
Authors and Affiliations
Contributions
Andrea Pilotto: study concept and design, acquisition of data, statistical analysis design and execution, interpretation of data, drafting/revising the manuscript for content.
Francesca Schiano di Cola: acquisition of data, statistical analysis execution, interpretation of data, drafting/revising the manuscript for content.
Enrico Premi: statistical analysis design and interpretation of data, revising the manuscript for content.
Roberto Grasso: acquisition of data, revising the manuscript for content.
Rosanna Turrone: acquisition of data, revising the manuscript for content.
Stefano Gipponi: acquisition of data, revising the manuscript for content.
Andrea Scalvini acquisition of data, revising the manuscript for content.
Elisabetta Cottini: acquisition of data, revising the manuscript for content.
Barbara Paghera: acquisition of data, revising the manuscript for content.
Valentina Garibotto: revising the manuscript for content.
Maria Cristina Rizzetti: revising the manuscript for content.
Laura Bonanni: revising the manuscript for content.
Barbara Borroni: revising the manuscript for content.
Silvia Morbelli: revising the manuscript for content.
Flavio Nobili: revising the manuscript for content.
Ugo Paolo Guerra: revising the manuscript for content.
Daniela Perani: revising the manuscript for content.
Alessandro Padovani: study concept and design, acquisition of data, analysis and interpretation of data, drafting/revising the manuscript for content.
Corresponding author
Ethics declarations
Conflicts of interest
Andrea Pilotto received speaker honoraria from BioMarin Pharmaceutical, Chiesi Pharmaceuticals, Nutricia Pharmaceuticals, UCB Pharma and Zambon Pharmaceuticals. He received travel grants from AbbVie Pharmaceuticals, BioMarin Pharmaceutical, Nutricia Pharmaceuticals, Zambon Pharmaceuticals and the Italian Movement Disorder Society.
Valentina Garibotto is funded by a grant from the Swiss National Science Foundation (SNF 320030_169876) and from the Velux Foundation (project 1123).
Silvia Morbelli acted as consultant for Eli Lilly in 2014 and for Avid Radiopharmaceuticals in 2016. She received speaker honoraria from General Electric Healthcare in 2017.
Daniela Perani is funded by a grant from Fondazione Cariplo, Bando Ricerca 2014 Malattie Invecchiamento, project title “Evaluation of autonomic, genetic, imaging and biochemical markers for Parkinson-related dementia,” 2015–2017, and the EU FP7 INMIND project (FP7-HEALTH-2013, grant agreement 278,850).
Alessandro Padovani is a consultant for and served on the scientific advisory board of GE Healthcare, Eli Lilly, and Actelion Ltd. Pharmaceuticals, received speaker honoraria from Nutricia, PIAM, Langstone Technology, GE Healthcare, Lilly, UCB Pharma, Zambon and Chiesi Pharmaceuticals. He is funded by a grant from the Ministry of University and Research (MURST).
All other authors declare no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Pilotto, A., Schiano di Cola, F., Premi, E. et al. Extrastriatal dopaminergic and serotonergic pathways in Parkinson’s disease and in dementia with Lewy bodies: a 123I-FP-CIT SPECT study. Eur J Nucl Med Mol Imaging 46, 1642–1651 (2019). https://doi.org/10.1007/s00259-019-04324-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04324-5