Abstract
Purpose
The aim of this study is to test the hypothesis that positron emission tomography (PET) with 3′-deoxy-3′-[18F]-fluorothymidine (18F-FLT) can differentiate malignancy from benign leiomyoma better than PET with 2-deoxy-2-[18F]fluoro-d-glucose (18F-FDG), and to evaluate whether 18F-FLT and 18F-FDG uptake correlate with immunohistochemical index of cell proliferation.
Methods
The protocol of this prospective study was approved by the institutional ethics committee, and all patients gave written informed consent. Fifteen patients (aged 26–65 years, median 44 years) with uterine corpus tumor which has the possibility of being leiomyosarcoma underwent 18F-FLT and 18F-FDG PET scans. Maximum standard uptake value (SUVmax) of PET scans and Ki-67 labeling index of surgical specimens were evaluated. Mann–Whitney’s U test was used for comparing uptakes between benign and malignant, and linear regression analysis was used for evaluating the correlation between Ki-67 labeling index and SUVmax.
Results
Five cases were diagnosed as malignant (leiomyosarcoma for 3 cases, and carcinoma for 2 cases), and the others were benign leiomyoma. Sensitivity and negative predictive value of both tracers for detecting malignancy was 100 %. Specificity, positive predictive value and accuracy of 18F-FLT PET were higher than those of 18F-FDG PET. Difference in SUVmax between malignant and benign was significant for 18F-FLT PET (P < 0.01), but not for 18F-FDG PET. While all the malignant cases showed positive uptake in both tracers, a case of leiomyosarcoma with huge necrosis showed relatively low uptake. Uptake of 18F-FLT showed better correlation with Ki-67 labeling index compared with 18F-FDG (R 2 = 0.91 vs. R 2 = 0.26).
Conclusion
Negative findings on additional 18F-FDG or 18F-FLT PET may rule out the possibility of malignancy for the patients with suspected leiomyosarcoma diagnosed by conventional methods. 18F-FLT PET is superior to 18F-FDG PET in differentiating malignant from benign leiomyoma. Moreover, 18F-FLT uptake correlated well with the immunohistochemical index of cell proliferation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Benign leiomyoma occasionally resembles malignant tumors such as leiomyosarcoma and endometrial carcinoma. Although the incidence of uterine leiomyosarcoma is extremely low, accurate diagnosis is essential because of its aggressiveness and subsequent poor prognosis. Magnetic resonance imaging (MRI) is the representative imaging modality for diagnosing gynecological disorder. However, no acceptable consensus exists on differentiating malignancy from benign leiomyoma [1].
Positron emission tomography (PET) using 2-deoxy-2-[18F]fluoro-d-glucose (18F-FDG) is effective for differentiating benign tumor from malignancy of various organs including lung [2], adrenal gland [3], and pancreas [4]. Although effectiveness has also been discussed in uterine corpus tumors by 18F-FDG PET, discriminating malignancy from uterine leiomyoma is difficult because of high incidence of false-positive scans [5].
From the pathological approach, differential diagnosis between leiomyosarcoma and leiomyoma is basically based on the findings of high mitotic index, presence of coagulation necrosis, and the degree of nuclear pleomorphism [6]. However, the histopathological criteria have been controversial especially for the low grade leiomyosarcoma. Recent advances in immunohistochemical analysis are expected to improve the diagnosis. Several immunohistochemical studies on uterine leiomyosarcoma have been reported recently, and Ki-67 labeling index is considered to be one of the representative marker.
Ki-67 antigen is a cell proliferation-associated protein that is expressed in all stages of the cell cycle except for G0 phase. The Ki-67 protein levels are comparatively low from G1 to early S phase, and gradually increase during mitosis. Ki-67 protein expression can be visualized by immunohistochemical staining, and it can be a useful marker of cell proliferation. Several studies have reported that expression of Ki-67 was useful for differentiating leiomyosarcoma from leiomyoma [7, 8].
Besides 18F-FDG, 3′-deoxy-3′-[18F]-fluorothymidine (18F-FLT) was introduced as a PET tracer for tumor imaging. As an analog of thymidine, 18F-FLT is phosphorylated by thymidine kinase 1 and accumulates within the cells with increased proliferation. Some papers showed that 18F-FLT uptake was well correlated to Ki-67 immunohistochemical staining in various kinds of tumor [9–11]. Therefore, 18F-FLT uptake has the possibility to reflect the malignant status of uterine corpus tumor directly.
The aim of this study is to test the hypothesis that 18F-FLT PET can differentiate malignancy from benign leiomyoma better than 18F-FDG, and to evaluate whether 18F-FLT and 18F-FDG uptake correlate with immunohistochemical index of cell proliferation.
Materials and methods
Patients population
The protocol of this prospective study was approved by the institutional ethics committee, and all patients gave written informed consent before entering the study.
Between March 2009 and March 2011, a total of 15 patients (median age 44 years; range 26–65 years) met the inclusion criteria (20 years or older, uterine corpus tumor with possibility of being leiomyosarcoma, surgical operation being considered without evidence of carcinoma cells detected by transvaginal cytology or biopsy prior to the PET scan) were enrolled in this study. Of these patients, 9 were premenopausal and 6 were postmenopausal. The subjects satisfied at least one of the detailed criteria for suspected leiomyosarcoma: tumor of 5 cm or larger and enlarging; resistance to hormonal therapy; heterogeneous high intensity lesions visualized in T2 weighted image of MRI; bleeding or necrosis suspected on MRI or ultrasound; and invasions to surrounding tissue or metastasis to other organs suspected. Even if the other differential diagnosis besides leiomyosarcoma was considered, cases completely filled the above-mentioned criteria without definitive diagnosis by the transvaginal histopathological approach were not excluded for this study.
PET protocol
The patients underwent 18F-FLT and 18F-FDG PET scans. Interval of the two scans was 1–34 days (median 4 days). PET scans were performed using a PET scanner (ECAT EXACT HR+, Siemens, Erlangen, Germany) except for 2 18F-FDG-PET scans performed using a PET/CT scanner (Discovery STEP, GE Healthcare, Milwaukee, WI, USA).
The patients fasted for 6 h or longer before the PET scans. For the ECAT PET scanner, images were obtained in 2-dimensional mode from 60 min post injection of 18F-FLT (319-424 MBq) or 18F-FDG (336–406 MBq), and reconstructed with ordered subsets-expectation maximization which provided image resolution of 8 mm full width at half maximum (FWHM). For the Discovery PET/CT scanner, images were obtained in 3-dimensional mode from 60 min post injection of 18F-FDG (158 and 154 MBq), and reconstructed with VUE point plus (HD) which provided image resolution of 5.14 mm FWHM.
All the images were acquired to cover the entire tumor. MRI images were used to correlate PET with morphological structure using the image fusion software (VOX-BASE II, J-MAC SYSTEM, INC., Sapporo, Japan). Regions of interest (ROI) were placed over the PET images, and maximum index of standardized uptake value (SUVmax) of the tumor was acquired for each tracer.
Histopathological finding
All histopathologic analysis was performed by a pathologist who was unaware of the PET results. Surgical specimens were fixed in 10 % buffered formalin and processed to create paraffin blocks in routine method. The sections (4 μm thick) were de-paraffinized in xylene and rehydrated thorough an ethanol series. The sections were treated with microwave in 0.1 mol/L citrate (pH 6.0) buffer for antigen retrieval before immunohistochemical staining. Then the sections were incubated with the primary antibodies, anti-Ki-67 antibody (Dako Japan, Tokyo, Japan), for 20 min. Immunostaining was performed with Dako Autostainer (Dako Japan, Tokyo, Japan) according to the instruction manual, and the slides were counterstained with hematoxylin. Ki-67 index was determined as percentage of the cells with Ki-67-positive nuclei per 500–1000 tumor cells in the regions of the tumor with the greatest density of staining.
Analyzed items and statistics
Final diagnosis was determined by the conventional histopathological method in 14 cases who had a surgical resection of the tumor. The 15th case was diagnosed as benign on the follow up of at least 6 month.
Based on the final diagnosis and SUVmax of each PET scan, receiver operating characteristic (ROC) analysis was performed to determine area under curve (AUC) and the cut-off SUVmax for differentiating between benign and malignant.
In addition, diagnostic capability such as sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy were evaluated by the cut-off SUVmax. Also the difference in SUVmax between malignant and benign was statistically analyzed by Mann–Whitney’s U test. In addition, correlation between PET tracers uptake and Ki-67 expression was evaluated by linear regression analysis on the cases whose surgical specimens were acquired. For the statistical analyses, P value of less than 0.05 was considered to be significant. Statistical analysis was implemented in JMP 7.0 (SAS Institute Japan, Tokyo, Japan).
Results
Final diagnosis was malignant in 5 cases (leiomyosarcoma for 3 cases, adenosquamous cell carcinoma for 1 case and endometrioid adenocarcinoma for 1 case), and benign in 10 cases (all cases were leiomyoma). Two cases of carcinoma were initially suspected of leiomyosarcoma, because endometrial biopsy preceded by the enrollment in this study could not prove carcinoma cells.
In the ROC analysis, AUC of 18F-FLT was larger than 18F-FDG, and the cut off SUVmax was 2.07 in 18F-FLT, and 4.32 in 18F-FDG (Fig. 1).
As the diagnostic capability, sensitivity for 18F-FLT and 18F-FDG was 100 % (5/5) each. NPV for 18F-FLT (9/9) and 18F-FDG (7/7) was also 100 % each. Specificity, PPV and accuracy of 18F-FLT were 90.0 % (9/10), 83.9 % (5/6) and 93.3 % (14/15), respectively. Meanwhile, specificity, PPV and accuracy of 18F-FDG were 70.0 % (7/10), 62.5 % (5/8) and 80.0 % (12/15), respectively.
Difference in SUVmax between malignant and benign was significant for 18F-FLT, but not for 18F-FDG (Fig. 2). A case of leiomyoma with extremely high 18F-FDG uptake showed a low 18F-FLT uptake (Fig. 3). All the malignant cases showed positive uptake in both tracers (Fig. 4). However, a case of leiomyosarcoma with huge necrotic tissue showed a lower uptake than the other malignant cases (Fig. 5).
SUVmax of a 18F-FLT and b 18F-FDG PET. Difference in SUVmax between malignant (leiomyosarcoma and carcinoma) and benign (leiomyoma) was significant in for 18F-FLT (P < 0.01, Mann–Whitney’s U test), but not for 18F-FDG. (Open circles LM, leiomyoma; open triangles LMS, leiomyosarcoma; multisymbols CA, carcinoma)
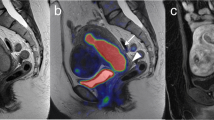
A representative case of leiomyoma. Maximum intensity projection PET images and transaxial PET images fused with MRI acquired with a 18F-FLT and b 18F-FDG PET, and c Ki-67 immunohistochemical staining. Although 18F-FLT uptake was not apparent (SUVmax 1.8), 18F-FDG accumulated in the tumor strongly (SUVmax 14.8). Ki-67 labeling index was low (1.7 %)
A case of leiomyosarcoma. Maximum intensity projection PET images and transaxial PET images fused with MRI acquired with a 18F-FLT and b 18F-FDG PET, and c Ki-67 immunohistochemical staining. Both 18F-FLT and 18F-FDG uptake were positive (SUVmax 3.9 and 6.5, respectively). Ki-67 labeling index was high (53.4 %)
A case of leiomyosarcoma with a huge necrotic area. Maximum intensity projection and transaxial images of a 18F-FLT and b 18F-FDG PET, and c MRI images (upper T1 weighted image, lower: T2 weighted image) of the same slice. Most of the tumor was occupied by non-active necrotic tissue, and leiomyosarcoma cells existed only on the edge of the tumor. Compared with Ki-67 labeling index (19.1 %), 18F-FLT uptake was low. While metastatic lesions in vertebrae are visualized in 18F-FDG PET images, they cannot be confirmed in 18F-FLT PET images because of physiological 18F-FLT uptake of the bone marrow
18F-FLT showed better correlation with the expression of Ki-67 compared with 18F-FDG by the linear regression analysis of the cases for which operation was performed (Fig. 6).
Correlation between Ki-67 labeling index and SUVmax for a 18F-FLT and b 18F-FDG PET in uterine corpus tumors (Open circles leiomyoma, open triangles leiomyosarcoma, multisymbols carcinoma). 18F-FLT showed better and significant linear correlation (R 2 = 0.91, P < 0.001) compared with 18F-FDG (R 2 = 0.26, P = 0.06)
Discussion
Uterine leiomyoma, the most common gynecological tumor, is usually asymptomatic and needs no treatment, except in some cases presenting serious symptoms such as atypical genital bleeding, abdominal pain and infertility. While hysterectomy is one of the radical treatment methods, patients who wish to preserve the uterus have other options recently. In addition to the conventional myomectomy, intervention radiological methods such as uterine artery embolization and high-intensity focused ultrasound are notable for less-invasive treatment [12, 13]. However, according to recent reports, some patients who underwent uterine artery embolization based on the diagnosis of leiomyoma were later diagnosed as leiomyosarcoma [14, 15]. There is a great benefit especially for the patients who wish to preserve their fertility if malignancy is correctly ruled out and unnecessary operation is avoided. Unfortunately 18F-FDG PET is not perfect because it sometimes presents a false positive scan. In the present study, 18F-FLT proved to be potentially useful for the differential diagnosis and it may contribute to select an optimal treatment method.
Incidence of positive 18F-FDG uptake in uterine leiomyoma has been reported as 10.4 % in premenopausal women, and 1.2 % in postmenopausal women [16]. However, most of the leiomyoma cases in the present study showed positive 18F-FDG uptake. One of the reasons for the differences may be explained by the differences in the subjects. All subjects in the present study were suspected leiomyosarcoma, while most of the subjects in the former study were asymptomatic women.
In some case of benign leiomyoma, 18F-FDG was positive and showed higher uptake than 18F-FLT. Therefore, 18F-FLT was superior to 18F-FDG in specificity and PPV. While 18F-FDG PET is widely used for various kinds of tumors, physicians are occasionally bothered by the unexpected 18F-FDG-avid finding in the uterus in managing the original tumor. Therefore, additional 18F-FLT PET may facilitate the process of differential diagnosis for the possible uterine tumor. Although AUC of 18F-FLT was larger than that of 18F-FDG in the ROC analysis in the present study, SUVmax of 18F-FLT were relatively lower than 18F-FDG, suggesting lower contrast. Furthermore as SUV should be measured properly, standard way of ROI definition and SUV measurement such as SUVpeak [17] may be considered for the sake of further multi-institutional studies.
In this study, NPVs for both tracers were 100 %. Therefore, negative result in 18F-FLT or 18F-FDG PET may rule out the possibility of malignancy. Previous papers on 18F-FDG PET of leiomyosarcoma also showed positive finding for primary lesion in most of the cases [18–20]. However, we need to pay attention to the existence of necrotic area in leiomyosarcoma. In a case of leiomyosarcoma shown in Fig. 5, majority of the tumor was occupied by central necrosis. Although the Ki-67 labeling index was not low, 18F-FLT and 18F-FDG uptake was not significantly increased due to partial volume effect. Therefore, leiomyosarcoma with huge necrosis have a possibility to be underestimated in interpretation of PET results.
18F-FLT uptake was better correlated with the expression of Ki-67 than 18F-FDG, which may be an evidence of the correlation between 18F-FLT uptake and malignancy in uterine corpus tumor. In addition, some recent studies indicate that the high Ki-67 labeling index is correlated with poor prognosis [21, 22]. Therefore, 18F-FLT may be a possible biomarker for predicting prognosis of uterine tumor.
Two cases of carcinoma in the present study showed higher uptake than leiomyoma in both tracers. High Ki-67 labeling index was confirmed in these cases. As there are few previous studies concerning 18F-FLT uptake or Ki-67 labeling index, carcinoma cases must be re-evaluated with a large number of patients.
The limitation of this study is the small number of patients, because there is wide variation and heterogeneity both in leiomyosarcoma and leiomyoma. Low-proliferating malignant tumors may not show up at 18F-FLT PET, leading to an increased false negative rate. Actually, uterine leiomyomas often shows various histopathological changes such as coagulation necrosis, hemorrhage, calcification, hyalinization, myxoid change and hydropic degeneration [23]. In addition, we could not evaluate the other types of intramuscular malignancies such as endometrial stromal sarcoma. With various types of tumors, further examination is required to evaluate the relationship between 18F-FLT uptake and malignancy of uterine corpus tumor.
Conclusion
Negative findings on additional 18F-FDG or 18F-FLT PET may rule out the possibility of malignancy for the patients with suspected leiomyosarcoma diagnosed by conventional methods. However, leiomyosarcoma with extensive necrosis has a possibility of false negative diagnosis. 18F-FLT PET is superior to 18F-FDG PET in efficiently selecting benign leiomyoma out of possible malignant tumors. Moreover, 18F-FLT uptake correlated well with the histopathological index of cell proliferation.
References
Sahdev A, Sohaib SA, Jacobs I, Shepherd JH, Oram DH, Reznek RH. MR imaging of uterine sarcomas. AJR Am J Roentgenol. 2001;177:1307–11.
Grgic A, Yuksel Y, Groschel A, Schafers HJ, Sybrecht GW, Kirsch CM, et al. Risk stratification of solitary pulmonary nodules by means of PET using 18F-fluorodeoxyglucose and SUV quantification. Eur J Nucl Med Mol Imaging. 2010;37:1087–94.
Boland GW, Dwamena BA, Jagtiani Sangwaiya M, Goehler AG, Blake MA, Hahn PF, et al. Characterization of adrenal masses by using FDG PET: a systematic review and meta-analysis of diagnostic test performance. Radiology 2011;259:117–126.
Ozaki Y, Oguchi K, Hamano H, Arakura N, Muraki T, Kiyosawa K, et al. Differentiation of autoimmune pancreatitis from suspected pancreatic cancer by fluorine-18 fluorodeoxyglucose positron emission tomography. J Gastroenterol. 2008;43:144–51.
Kitajima K, Murakami K, Yamasaki E, Kaji Y, Sugimura K. Standardized uptake values of uterine leiomyoma with 18F-FDG PET/CT: variation with age, size, degeneration, and contrast enhancement on MRI. Ann Nucl Med. 2008;22:505–12.
Tavassoli FA, Devilee P. Pathology and genetics of tumours of the breast and female genital organs (Who/IARC Classification of Tumours). Lyon: IARC Press; 2003.
Gokaslan H, Turkeri L, Kavak ZN, Eren F, Sismanoglu A, Ilvan S, et al. Differential diagnosis of smooth muscle tumors utilizing p53, pTEN and Ki-67 expression with estrogen and progesterone receptors. Gynecol Obstet Invest. 2005;59:36–40.
O’Neill CJ, McBride HA, Connolly LE, McCluggage WG. Uterine leiomyosarcomas are characterized by high p16, p53 and MIB1 expression in comparison with usual leiomyomas, leiomyoma variants and smooth muscle tumours of uncertain malignant potential. Histopathology. 2007;50:851–8.
Buck AK, Halter G, Schirrmeister H, Kotzerke J, Wurziger I, Glatting G, et al. Imaging proliferation in lung tumors with PET: 18F-FLT versus 18F-FDG. J Nucl Med. 2003;44:1426–31.
Buck AK, Bommer M, Stilgenbauer S, Juweid M, Glatting G, Schirrmeister H, et al. Molecular imaging of proliferation in malignant lymphoma. Cancer Res. 2006;66:11055–61.
Hatakeyama T, Kawai N, Nishiyama Y, Yamamoto Y, Sasakawa Y, Ichikawa T, et al. 11C-methionine (MET) and 18F-fluorothymidine (FLT) PET in patients with newly diagnosed glioma. Eur J Nucl Med Mol Imaging. 2008;35:2009–17.
Bradley LD. Uterine fibroid embolization: a viable alternative to hysterectomy. Am J Obstet Gynecol. 2009;201:127–35.
Stovall DW. Alternatives to hysterectomy: focus on global endometrial ablation, uterine fibroid embolization, and magnetic resonance-guided focused ultrasound. Menopause. 2011;18:437–44.
Goldberg J, Burd I, Price FV, Worthington-Kirsch R. Leiomyosarcoma in a premenopausal patient after uterine artery embolization. Am J Obstet Gynecol. 2004;191:1733–5.
Iihara K, Hirano K, Fujioka Y, Sakamoto A. Leiomyosarcoma with dedifferentiation in a premenopausal patient discovered after uterine artery embolization. Pathol Int. 2007;57:681–7.
Nishizawa S, Inubushi M, Kido A, Miyagawa M, Inoue T, Shinohara K, et al. Incidence and characteristics of uterine leiomyomas with FDG uptake. Ann Nucl Med. 2008;22:803–10.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–50S.
Ho KC, Lai CH, Wu TI, Ng KK, Yen TC, Lin G, et al. 18F-fluorodeoxyglucose positron emission tomography in uterine carcinosarcoma. Eur J Nucl Med Mol Imaging. 2008;35:484–92.
Tsujikawa T, Yoshida Y, Mori T, Kurokawa T, Fujibayashi Y, Kotsuji F, et al. Uterine tumors: pathophysiologic imaging with 16alpha-[18F]fluoro-17beta-estradiol and 18F fluorodeoxyglucose PET—initial experience. Radiology. 2008;248:599–605.
Yoshida Y, Kiyono Y, Tsujikawa T, Kurokawa T, Okazawa H, Kotsuji F. Additional value of 16alpha-[18F]fluoro-17beta-oestradiol PET for differential diagnosis between uterine sarcoma and leiomyoma in patients with positive or equivocal findings on [18F]fluorodeoxyglucose PET. Eur J Nucl Med Mol Imaging. 2011;38:1824–31.
Akhan SE, Yavuz E, Tecer A, Iyibozkurt CA, Topuz S, Tuzlali S, et al. The expression of Ki-67, p53, estrogen and progesterone receptors affecting survival in uterine leiomyosarcomas. A clinicopathologic study. Gynecol Oncol. 2005;99:36–42.
D’Angelo E, Espinosa I, Ali R, Gilks CB, Rijn M, Lee CH, et al. Uterine leiomyosarcomas: tumor size, mitotic index, and biomarkers Ki67, and Bcl-2 identify two groups with different prognosis. Gynecol Oncol. 2011;121:328–33.
Sreenan JJ, Prayson RA, Biscotti CV, Thornton MH, Easley KA, Hart WR. Histopathologic findings in 107 uterine leiomyomas treated with leuprolide acetate compared with 126 controls. Am J Surg Pathol. 1996;20:427–32.
Acknowledgments
This study was partly supported by Grant-in-Aid for Young Scientists (B), KAKENHI (22791241).
Author information
Authors and Affiliations
Corresponding author
Additional information
Registration identification number for the trial’s registry: UMIN-CTR: UMIN000003338 (www.umin.ac.jp/ctr/index/htm).
Rights and permissions
About this article
Cite this article
Yamane, T., Takaoka, A., Kita, M. et al. 18F-FLT PET performs better than 18F-FDG PET in differentiating malignant uterine corpus tumors from benign leiomyoma. Ann Nucl Med 26, 478–484 (2012). https://doi.org/10.1007/s12149-012-0597-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-012-0597-0