Abstract
α-Synucleinopathies, such as Parkinson’s disease (PD) and dementia with Lewy bodies (DLB), are characterized by α-synuclein accumulation from brainstem structures to the neocortex. PD and DLB are clinically distinguishable, while discrimination between Parkinson Disease Dementia (PDD) and DLB can be subtle and based on the temporal relationship between motor and cognitive symptoms. To explore patterns of subcortical atrophy in PD, PDD and DLB, and assess specific differences between PD and PDD, and between DLB and PDD. 16 PD, 11 PDD and 16 DLB patients were recruited and underwent 1.5 Tesla structural MRI scanning. Segmentation of subcortical structures was performed with a well-validated, fully-automated tool, and volume and shape for each structure were compared between groups. PDD and DLB patients showed global subcortical atrophy compared to PD patients. Greater hippocampal atrophy was the specific trait that distinguished PDD from PD, while greater atrophy of the pallidi discriminated DLB from PDD. Vertex analysis revealed specific shape differences in both structures. Our results suggest that automated, time-sparing, subcortical volumetry may provide diagnostically useful information in α-synucleinopathies. Future studies on larger samples and with iron-sensitive MRI contrasts are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Abnormal accumulation of inclusions containing α-synuclein is the neuropathological hallmark of synucleinopathies, e.g., Parkinson disease (PD), Dementia with Lewy bodies (DLB) and Parkinson Disease Dementia (PDD), among others. The pathological hallmarks of these three conditions are the loss of dopaminergic neurons in the substantia nigra along with inclusions of Lewy bodies and Lewy neurites. Lewy bodies’ deposition is thought to start in the medulla oblongata and progress to diencephalic, telencephalic and cortical structures in the final stages of PD [1].
Clinical findings reflect the ascending spreading of pathology: extrapyramidal features of PD are related to basal ganglia involvement in early disease stages, while cognitive complaints in PDD and DLB are thought to be a consequence of cortical Lewy body burden [2, 3].
When extrapyramidal and cognitive symptoms occur in close succession, classification between PDD and DLB becomes difficult. The most widely used diagnostic criteria in this context is the “1 year role”: a case is classified as DLB if dementia occurs within 1 year from motor symptom onset, whereas it is diagnosed as PDD if dementia is identified more than 1 year after motor symptom onset [4]. However, the existence of PDD and DLB as two distinct entities is still a matter of debate; it has been shown that the severity of Lewy body distribution in PDD and DLB is comparable, and cognitive assessment and clinical progression follow a similar trajectory in both diseases [5–8]. Conversely, whole-brain volumetric MRI studies have found differential patterns of cortical involvement between PDD and DLB [9, 10]. Measures of cortical atrophy in PD are inconclusive when compared to those in age-matched controls [10, 11]; only structural measurements of the basal ganglia have shown some promise [12–16]. Despite some already existing evidence of basal ganglia involvement in the transition from PD to PD-MCI [17–19], studies comparing subcortical atrophy patterns between PD and PDD are limited [20, 21].
In this study, we tested two hypotheses: (1) whether subcortical volumes might characterize PD-dementia (PDD) relative to PD, and (2) whether PDD and DLB might be distinguished by their subcortical atrophy pattern. To this end, we studied both global (volumetric) and regional (shape based) measurements of atrophy in several subcortical structures using a validated, fully-automated segmentation method for structural MRI.
Methods
Subjects
Patients were consecutively recruited at the Centre for Ageing Brain and Neurodegenerative Disorders and the Centre for Movement Disorders of the Neurology Unit, Department of Clinical and Experimental Sciences, University of Brescia, Italy and at S. Isidoro Hospital—FERB Onlus, Trescore Balneario, Bergamo, Italy.
A diagnosis of probable PD was made according to the Queen Square Brain Bank clinical criteria [22], defined by the presence of bradykinesia and at least one between muscular rigidity, rest tremor or postural instability. Diagnosis of PD was further accomplished by documented response to levodopa and absence of cognitive impairment on the basis of standardized neuropsychological assessment. Diagnosis of PDD was made in those patients fulfilling PD criteria, who developed multi-domain dementia more than 1 year after diagnosis of PD, according to the MDS criteria [23, 24]. DLB diagnosis was made in those patients who developed parkinsonism and cognitive decline at onset, fulfilling the DLB consortium clinical criteria for probable DLB [4].
To rule out a probable Alzheimer ’s disease diagnosis, each DLB patient underwent DATSCAN SPECT imaging, which showed reduced bilateral striatal dopaminergic binding [4].
Each patient underwent general and neurological examination, neuropsychological assessment and brain structural MRI scanning. Patients were followed-up for at least 1 year to ensure a more consistent clinical diagnosis.
Stringent exclusion criteria were applied as follows: (1) cerebrovascular disorders, previous stroke, normal pressure hydrocephalus, and intra-cranial mass lesions documented by MRI; (2) diagnosis of other type of neurodegenerative disorders (i.e., Multiple System Atrophy, Corticobasal Syndrome, Progressive Supranuclear Palsy) according to current clinical criteria; (3) other neurological disorders or significant medical problems (i.e., hepatic or renal failure, chronic respiratory insufficiency) potentially responsible for encephalopathy; or (4) use of antipsychotic treatment or iatrogenic parkinsonism. The work conformed to the Helsinki Declaration and patients were enrolled according to the Local Ethics Committee criteria and with appropriate informed consent.
Clinical, behavioural and neuropsychological assessment
Staging of PD, PDD and DLB motor symptoms was assessed by the Unified Parkinson Disease Rating Scale, motor part (UPDRS-III). Behavioural disturbances were recorded according to the Neuropsychiatric Inventory [25]. A comprehensive neuropsychological and behavioural assessment, including Basic Activities of Daily Living (BADL) and Instrumental Activities of Daily Living (IADL), was carried out. Neuropsychological testing was performed by a standardized neuropsychological battery including Mini-Mental State Examination (MMSE), Rey Auditory Verbal Learning Test (RAVLT), Rey Complex Figure Copy and Recall, Stroop Color-Word Test, Phonological and Category Fluency, Visual Object and Space Perception Battery (VOSP) subtests (Number Location; Cube Analysis; Silhouette and Progressive Silhouettes), Trail Making Test A and B and Clock Drawing Test [26].
MRI data, acquisition and processing
All imaging was obtained using a 1.5 Tesla Siemens Avanto magnetic resonance scanner (Siemens, Erlangen, Germany), equipped with a circularly polarized single channel transmit-receive coil.
In a single session, the following scans were collected from each studied subject: (1) T2-weighted TSE scan (TR/TE = 2500 ms/50 ms) to exclude the presence of macroscopic brain abnormalities, according to exclusion criteria; (2) 3D magnetization-prepared rapid gradient echo (MPRAGE) T1-weighted scan (TR = 2050 ms, TE = 2.56 ms, inversion time = 1100 ms, Matrix = 1 × 1 × 1 mm3, in-plane field of view FOV = 256 × 256 × 256 mm3, flip angle = 15°).
Preprocessing steps included conversion of DICOM images to NIFTI format and reorientation to MNI 152 space.
Segmentation of gray matter (GM), white matter (WM) and cerebro-spinal fluid (CSF) was performed in the native space, to obtain the Total Intracranial Volume (defined as the sum of GM, WM and CSF) and the Total Brain Volume (defined as the sum of GM and WM) which were subsequently used to normalize subcortical volumes [27]. We used the Advanced Data Processing Assistant for Resting-State fMRI (DPARSFA) (http://rfmri.org/DPARSF) with the “Unified Segmentation” option, which relies on SPM8 scripts.
Segmentation of subcortical structures was performed with the fully-automated FMRIB’s Integrated Registration and Segmentation Tool (FIRST), implemented in FSL 5.0 [28]. The following regions were segmented: caudate, putamen, pallidus, thalamus, amygdala, accumbens and hippocampus, bilaterally.
FIRST registers a model composed of manually segmented volumes of 336 subjects (healthy and pathological subjects ranging from 4 to 87 years of age, provided by the Center for Morphometric Analysis, MGH, Boston) to each subject’s T1 image.
It uses a two-stage affine process to register each brain image onto an MNI 152 template. The first stage of this registration is performed on the whole brain. In the second stage, this intermediate brain image is weighted by a subcortical mask and affine registration is applied one more time to achieve optimal registration of the subcortical structures. Once the registration is estimated, inverse transformation is calculated and applied to the MNI 152 template to bring it to the native space so that the original image voxels do not have to be interpolated. After a general registration is achieved, Bayesian Active Appearance Model (AAM) is used to register and segment each subcortical structure precisely. AAM is an extension of Active Shape Model (ASM) [29] and it incorporates intensity information in addition to the geometrical shape model [30].
FIRST creates a surface mesh for each subcortical structure using a deformable mesh model. Each mesh is made up of triangles and the apex of connecting triangles represents a vertex [15, 28]. In this way, FIRST allows for both an estimation of volume and shape of each structure.
Comparing FIRST segmentation to manual segmentation (considered as the gold standard) with a leave-one-out (LOO) cross-validation method, Patenaude et al. found a good concordance among the two methods, ranging from 0.7 to 0.9. Best concordance was found for thalamus and putamen, while smaller structures such as accumbens and amygdala showed a lower median Dice overlap [28].
Each subject’s segmentation output was visually inspected using the “first_slice_dir” script, but no gross inaccuracies were identified.
Statistical analysis
Differences in clinical and demographic data among groups were assessed using one-way ANOVA or Chi-square test, as appropriate. For continuous variables, Bonferroni post hoc test was performed to assess differences among paired groups. Values of p < 0.05 were considered significant.
For imaging analysis, the volume of each structure was extracted. TIV and TBV were calculated to normalize each subcortical volume to head size. Normalized subcortical volumes (reported in mm3) were calculated with the following formula: [average TIV of the total population × (total brain or subcortical volume of the participant/TIV of the participant)] [27].
Then, the mean between the left and right volume of each structure was considered, and differences for each structure were probed with a multivariate analysis of equal mean where age, gender and disease duration were nuisance factors.
Values of p < 0.05, Fisher’s least significant difference (LSD) post hoc corrected, were considered significant.
For shape analysis, we adopted the ReconNative + RigidAlign option, which reconstructs the meshes in the native space of the images. It removes the pose with a six degree-of-freedom (DOF) registration, retaining both volume and shape. Statistical analysis was performed using two-sample t-tests (PD vs PDD and PDD vs DLB), with age, gender and disease duration as nuisance variables. p values <0.05 corrected for the false discovery rate were considered significant [31].
Results
As reported in Table 1, our cohort of patients consisted of 16 PD, 11 PDD and 16 DLB patients.
PD patients were cognitively unimpaired as compared to both PDD and DLB.
PDD patients were younger at disease onset and had a longer disease course as compared to DLB patients.
Results from volumetric analysis are summarized in Table 2. PDD and DLB patients returned significantly smaller volumes in the whole brain, caudati, pallidi, accumbens nuclei, thalami and hippocampi compared to the PD group.
When we considered the differences between PD and PDD, the regions found to be the most affected were caudati, accumbens nuclei, thalami and hippocampi. The logistic regression analysis revealed that hippocampus was the only significant predictor differentiating PD and PDD (p < 0.001, β = 0.688), thus being the best predictor of PD-related dementia (see Fig. 1a).
When comparing PDD and DLB, the only significant difference was found in the pallidi; these being more impaired in DLB (Fig. 1b).
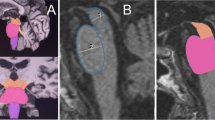
We then performed shape analysis in the most predictive regions, namely the hippocampus (PD vs PDD) and globus pallidus (PDD vs DLB) (see Fig. 2a, b).
Shape analysis of hippocampi and pallidi in α-synucleinopathies. a Dorsal and ventral view of left and right hippocampus in the PDD <PD contrast. b dorsal and ventral view of left and right globus pallidus in the DLB <PDD contrast. Light blue structure shape, orange shape difference. p < 0.05 FDR corrected. A anterior, P posterior, L left, R right
Both left and right hippocampi showed supra-threshold differences in the PDD < PD contrast, with left hippocampus being extensively affected by regional atrophy in its dorsolateral and ventrolateral surfaces. No significant shape alterations were found for the opposite contrast (PDD greater than PD).
DLB patients showed deformation of both dorsal and ventral pallidal surface when compared to PDD. No significant deformations in the PDD < DLB contrast were found.
Discussion
In this work, our aim was to study subcortical differences in the three most common alpha-synucleinopathies. PD, PDD and DLB are characterized by the same neuropathological substrate, and overlapping clinical symptoms [32]. Dementia occurs in about 85 % of PD patients over disease course [23], and PDD and DLB may be distinguished only by the controversial “1 year rule” criterion [4].
To unravel differences between these disorders, we focused on the basal ganglia.
Different neuropathological studies in the PD spectrum have demonstrated that the striatum is commonly affected by α-synuclein pathology, with higher burden and co-occurrence of Aβ and Tau deposition in the striatum of PDD and DLB patients [5, 33, 34]. Among these two groups, DLB patients seem to be more severely and frequently affected [34].
We used FIRST, an algorithm implemented in FSL, which enables fast automated segmentation of several subcortical structures with good accuracy and reliability [28]. As compared to manual segmentation, actually considered the gold standard, software like FIRST and FreeSurfer have shown to provide good estimates of subcortical volumes, particularly with higher intraclass correlation coefficient (ICC) for larger structures [35, 36]. With regard to FIRST, high inter- and intra-scanner reliability has been demonstrated, proposing it as a robust and computationally rapid alternative to FreeSurfer segmentation [37].
Two main results were obtained by the present study. First, we observed the selective involvement of hippocampus in the transition from PD to PD-dementia. Preferential involvement of this region can be justified both by neuritic dystrophy in limbic structures and by the possible co-occurrence of Alzheimer’s disease pathology and cholinergic dysfunction [38].
The second finding was the evidence of PDD and DLB differences at subcortical level. Indeed, in PDD patients, despite extensive cortical and subcortical atrophy, the only preserved structure was the pallidus, whose volume was comparable to that of PD patients. Globus pallidus is known to be the major output of the basal ganglia circuitry; it receives both dopaminergic (from substantia nigra and ventral tegmental area), and cholinergic inputs, and projects to thalamic nuclei and back to the cortex [39, 40]. At our knowledge, only one previous neuropathological work explored globus pallidus in DLB and PDD in a series of 34 patients. The authors showed only one isolated DLB case with Aß plaques in the pallidus, and none of the PDD patients [34].
PDD and DLB are the focus of the on-going debate whether they are in essence the same disorder or they represent truly distinct phenotypes. The present results, along with previous structural and functional MRI works exploring cortical structures [9, 10], might be supportive of the latter hypothesis.
In conclusion, the results of this pilot study, if confirmed, argued that probing subcortical structures with structural MRI might be useful to define PD patients at-risk for dementia and to discriminate between PDD and DLB condition in uncertain cases.
However, some limitations should be acknowledged. First of all, the small sample of subjects and the necessity to covariate for parameters known to influence brain volumes (i.e., age, gender, disease duration) greatly limits the statistical power. To confirm these results, larger samples are required.
Furthermore, despite the careful patients’ selection and acquisition of DATSCAN imaging to define DLB pathology, this study lacks of neuropathological confirmation.
Finally, it is worth discussing a technical point: the caveat that the pallidum—together with the amygdala and nucleus accumbens—are the least reliable structures for automated segmentation [28]. The issue of segmenting the pallidum with a T1-weighted contrast is that it is one of the most iron-rich gray matter structures in the adult brain [41], thereby its gray/white matter contrast is poorer compared with, e.g., the cortex. In fact, there is evidence suggesting that iron accumulation might drive mis-segmentation of basal ganglia structures from structural scans due to T1-shortening [42]; thus, it is at least conceivable the present results might also reflect increased metal accumulation in DLB’s pallidum. Future research, utilizing more specific iron-sensitive MRI contrasts such as quantitative susceptibility mapping (QSM) and/or R2* mapping, might shed some light on this observation.
References
Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211
Emre M (2003) Dementia associated with Parkinson’s disease. Lancet Neurol 2:229–237
Hurtig HI, Trojanowski JQ, Galvin J et al (2000) Alpha-synuclein cortical Lewy bodies correlate with dementia in Parkinson’s disease. Neurology. 54:1916–1921
McKeith IG, Dickson DW, Lowe J et al (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 65:1863–1872
Tsuboi Y, Dickson DW (2005) Dementia with Lewy bodies and Parkinson’s disease with dementia: are they different? Parkinsonism Relat Disord. 11(Suppl 1):S47–51
Guo L, Itaya M, Takanashi M, Mizuno Y, Mori H (2005) Relationship between Parkinson disease with dementia and dementia with Lewy bodies. Parkinsonism Relat Disord. 11:305–309
Goldman JG, Williams-Gray C, Barker RA, Duda JE, Galvin JE (2014) The spectrum of cognitive impairment in Lewy body diseases. Mov Disord 29:608–621
Aarsland D, Ballard CG, Halliday G (2004) Are Parkinson’s disease with dementia and dementia with Lewy bodies the same entity? J Geriatr Psychiatry Neurol 17:137–145
Beyer MK, Larsen JP, Aarsland D (2007) Gray matter atrophy in Parkinson disease with dementia and dementia with Lewy bodies. Neurology. 69:747–754
Borroni B, Premi E, Formenti A et al (2015) Structural and functional imaging study in dementia with Lewy bodies and Parkinson’s disease dementia. Parkinsonism Relat Disord. 21:1049–1055
Planetta PJ, Kurani AS, Shukla P et al (2015) Distinct functional and macrostructural brain changes in Parkinson’s disease and multiple system atrophy. Hum Brain Mapp 36:1165–1179
Menke RA, Szewczyk-Krolikowski K, Jbabdi S et al (2014) Comprehensive morphometry of subcortical grey matter structures in early-stage Parkinson’s disease. Hum Brain Mapp 35:1681–1690
Garg A, Appel-Cresswell S, Popuri K, McKeown MJ, Beg MF (2015) Morphological alterations in the caudate, putamen, pallidum, and thalamus in Parkinson’s disease. Front Neurosci. 9:101
Sterling NW, Du G, Lewis MM et al (2013) Striatal shape in Parkinson’s disease. Neurobiol Aging 34:2510–2516
Nemmi F, Sabatini U, Rascol O, Peran P (2015) Parkinson’s disease and local atrophy in subcortical nuclei: insight from shape analysis. Neurobiol Aging 36:424–433
Lee HM, Kwon KY, Kim MJ et al (2014) Subcortical grey matter changes in untreated, early stage Parkinson’s disease without dementia. Parkinsonism Relat Disord. 20:622–626
Melzer TR, Watts R, MacAskill MR et al (2012) Grey matter atrophy in cognitively impaired Parkinson’s disease. J Neurol Neurosurg Psychiatry 83:188–194
Mak E, Bergsland N, Dwyer MG, Zivadinov R, Kandiah N (2014) Subcortical atrophy is associated with cognitive impairment in mild Parkinson disease: a combined investigation of volumetric changes, cortical thickness, and vertex-based shape analysis. AJNR Am J Neuroradiol 35:2257–2264
Pereira JB, Junque C, Bartres-Faz D, Ramirez-Ruiz B, Marti MJ, Tolosa E (2013) Regional vulnerability of hippocampal subfields and memory deficits in Parkinson’s disease. Hippocampus. 23:720–728
Apostolova LG, Beyer M, Green AE et al (2010) Hippocampal, caudate, and ventricular changes in Parkinson’s disease with and without dementia. Mov Disord 25:687–695
Mak E, Su L, Williams GB, O’Brien JT (2015) Neuroimaging correlates of cognitive impairment and dementia in Parkinson’s disease. Parkinsonism Relat Disord. 21:862–870
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Emre M, Aarsland D, Brown R et al (2007) Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 22:1689–1707
Goetz CG, Emre M, Dubois B (2008) Parkinson’s disease dementia: definitions, guidelines, and research perspectives in diagnosis. Ann Neurol. 64(Suppl 2):S81–92
Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J (1994) The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 44:2308–2314
Lezak MD (1995) Neuropsychological assessment, 3rd edn. Oxford University Press, New York
van Norden AG, Fick WF, de Laat KF et al (2008) Subjective cognitive failures and hippocampal volume in elderly with white matter lesions. Neurology. 71:1152–1159
Patenaude B, Smith SM, Kennedy DN, Jenkinson M (2011) A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 56:907–922
Taylor CJ, Cootes TF, Lanitis A, Edwards G, Smyth P, Kotcheff AC (1997) Model-based interpretation of complex and variable images. Philos Trans R Soc Lond B Biol Sci 352:1267–1274
Sandman CA, Head K, Muftuler LT, Su L, Buss C, Davis EP (2014) Shape of the basal ganglia in preadolescent children is associated with cognitive performance. Neuroimage. 99:93–102
Genovese CR, Lazar NA, Nichols T (2002) Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 15:870–878
Berg D, Postuma RB, Bloem B et al (2014) Time to redefine PD? Introductory statement of the MDS Task Force on the definition of Parkinson’s disease. Mov Disord 29:454–462
Tsuboi Y, Uchikado H, Dickson DW (2007) Neuropathology of Parkinson’s disease dementia and dementia with Lewy bodies with reference to striatal pathology. Parkinsonism Relat Disord. 13(Suppl 3):S221–224
Jellinger KA, Attems J (2006) Does striatal pathology distinguish Parkinson disease with dementia and dementia with Lewy bodies? Acta Neuropathol 112:253–260
Morey RA, Petty CM, Xu Y et al (2009) A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage. 45:855–866
Morey RA, Selgrade ES, Wagner HR 2nd, Huettel SA, Wang L, McCarthy G (2010) Scan-rescan reliability of subcortical brain volumes derived from automated segmentation. Hum Brain Mapp 31:1751–1762
Nugent AC, Luckenbaugh DA, Wood SE, Bogers W, Zarate CA Jr, Drevets WC (2013) Automated subcortical segmentation using FIRST: test-retest reliability, interscanner reliability, and comparison to manual segmentation. Hum Brain Mapp 34:2313–2329
Ruffmann C, Calboli FC, Bravi I et al (2015) Cortical Lewy bodies and Abeta burden are associated with prevalence and timing of dementia in Lewy body diseases. Neuropathol Appl Neurobiol. doi:10.1111/nan.12294
Hegeman DJ, Hong ES, Hernandez VM, Chan SC (2016) The external globus pallidus: progress and perspectives. Eur J Neurosci 43(10):1239–1265
Gittis AH, Berke JD, Bevan MD et al (2014) New roles for the external globus pallidus in basal ganglia circuits and behavior. J Neurosci 34:15178–15183
Acosta-Cabronero J, Betts MJ, Cardenas-Blanco A, Yang S, Nestor PJ (2016) In Vivo MRI mapping of brain iron deposition across the adult lifespan. J Neurosci 36:364–374
Goto M, Abe O, Miyati T et al (2013) Association between iron content and gray matter missegmentation with voxel-based morphometry in basal ganglia. J Magn Reson Imaging 38:958–962
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical standards
All procedures were approved by the Local Ethics Commitee and were performed in accordance with the ethical principles and guidelines of the 1964 Helsinki declaration and its later amendments.
Rights and permissions
About this article
Cite this article
Gazzina, S., Premi, E., Turrone, R. et al. Subcortical matter in the α-synucleinopathies spectrum: an MRI pilot study. J Neurol 263, 1575–1582 (2016). https://doi.org/10.1007/s00415-016-8173-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-016-8173-5