Abstract
Purpose
We investigated the prognostic value of the tumour heterogeneity index determined on preoperative [18F]FDG PET/CT in patients with uterine leiomyosarcoma (LMS).
Methods
We retrospectively reviewed patients with uterine LMS who underwent preoperative [18F]FDG PET/CT scans at three tertiary referral hospitals. The PET/CT parameters maximum standardized uptake value of the primary tumour (SUVmax), metabolic tumour volume (MTV) and total lesion glycolysis were assessed. The negative values of the MTV linear regression slope (nMLRS) according to the SUV thresholds of 2.5 and 3.0 were determined as the tumour heterogeneity index. The value of PET/CT-derived parameters in predicting progression-free survival (PFS) and overall survival (OS) were determined in regression analyses.
Results
Clinicopathological and PET/CT data from 16 patients were reviewed. The median postsurgical follow-up was 21 months (range 4–82 months), and 12 patients (75.0%) experienced recurrence. Tumour size (P = 0.017), SUVmax (P = 0.019), MTV (P = 0.016) and nMLRS (P = 0.008) were significant prognostic factors for recurrence. MTV (P = 0.048) and nMLRS (P = 0.045) were significant prognostic factors for patient survival. nMLRS was correlated with clinicopathological parameters including tumour size (Pearson’s correlation coefficient γ = 0.825, P < 0.001) and lymph node metastasis (γ = 0.721, P = 0.004). Patient groups categorized according to the nMLRS cut-off value showed significant differences in PFS (P = 0.033) and OS (P = 0.044).
Conclusion
The preoperative tumour heterogeneity index obtained using the MTV linear regression slope may be a novel and useful prognostic marker in uterine LMS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Uterine sarcomas are a group of rare tumours with differing tumour biology, natural history and response to treatment. Uterine sarcomas arise from smooth muscles and connective tissue of the uterus. They account for up to 3–7% of all uterine malignancies and are associated with a poorer prognosis than endometrial carcinoma [1]. Leiomyosarcoma (LMS) is the most common histological subtype of uterine sarcoma [2, 3]. The annual incidence of uterine LMS is less than two women per 100,000 based on the population-based Surveillance, Epidemiology and End Results (SEER) database of the National Cancer Institute [4], and the incidence of uterine LMS appears to increase with age, and to be higher in African-American women and in women who have use of tamoxifen for more than 5 years [4, 5].
Few studies have investigated the value of PET imaging in LMS. In studies examining patients with chondrosarcoma, liposarcoma and synovial sarcoma, tumour FDG uptake has been shown to correlate with tumour grade and disease progression [6,7,8]. Moreover, due to the rarity of the tumour, few studies of uterine LMS have been performed, and most are case reports. [18F]FLT PET has been shown to be superior to [18F]FDG PET in differentiating malignant from benign leiomyoma, and [18F]FLT uptake correlates well with the immunohistochemical index of cell proliferation [9]. We recently found that a high maximum standardized uptake value (SUVmax) on preoperative [18F]FDG PET/CT was associated with a worse outcome in patients with uterine LMS [10].
Research over decades has demonstrated that cancer is a heterogeneous disease. The phenomenon of intratumoral heterogeneity arises as a result of the genetic instability of cancer cells and the tumour microenvironment [11, 12], and these biological features have a strong impact on patient treatment and outcome. There has been increasing interest in assessing tumour heterogeneity and specifically heterogeneity in intratumoral [18F]FDG uptake [13]. [18F]FDG PET can reflect metabolic tumour heterogeneity by demonstrating variations in metabolism of different tumour regions, and this metabolic heterogeneity is considered to reflect biological and clinical tumour properties. Tumour heterogeneity is a major challenge to personalized medicine and biomarker development, and it may contribute to treatment failure [14]. Several heterogeneity indices have been suggested for use with [18F]FDG PET/CT, including coefficient of variation [15,16,17,18,19] or the slope of linear regression [20,21,22]. However, the prognostic value of tumour heterogeneity indices measured on [18F]FDG PET/CT has never been evaluated in uterine LMS.
In the current study, we investigated the relationship between tumour heterogeneity measured in terms of metabolic tumour volume (MTV) linear regression slope (MLRS) and clinicopathological parameters, and determined the prognostic value of tumour heterogeneity in uterine LMS.
Materials and methods
Patients
We retrospectively identified patients with biopsy-proven uterine LMS who had undergone preoperative [18F]FDG PET/CT imaging at Samsung Medical Center, Asan Medical Center, and Seoul National University Hospital between April 2009 and October 2016. The diagnoses were established by preoperative endometrial biopsy and verified on hysterectomy specimens, and stage was assessed according to the FIGO 2009 criteria for surgical staging [23]. Although LMS is a tumour that develops in the myometrium, the tumour tissues were obtained from the endometrial biopsy. All the patients in the current study had a tumour more than 3 cm in size with extension to the endometrium found at the final pathological examination. Patients were required to have undergone both preoperative integrated [18F]FDG PET/CT imaging in the 2 weeks prior to surgery. Patients were excluded from the analysis if they (1) were previously diagnosed with another malignant disease, (2) had a follow-up duration <3 months, or (3) had received a primary treatment other than surgery, such as chemotherapy or preoperative radiation. After surgery, all patients were clinically and radiologically followed up according to each institution’s clinical protocol. All clinicopathological and survival data were obtained from electronic medical records and the institutional tumour registry. Data from ten patients in a previous study were included in the current analysis [10]. The study protocol was approved by the institutional review board, and informed consent was waived due to its retrospective design.
[18F]FDG PET/CT scan
[18F]FDG PET/CT was performed using the following scanners: a dedicated Biograph (Siemens Medical Solutions, Malvern, PA) or a Gemini scanner (Philips Medical Systems, Andover, MA) at Seoul National University Hospital, a Discovery STE 8, a Discovery PET/CT 690 or 710 (GE Healthcare, Waukesha, WI, USA), or a Biograph TruePoint 16 or TruePoint 40 scanner (Siemens Healthcare, Germany) at Asan Medical Center, or a Discovery LS or STE scanner (GE Healthcare, Milwaukee, WI, USA) at Samsung Medical Center. Both PET and low-dose CT scanning covered the skull to the proximal thighs. The protocol required patients to fast for at least 4 h before image acquisition, and all patients were asked to void before undergoing scanning, and a Foley catheter was inserted to minimize bladder activity. One hour before scanning, 125 mL of a barium sulphate solution was administered orally to opacify the bowel for the CT portion of the study at Seoul National University Hospital. [18F]FDG (5–5.18 MBq/kg) was administered intravenously 45 min to 1 h before the CT scan. All patients rested in a darkened room at a comfortable temperature between injection and scanning.
At Seoul National University Hospital, prior to PET scanning, a CT scan without contrast administration was performed using the following parameters: 80 mAs, 120 kVp, slice thickness 5 mm, 0.5 s per rotation and reconstruction onto a 512 × 512 matrix. The CT results were used to generate an attenuation correction map for PET, and the PET images were reconstructed. Each PET scan was acquired from the skull base to the proximal thighs in three-dimensional row-action maximum-likelihood algorithm mode, with four iterations, eight subsets and 4.8-mm full-width half-maximum reconstruction onto a 512 × 512 matrix. A total of seven to nine bed positions were used for the PET acquisition, with 2.5 min per bed position.
At Asan Medical Center, the patients were injected with 370–555 MBq of FDG (0.14 mCi/kg for the Discovery STE 8, the Discovery PET/CT 690 and 710 and the Biograph TruePoint 40 scanners, and 0.2 mCi/kg for the Biograph TruePoint 16 scanner) and allowed to rest in a sitting or supine position for 60 min prior to scanning. Acquisition times per bed position were 3 min for the Biograph TruePoint 16 and Discovery STE 8 scanners, 2.5 min for the Biograph TruePoint 40 scanner and 2 min for the Discovery PET/CT 690 and 710 scanners.
At Samsung Medical Center, CT images were acquired using a 16-slice helical CT scanner (140 KeV, 30–170 mAs with Auto A mode; slice thickness 3.75 mm) with the STE scanner or an eight-slice helical CT scanner (140 keV, 40–120 mAs adjusted to body weight, slice thickness 5 mm) with the LS scanner. Emission PET images were then acquired from the thighs to the head for 2.5 min per frame in three-dimensional mode with the STE scanner or 4 min per frame in two-dimensional mode with the LS scanner. Attenuation-corrected PET images (voxel size 3.9 × 3.9 × 3.3 mm with the STE scanner, 4.3 × 4.3 × 3.9 mm with the LS scanner) were reconstructed using CT data with an ordered-subsets expectation maximization algorithm (20 subsets, two iterations with the STE scanner; 28 subsets, two iterations with the LS scanner).
Image analysis
Intensity values (radioactivity concentrations) from the [18F]FDG PET/CT data were converted to SUVs on the workstation, and maximum SUVs (SUVmax) were used to determine [18F]FDG avidity quantitatively. SUV was defined as the concentration of [18F]FDG divided by the injected dose, corrected for the body weight of the patient and radioactive decay at the time of scanning: SUV = activity concentration/(injected dose/body weight).
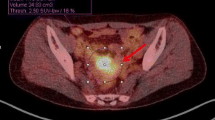
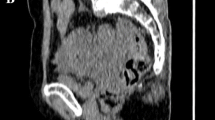
MTV (centimetres cubed) was measured from attenuation-corrected [18F]FDG PET/CT images using an SUV-based automated contouring program (syngo MI Applications, volumetric analysis 6.0.14.4; Siemens Medical Solutions). MTVs were automatically calculated by summing the total volumes of the voxels with threshold SUVs of 2.5 and 3.0 in the tumour VOI, and Fig. 1 shows representative images of MTVs. Total lesion glycolysis (TLG) was calculated by multiplying the average SUV by the MTV.
Representative [18F]FDG PET/CT images showing measurement of metabolic tumour volume (MTV) using an SUV-based automated contouring program. The contour inside the target volume of interest was automatically produced and the voxels with SUV >2.5 or >3.0 were incorporated to define the volumes of the uterine corpus tumours
Tumour heterogeneity analysis
There are several ways to calculate heterogeneity by statistical methods. Statistical heterogeneity parameters are categorized based on the scale of the analysis as global, regional or local. In this analysis, we calculated the MLRS according to different SUV thresholds (2.5 and 3.0) using a previously reported method with modification [21, 22], and used the negative value of the MLRS (nMLRS) for convenience and analysis.
Clinical endpoints and follow-up
All patients underwent clinical follow-up that included diagnostic imaging studies and blood tests as described in a previous study [10]. Progression-free survival (PFS) was analysed using time-to-event regression. PFS was calculated from the date of operation to the date of documented recurrence. Recurrence of disease was defined as the development of tumour seen on physical examination and/or CT scan that was considered consistent with recurrent LMS.
Statistical analysis
We sought to determine the value of tumour heterogeneity for predicting recurrence and survival in patients with uterine LMS. The Cox proportional hazards model was used to evaluate prognostic variables, and estimated hazard ratios (HR) with 95% confidence intervals (95% CI) are presented, and P < 0.05 was considered statistically significant. Due to the limited number of patients available for analysis, only univariate Cox regression models were used to assess the prognostic factors. With respect to PET/CT parameters, receiver operating characteristic (ROC) curve analysis was performed to determine the cut-off values for predicting recurrence. Independent sample t tests were used to compare the means of clinicopathological parameters in the high and low nMLRS groups [24]. All analyses were performed using SASS software for Windows, version 19.0 (IBM SASS, Armonk, NY).
Results
Patient characteristics
Between April 2009 and October 2016, data from 26 patients were collected at three participating institutions. Of these patients, 7 had received surgical staging without lymph node (LN) dissection, 3 had received radiation therapy and/or chemotherapy as first-line treatment, and 16 were finally enrolled for analysis. As shown in Table 1, the median size of the primary uterine tumours was 11 cm (range, 3.0–18.5 cm), and the median duration of follow-up was 21 months (range 4–82 months). Of the 16 patients, 12 (75.0%) developed recurrent disease and 7 (43.8%) died of disease. All scans were eligible for calculation of nMLRS according to the SUV thresholds of 2.5 and 3.0. Table 1 also shows the preoperative [18F]FDG PET/CT parameters of the patients.
Measuring tumour heterogeneity
For measurement of MLRS, MTV was assessed according to two SUV thresholds (2.5 and 3.0) and linear regression analysis was performed to find the slope. In this analysis, the average MLRS in all 16 patients was −116.38, and nMLRS was 116.38 (Fig. 2).
Method for calculating tumour heterogeneity. For measurement of metabolic tumour volume (MTV) linear regression slope (MLRS), MTV was determined according to two SUV thresholds (2.5 and 3.0) and linear regression analysis was performed to find the slope. In this analysis, MLRS of the current study population was −116.38, and its negative form (nMLRS) was 116.38
Correlation between parameters
In this analysis, nMLRS was correlated with clinicopathological parameters including tumour size (Pearson’s correlation coefficient γ = 0.813, P < 0.001), LN metastasis (γ = 0.707, P = 0.002), and mitotic count (γ = 0.708, P = 0.033). MTV was correlated with FIGO stage (γ = 0.603, P = 0.013), tumour size (γ = 0.579, P = 0.019), and adnexal tumour involvement (γ = 0.537, P = 0.039).
Measurement of nMLRS cut-off value
ROC curve analysis identified that the areas under the curve (AUC) for recurrence and survival were maximal when the threshold nMLRS were 63.29 and 97.68, respectively. The AUC for PFS at the cut-off nMLRS (63.29) was 0.958 (P = 0.008, 95% CI 0.859–1.000), and the AUC for overall survival (OS) at the cut-off nMLRS (97.68) was 0.746 (P = 0.101, 95% CI 0.491–1.000).
Differences between patients with high and low nMLRS
Table 2 shows the clinicopathological and [18F]FDG PET/CT imaging characteristics of patients grouped according to the nMLRS cut-off value for OS. There were significant differences in mean tumour size (P = 0.001), SUVmax (P = 0.004), MTV (P = 0.017) and OS (P = 0.032) between the two groups.
Prediction of recurrence and survival
Table 3 presents the results of the univariate Cox regression analysis of the prognostic factors for PFS. Among clinicopathological variables, only tumour size (P = 0.017, HR 1.200, 95% CI 1.033–1.394) was significant. Most [18F]FDG PET/CT parameters including SUVmax (P = 0.019, HR 1.052, 95% CI 1.008–1.098), MTV (P = 0.016, HR 1.002, 95% CI 1.000–1.003), and nMLRS (P = 0.008, HR 1.009, 95% CI 1.002–1.016) were significant. The resulting Kaplan-Meier survival curves according to the nMLRS cut-off value showed a significant difference in PFS (P = 0.033, log-rank test; Fig. 3).
Kaplan-Meier survival curves of progression-free survival (PFS) rate in patients with uterine LMS stratified according to negative MTV linear regression slope (nMLRS). There is a statistically significant difference in survival between patients with nMLRS <63.29 (blue line) and those with nMLRS ≥63.29 (green line; P = 0.033, log-rank test)
Table 3 also presents the regression analysis of the prognostic factors for OS. None of the clinicopathological parameters showed significance in the regression analysis. Only two [18F]FDG PET/CT parameters, SUVmax (P = 0.048, HR 1.051, 95% CI 1.000–1.105) and nMLRS (P = 0.045, HR 1.007, 95% CI 1.000–1.015), were significant prognostic factors. The resulting Kaplan-Meier survival curves showed a significant difference in OS between the groups (P = 0.044, log-rank test; Fig. 4).
Kaplan-Meier survival curves of overall survival (OS) rate in patients with uterine LMS stratified according to negative MTV linear regression slope (nMLRS). There is a statistically significant difference in survival between patients with nMLRS <97.68 (blue line) and nMLRS ≥97.68 (green line; P = 0.044, log-rank test)
Discussion
The aim of the current study was to investigate the prognostic value of tumour heterogeneity calculated in terms of MLRS on preoperative [18F]FDG PET/CT in patients with uterine LMS. The principle finding of the study was that MLRS on preoperative [18F]FDG PET/CT was a useful and novel prognostic marker for prediction of recurrence and survival in patients with uterine LMS.
The results indicate that preoperative evaluation of MLRS is an accurate prognostic tool for both recurrence and OS. To the best of our knowledge, this is the first study investigating the prognostic value of preoperative nMLRS in uterine LMS. Tumour heterogeneity of uterine LMS was also found to be correlated with clinicopathological prognostic parameters. nMLRS was significantly correlated with tumour size and LN metastasis. These findings suggest that nMLRS may correlate with traditional clinicopathological prognostic markers. Tumour heterogeneity generally increases with tumour size, and hence results in greater aggressiveness of the tumour. This correlation implies a consistent cascade of tumour size, tumour heterogeneity and tumour metastatic aggressiveness, and this in turn indicates that the MTV-based tumour heterogeneity index may be a promising predicator of patient prognosis.
In the current study, the risk of tumour recurrence and death increased with increasing tumour heterogeneity. This highlights the importance of tumour heterogeneity and the possibility that it may be a useful prognostic marker. We assessed nMLRS using absolute SUV thresholds (2.5 and 3.0) to obtain more exact identification of the cancer margin without physiological uptake in adjacent bowel, which are widely used in the determination of absolute MTV [19, 25].
Among the clinicopathological parameters, tumour size and LN metastasis were significant prognostic factors. It is interesting that the [18F]FDG PET/CT parameters (SUVmax and MTV) and nMLRS were found to be significant prognostic factors. Moreover, only SUVmax and nMLRS were found to be significant prognostic factors for OS. Considering the small number of study participants, these results emphasize the significance of the heterogeneity index in prognosis. Information on tumour size and LN metastasis can be obtained on histopathological examination after surgery, but [18F]FDG PET/CT parameters and the heterogeneity index are readily available immediately after scanning, and may help establish both treatment and the surveillance strategy. In this respect, we cannot overestimate the clinical value of preoperative functional parameters and the heterogeneity index.
There are several possible methods for evaluating heterogeneity using [18F]FDG PET/CT. There is increasing evidence that tumour heterogeneity measured using [18F]FDG PET/CT texture analysis is associated with response to therapy and prognosis. However, no textural analysis parameters are widely accepted for measuring tumour heterogeneity in clinical practice. In addition, texture is difficult to assess due to the difficulty in acquiring the measurements. Compared with textural analysis, nMLRS used in the current study, is simple to calculate and can be readily applied in clinical practice. MLRS can easily be determined on a workstation or calculated with any conventional statistics program. In this respect, the results of the current study demonstrated the clinical usefulness of nMLRS as a novel prognostic factor that compensates for the inherent limits of conventional clinicopathological markers and investigational textural analysis.
This study had several limitations. First, the findings should be interpreted cautiously because of the retrospective nature of the study and the rarity of the malignancy. Due to the general rarity of uterine LMS, only a small number of patients were eligible for analysis. Selection bias was inherent due to the retrospective nature of the study, and the general applicability of the findings may be limited. Most patients did not undergo a preoperative PET/CT scan due to the difficulty in pretreatment diagnosis. Nevertheless, this study is noteworthy because it is the first to show the prognostic value of the preoperative tumour heterogeneity index in patients with uterine LMS. Second, the use of various types of PET scanners during the study period in the different institutions might have affected the values of the quantitative parameters. Third, measurement of MTV and TLG was institution-based, and image acquisition and interpretation procedures differed among the institutions so that there is the possibility of interobserver differences that might have influenced the measurements. We performed a post-hoc central review of PET/CT scans from all three institutions, and reached the conclusion that the readings from each institutions were in accordance with the central review. Fourth, we were not able to perform partial volume correction as the segmentation software of the system did not support this option. Lastly, most patients in this analysis had stage I or IV disease, and this abnormal population distribution might also have influenced the results of the survival analysis.
Although the tumour heterogeneity parameter is not well standardized, and various methods for its determination are still under investigation, the strength of this study lies in the fact that it is the first to show the prognostic value of tumour heterogeneity measured on preoperative [18F]FDG PET/CT in patients with uterine LMS. Although these findings are preliminary and limited by the small sample size, they are promising because they show the potential of heterogeneity not only as a functional marker, but also for patient stratification for clinical trials. The direct clinical impact of the use of nMLRS in predicting recurrence and OS needs to be investigated and verified in future studies.
In conclusion, we provide the first report of the prognostic value of tumour heterogeneity, showing that nMLRS on preoperative [18F]FDG PET/CT imaging is significantly associated with recurrence and survival in patients with uterine LMS. The method for determining nMLRS is simple and convenient and nMLRS shows prognostic value. Preoperative assessment of nMLRS using [18F]FDG PET/CT may be a promising novel prognostic marker for identifying patients with uterine LMS with a poor prognosis.
References
D'Angelo E, Prat J. Uterine sarcomas: a review. Gynecol Oncol. 2010;116(1):131–9. https://doi.org/10.1016/j.ygyno.2009.09.023.
Abeler VM, Royne O, Thoresen S, Danielsen HE, Nesland JM, Kristensen GB. Uterine sarcomas in Norway. A histopathological and prognostic survey of a total population from 1970 to 2000 including 419 patients. Histopathology. 2009;54(3):355–64. https://doi.org/10.1111/j.1365-2559.2009.03231.x.
Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. 2005;366(9484):491–505. https://doi.org/10.1016/S0140-6736(05)67063-8.
Brooks SE, Zhan M, Cote T, Baquet CR. Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989-1999. Gynecol Oncol. 2004;93(1):204–8. https://doi.org/10.1016/j.ygyno.2003.12.029.
Gockley AA, Rauh-Hain JA, del Carmen MG. Uterine leiomyosarcoma: a review article. Int J Gynecol Cancer. 2014;24(9):1538–42. https://doi.org/10.1097/IGC.0000000000000290.
Brenner W, Conrad EU, Eary JF. FDG PET imaging for grading and prediction of outcome in chondrosarcoma patients. Eur J Nucl Med Mol Imaging. 2004;31(2):189–95. https://doi.org/10.1007/s00259-003-1353-4.
Brenner W, Eary JF, Hwang W, Vernon C, Conrad EU. Risk assessment in liposarcoma patients based on FDG PET imaging. Eur J Nucl Med Mol Imaging. 2006;33(11):1290–5. https://doi.org/10.1007/s00259-006-0170-y.
Lisle JW, Eary JF, O'Sullivan J, Conrad EU. Risk assessment based on FDG-PET imaging in patients with synovial sarcoma. Clin Orthop Relat Res. 2009;467(6):1605–11. https://doi.org/10.1007/s11999-008-0647-z.
Yamane T, Takaoka A, Kita M, Imai Y, Senda M. 18F-FLT PET performs better than 18F-FDG PET in differentiating malignant uterine corpus tumors from benign leiomyoma. Ann Nucl Med. 2012;26(6):478–84. https://doi.org/10.1007/s12149-012-0597-0.
Park JY, Lee JW, Lee HJ, Lee JJ, Moon SH, Kang SY, et al. Prognostic significance of preoperative 18F-FDG PET/CT in uterine leiomyosarcoma. J Gynecol Oncol. 2017;28(3):e28. https://doi.org/10.3802/jgo.2017.28.e28.
Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194(4260):23–8.
Heppner GH. Tumor heterogeneity. Cancer Res. 1984;44(6):2259–65.
Tixier F, Le Rest CC, Hatt M, Albarghach N, Pradier O, Metges JP, et al. Intratumor heterogeneity characterized by textural features on baseline 18F-FDG PET images predicts response to concomitant radiochemotherapy in esophageal cancer. J Nucl Med. 2011;52(3):369–78. https://doi.org/10.2967/jnumed.110.082404.
Gerlinger M, Rowan AJ, Horswell S, Math M, Larkin J, Endesfelder D, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366(10):883–92. https://doi.org/10.1056/NEJMoa1113205.
Asselin MC, O'Connor JP, Boellaard R, Thacker NA, Jackson A. Quantifying heterogeneity in human tumours using MRI and PET. Eur J Cancer. 2012;48(4):447–55. https://doi.org/10.1016/j.ejca.2011.12.025.
Lee M, Lee H, Cheon GJ, Kim HS, Chung HH, Kim JW, et al. Prognostic value of preoperative intratumoral FDG uptake heterogeneity in patients with epithelial ovarian cancer. Eur Radiol. 2017;27(1):16–23. https://doi.org/10.1007/s00330-016-4368-5.
Kang SY, Cheon GJ, Lee M, Kim HS, Kim JW, Park NH, et al. Prediction of recurrence by preoperative intratumoral FDG uptake heterogeneity in endometrioid endometrial cancer. Transl Oncol. 2017;10(2):178–83. https://doi.org/10.1016/j.tranon.2017.01.002.
Chung HH, Kang SY, Ha S, Kim JW, Park NH, Song YS, et al. Prognostic value of preoperative intratumoral FDG uptake heterogeneity in early stage uterine cervical cancer. J Gynecol Oncol. 2016;27(2):e15. https://doi.org/10.3802/jgo.2016.27.e15.
Kim YI, Kim YJ, Paeng JC, Cheon GJ, Lee DS, Chung JK, et al. Heterogeneity index evaluated by slope of linear regression on 18F-FDG PET/CT as a prognostic marker for predicting tumor recurrence in pancreatic ductal adenocarcinoma. Eur J Nucl Med Mol Imaging. 2017;44(12):1995–2003. https://doi.org/10.1007/s00259-017-3755-8.
Huang B, Chan T, Kwong DL, Chan WK, Khong PL. Nasopharyngeal carcinoma: investigation of intratumoral heterogeneity with FDG PET/CT. AJR Am J Roentgenol. 2012;199(1):169–74. https://doi.org/10.2214/AJR.11.7336.
Kim TH, Yoon JK, Kang DK, Lee SJ, Jung YS, Yim H, et al. Correlation between F-18 fluorodeoxyglucose positron emission tomography metabolic parameters and dynamic contrast-enhanced MRI-derived perfusion data in patients with invasive ductal breast carcinoma. Ann Surg Oncol. 2015;22(12):3866–72. https://doi.org/10.1245/s10434-015-4526-z.
Kwon SH, Yoon JK, An YS, Shin YS, Kim CH, Lee DH, et al. Prognostic significance of the intratumoral heterogeneity of 18F-FDG uptake in oral cavity cancer. J Surg Oncol. 2014;110(6):702–6. https://doi.org/10.1002/jso.23703.
Prat J. FIGO staging for uterine sarcomas. Int J Gynaecol Obstet. 2009;104(3):177–8. https://doi.org/10.1016/j.ijgo.2008.12.008.
Altman DG, Gore SM, Gardner MJ, Pocock SJ. Statistical guidelines for contributors to medical journals. Br Med J. 1983;286(6376):1489–93.
Lucignani G. SUV and segmentation: pressing challenges in tumour assessment and treatment. Eur J Nucl Med Mol Imaging. 2009;36(4):715–20. https://doi.org/10.1007/s00259-009-1085-1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. For this type of retrospective study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Lee, JW., Park, JY., Lee, H.J. et al. Preoperative [18F]FDG PET/CT tumour heterogeneity index in patients with uterine leiomyosarcoma: a multicentre retrospective study. Eur J Nucl Med Mol Imaging 45, 1309–1316 (2018). https://doi.org/10.1007/s00259-018-3975-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-018-3975-6