Abstract
The aims of this study were to assess the potential of fluorine-18 fluorodeoxyglucose positron emission tomography (FDG PET) for tumor grading in chondrosarcoma patients and to evaluate the role of standardized uptake value (SUV) as a parameter for prediction of patient outcome. FDG PET imaging was performed in 31 patients with chondrosarcoma prior to therapy. SUV was calculated for each tumor and correlated to tumor grade and size, and to patient outcome in terms of local relapse or metastatic disease with a mean follow-up period of 48 months. Chondrosarcomas were detectable in all patients. Tumor SUV was 3.38±1.61 for grade I (n=15), 5.44±3.06 for grade II (n=13), and 7.10±2.61 for grade III (n=3). Significant differences were found between patients with and without disease progression: SUV was 6.42±2.70 (n=10) in patients developing recurrent or metastatic disease compared with 3.74±2.22 in patients without relapse (P=0.015). Using a cut-off of 4 for SUV, sensitivity, specificity, and positive and negative predictive values for a relapse were 90%, 76%, 64%, and 94%, respectively. Combining tumor grade and SUV, these parameters improved to 90%, 95%, 90%, and 95%, respectively. Pretherapeutic tumor SUV obtained by FDG PET imaging was a useful parameter for tumor grading and prediction of outcome in chondrosarcoma patients. The combination of SUV and histopathologic tumor grade further improved prediction of outcome substantially, allowing identification of patients at high risk for local relapse or metastatic disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chondrosarcomas are chondrogenic tumors of the bone or soft tissue. Since many chondrosarcomas are, according to the commonly applied histologic grading criteria of Evans et al. [1], classified as low-grade tumors, Arsos and co-workers considered them “a difficult target for radionuclide imaging” [2]. Despite several previous publications on positron emission tomography (PET) imaging for preoperative planning, grading, and prediction of patient outcome in musculoskeletal tumors including chondrosarcoma [3, 4, 5, 6, 7, 8, 9], there is only one study that has focused on fluorine-18 fluorodeoxyglucose (FDG) PET imaging in chondrosarcoma. In this short report on six chondrosarcoma patients, Aoki et al. found a correlation of standardized uptake value (SUV) and tumor grade as well as a significant difference in SUV between benign cartilage tumors and chondrosarcomas [10]. In this group, four of the six patients suffered from grade II tumors and SUVaverage was low, ranging from 1.3 to 3.1. One patient with a grade III tumor had an SUV of 3.3. These low values likely reflect the low metabolic activity of cartilage tissue in general. Thus, even malignant dedifferentiation in cartilage tumors seems to result in low FDG metabolic rates compared with other musculoskeletal tumors.
Based on this preliminary report by Aoki and co-workers [10], the aims of our study were to retrospectively assess the potential of FDG PET imaging for tumor grading during the pretherapeutic clinical work-up in a larger chondrosarcoma patient population and to evaluate the role of SUV as a parameter for prediction of patient outcome.
Materials and methods
Patients
In this study, 31 patients with histologically proven chondrosarcomas who underwent PET imaging with FDG between May 1995 and May 2002 were retrospectively evaluated. Patients gave informed consent for the imaging study by signing Human Subjects and Radiation Safety Committee approved forms. They ranged in age from 23 to 85 years (median 48 years) and had a variety of chondrosarcoma subtypes covering all tumor grades. All patients underwent wide surgical excision of the tumor, and the tumor specimens were checked histologically for free surgical margins. No adjuvant or neoadjuvant chemotherapy was applied. A summary of the patient data is given in Table 1.
PET imaging
PET imaging was performed prior to therapy in all patients. Imaging studies were performed on an Advance Tomograph (General Electric Medical Systems, Waukesha, WI) operating in a two-dimensional high-sensitivity mode, with 35 imaging planes per axial field of view of 15 cm (plane thickness 4.25 mm) and an in-plane resolution of 4–5 mm [11, 12]. After the patients had been positioned in the tomograph, a 25-min attenuation scan over the tumor site was acquired. All patients had fasted for at least 12 h prior to intravenous injection of 370 MBq FDG. After a 45-min rest period, an emission scan of the tumor site and additional adjoining 15-cm fields of view of the greater tumor area were acquired. Images of the tumor site were corrected for attenuation, and were reconstructed into 128×128 matrices by filtered backprojection using a Hanning filter. The resulting images had a reconstructed resolution of 10 mm.
Data analysis
PET data were analysed by a region of interest (ROI) approach. Circular or elliptic ROIs were placed over the tumor site on transaxial images. Sagittal and coronal image reconstruction was performed to ensure correct ROI placement. The maximum tumor standardized uptake value (SUVmax) for each ROI was automatically calculated by the tomograph software according to the following expression:
where A is the maximum tissue activity within the ROI, ID is the injected dose, and m is the patient body weight.
SUVmax was correlated to tumor grade and size as established by a pathologic review of the final reports of surgical histopathology by an experienced bone pathologist. It was also correlated to patient outcome in terms of time to local relapse or metastasis with a mean follow-up period of 48 months since the first PET study (range 11–92 months); the follow-up included physical examination, X-ray, and computed tomography (CT) of the primary tumor site and the lungs in all patients at 3- to 6-month intervals.
Statistical analysis
SUV results are given as mean ±1 SD. Sensitivity, specificity, and positive and negative predictive values for subgroups based on histologic grading, tumor diameter, and tumor SUV were calculated. ANOVA analysis or Student’s t test for unpaired data was used to evaluate statistical differences between subgroups with P<0.05 considered to be statistically significant. Kaplan-Meier relapse-free survival curves were calculated for various grouping variables and the log-rank test was applied to evaluate statistical differences.
Results
Chondrosarcomas were clearly detectable by FDG PET imaging in all patients. SUVmax was 3.38±1.61 for tumor grade I (n=15), 5.44±3.06 for grade II (n=13), and 7.10±2.61 for grade III (n=3). ANOVA proved significant differences between the three subgroups (P=0.023). The differences between grades I and II were also statistically significant in Student’s t test (P=0.043).
In tumors of grade I, SUVmax ranged from 1.3 to 7.8. The three highest SUVmax were found for a multifocal extraskeletal myxoid tumor and for two extraskeletal myxoid chondrosarcomas while classic grade I central medullary chondrosarcomas had a maximum SUVmax of 4.1 with a mean value of 2.8. In patients with grade I tumors, recurrent disease was observed only in one subject with an initially large tumor of the rib (15×11×8 cm). No metastatic disease was observed in grade I patients.
SUVmax ranged from 3.1 to 12.2 in grade II tumors. Two of 13 patients presented with metastatic disease at the time of diagnosis, and four patients developed progression within 7–32 months. One of these patients died of this disease 77 months after diagnosis.
In grade III tumors, SUVmax were 4.6, 6.9, and 9.8. All patients developed recurrent and/or metastatic disease within 9 months after surgery, and two patients died of this disease within 2 years after initial diagnosis.
In patients suffering from local relapse or metastatic disease, we found a significant difference in SUVmax between patients with and patients without recurrences. In patients with any kind of relapse, SUVmax was 6.42±2.70 (n=10) compared with 3.74±2.22 in patients without relapse (P=0.015). Using SUVmax >4 to identify patients with a high risk for relapse, in only one patient with a large grade I tumor would the risk for relapse have been underestimated. In five patients a false high risk would have been predicted. Thus, an SUVmax ≤4 had a negative predictive value of 94% irrespective of the histopathologic tumor grade. The positive predictive value was 64%. An SUV of 4 also proved a discriminator in Kaplan-Meier analysis (Fig. 1), showing significant differences between the mean relapse-free survival time of 86 months for tumors with an SUVmax ≤4 and 61 months for tumors with an SUVmax >4 (P=0.026).
Prediction of relapse on the basis of histologic tumor grading, with grade I predictive for a low risk and grades II and III predictive for a high risk of tumor relapse, yielded results in a similar range as for SUVmax (Table 2), with a limited specificity and positive predictive value. Kaplan-Meier analysis (Fig. 1) showed significant differences for the mean relapse-free survival between patients with grade I tumors (86 months) and those with grade II or III tumors (59 months; P=0.004).
The combination of SUVmax and tumor histologic grade improved risk assessment, as shown in Table 2. According to these data, only patients with tumor grade II or III and an SUVmax higher than 4 are at a high risk for developing recurrent disease, while all the other patients are unlikely to suffer from a relapse. In this total of 31 patients, this risk stratification method resulted in only one false negative finding, in a patient with a large 15-cm grade I tumor, and one false positive finding, in a patient with a grade II chondrosarcoma. According to these results, Kaplan-Meier analysis (Fig. 1) had the highest power to prove differences for relapse-free survival between the two subgroups, with a P value of 0.0009. The mean relapse-free survival time was 86 months for low-risk tumors and 53 months for high-risk tumors.
Since tumor size is also known to be associated with poor outcome, we correlated the maximum tumor diameters with grade and SUVmax. No significant correlation of tumor size with grade or SUVmax was found, although larger diameters were predictive for tumor relapse. A maximum tumor diameter of ≥9 cm yielded a high sensitivity but low specificity and positive predictive value for outcome (Table 2). However, maximum tumor diameter was not a useful discriminator for Kaplan-Meier estimates (P=0.17). Combination of maximum tumor diameter with tumor grade or SUVmax or both parameters did not improve identification of patients at high risk for relapse.
Discussion
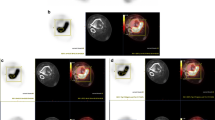
Although low-grade chondrosarcomas are sometimes difficult to detect by means of conventional scintigraphic imaging using thallium-201, technetium-99m sestamibi, or technetium-99m tetrofosmin, as reviewed by Arsos et al. in 2002 [2], chondrosarcomas could be detected by FDG PET in all our patients, including 15 grade I tumors. Figure 2 presents examples of a low-grade, low-uptake chondrosarcoma in the ribs as well as a highly FDG-avid grade II chondrosarcoma of the femur. Although our patient population covered a wide range of all tumor grades and subtypes of chondrosarcoma, SUVmax ranged only from 1.3 to 12.2, with a mean value of 4.6. Only 6 of 31 patients (19%) had an SUVmax higher than 6. These values are relatively low compared to the data provided for a large series of patients with various kinds of sarcoma [4]. In that study, Eary and co-workers [4] reported a mean SUVmax of 6 in 209 sarcoma patients, with a range from 1.4 to 60, and 51% of the patients showed values higher than 6. Chondrosarcomas in general are slow-growing matrix-producing tumors with a comparatively low mitotic activity and poor response to chemotherapy or radiation. They are usually low-aggressive tumors with a risk for metastases in 10% of grade II and 70% of grade III tumors within 5 years [1, 13]. They do, however, have high rates of local recurrence. The low FDG uptake in chondrosarcoma reflects the histologic features of this tumor entity, that is low cellularity and low mitotic rates combined with a large amount of inactive chondroid matrix tissue, and, thus, reflects tumor biology.
a Coronal FDG PET image of a grade I chondrosarcoma of the left anterior upper ribs (arrow) with an SUVmax of 1.5 in a 57-year-old female patient. This large tumor (9.5 cm diameter) showed an inhomogeneous, low FDG uptake with a maximum at the upper left tumor area and only faint uptake in the central, highly cartilaginous portions of the tumor. b Coronal FDG PET image of a grade II chondrosarcoma (6 cm diameter) of the right distal femur with a high SUVmax of 11.4 in a 45-year-old female patient. There is reduced uptake in the central parts of the tumor suggesting either necrosis or cartilage matrix with low cellularity
In a subset of three patients with low-grade extraskeletal myxoid chondrosarcoma, high SUVmax of 4.3, 4.9, and 7.8 were observed while no local relapse or metastatic disease occurred within a respective follow-up time of 19, 22, and 91 months. Whether the short follow-up period in two of these patients is the reason for finding no relapse despite the higher SUVmax, or whether extraskeletal myxoid chondrosarcomas in general have higher SUVmax values, cannot be decided on the basis of the data available so far. There is strong evidence, however, that extraskeletal myxoid chondrosarcoma is a unique tumor entity [14, 15]. These tumors typically posses a t(9;22) translocation which is not seen in conventional skeletal chondrosarcoma. They also have a unique clinical course with a high rate of local recurrence and eventually a high rate of late death due to tumor after 5 years of diagnosis. These features clearly distinguish low-grade extraskeletal myxoid chondrosarcomas from low-grade chondrosarcoma of the bone. High SUVmax values in extraskeletal myxoid chondrosarcoma therefore seem to reflect this more aggressive behavior in comparison to conventional grade I chondrosarcomas with low SUVmax and favorable outcome.
In our study, FDG uptake increased in parallel to tumor grade, yielding a mean SUVmax of 3.38, 5.44, and 7.10 in the case of grade I, II, and III tumors, respectively. These values are markedly higher than the results reported by Aoki and co-workers [10], who calculated average SUV values of the tumor while we report maximum SUV as a measure for tumor uptake. Average SUV is widely used as a semiquantitative measure for tumor metabolic activity. However, we believe that tumor metabolism in terms of a predictive oncologic parameter is better characterized by maximum SUV. Current thinking in tumor biology is that the highest measured SUV in a tumor reflects the most metabolically active area of the tumor. The most metabolically active areas are thought to reflect more aggressive tumor regions. The inherent assumption in this concept is that tumor grade and the overall behavior of the tumor are predicted by the activity of the most aggressive region. Therefore, SUVmax seems more appropriate for tumor characterization than SUVaverage. But there is one more practical point in favour of SUVmax: this measure, representing the pixel/voxel with the highest activity, is independent of the size and shape of the ROI for analysis as long as the whole tumor is covered by the ROI. Thus, it is independent of inherent errors of subjectively chosen tumor ROIs which significantly affect the average SUV. In calculating SUVaverage, the result will be higher the more closely the ROI is centered around the highest tumor uptake. The value will decrease with a larger ROI owing to partial volume effects when the ROI covers areas close to the tumor border, an ROI covering necrotic tumor tissue, or an ROI covering tumor-surrounding normal tissue. SUVmax values obtained by manually placed ROIs over a tumor site therefore should be more reproducible in general, especially in cases with different operators.
As already shown for musculoskeletal tumors [3, 5, 6, 8], there is also a good correlation of SUV and tumor grade in chondrosarcoma. SUVmax values were significantly lower in low-grade tumors than in tumors of grade II or III. This is clinically important since tumor grade and risk of local relapse or metastatic disease are closely linked. Grade I tumors are unlikely to develop metastases while tumors of grade II and grade III have a higher risk for metastases (10% and 70% within 5 years, respectively) [1, 13]. In our patient population, over a mean follow-up period of 48 months, only one out of 15 patients with a grade I tumor, but 3 out of 13 patients with a grade II tumor and all three patients with a grade III tumor developed metastases. The local recurrence rate was also higher in high-grade tumors: no patient with a grade I tumor but three patients with a grade II tumor suffered from local relapse.
Relapse is also known to occur more often in large tumors of more than 10 cm [16]. This was found to be true in our patients also. In nine of ten patients with recurrent disease, irrespective of tumor grade or SUVmax, maximum tumor diameters were larger than 9 cm. The only local relapse in the grade I tumor group occurred in a patient with a large tumor of the rib (15 cm, SUVmax 3.1, see patient 8, Table 1). However, a significant correlation of tumor size with grade or with SUVmax could not be found.
Despite a close correlation of SUVmax and tumor grade and despite statistically significant differences between SUVmax in grades I and II, an overlap in SUVmax between tumor grades was observed. SUVmax ranged from 1.3 to 7.8 in grade I, from 3.1 to 12.2 in grade II, and from 4.6 to 9.8 in grade III. Therefore, it was not possible to accurately grade chondrosarcomas using FDG PET-derived SUVmax as a substitute for histopathology grading assessment.
There are reports in the literature that initial, pretherapeutic SUV can be a reliable prognostic parameter for predicting patient outcome [17, 18, 19, 20]. Eary and co-workers [4] found that SUVmax, determined by FDG PET imaging in 209 patients with different types of sarcoma, was an independent and significant predictor of survival and disease progression. Interestingly, SUVmax had the same prognostic power as tumor grade with regard to prediction of overall survival, as shown by multivariate analysis. In the present study, assessment of differences in estimated Kaplan-Meier relapse-free survival curves based on tumor grading, size or SUVmax revealed significant differences for tumor grade and SUVmax as discriminators, but not for tumor diameter. According to these findings, we observed significant differences in SUVmax between patients with tumor progression and patients without any relapse, with mean SUVmax values of 6.42±2.70 and 3.74±2.22, respectively. Using a cut-off of 4 for SUVmax, the negative predictive value in patients below this cut-off to suffer from a relapse was 94%, independently of the histopathologic tumor grade. SUVmax values higher than 4 correctly predicted tumor relapse with a sensitivity of 90%. A false high risk would have been predicted in five patients, resulting in a limited specificity of 76%. Four of these five tumors were grade I chondrosarcomas, which are known to rarely relapse or metastasize. On the other hand, histopathologic tumor grading yielded almost the same results, with a negative predictive value of 93% and a sensitivity of 90%, while the specificity was only 67%. Interestingly, in six of seven false positive tumors by means of tumor grade, SUVmax was lower than 4. The problem of tumor grading in chondrosarcomas is that it is mainly based on assessment of cell atypia while the generally low mitotic activity and cellularity are not strong risk parameters. Thus, tumor histologic grading based on atypia of few cells within large and matrix-rich tumors seems to be less reliable than in other tumor types. Interestingly, tumor diameter as one of the most simple risk parameters provided similar results for prediction of relapse as obtained by tumor grading: diameters ≥9 cm had a sensitivity of 90% and a negative predictive value of 91% while specificity was 56%. However, tumor diameter was not a significant discriminator in Kaplan-Meier analysis.
In this study population, SUVmax was as useful a parameter to predict time to progression as tumor grade determined by histopathology, with both parameters resulting in a number of false positive findings (Table 2). The combination of tumor grade and SUVmax reduced the number of false positive results significantly while the combination of tumor diameter with grade or SUVmax did not improve the results. Nine out of ten patients with tumor grade II or III and an SUVmax higher than 4 developed recurrent disease while 20 out of 21 patients considered as at low risk (grade I irrespective of SUVmax, and grade II or III with an SUVmax ≤ 4) have shown no relapse so far. According to these data, only patients with tumor grade II or III and an SUVmax higher than 4 are at a high risk for developing progressive disease while all other patients are unlikely to suffer from a relapse. The mean relapse-free survival time according to this assessment scheme was 86 months in low-risk tumors and 53 months in high-risk tumors, revealing the highest power of all tested risk factors to discriminate between low- and high-risk tumors.
When the information on pretherapeutic SUVmax was added to tumor grade, prediction of patient outcome was significantly improved with regard to specificity and positive predictive value. This new risk stratification method might be clinically useful for the management of chondrosarcoma patients. In grade I and II tumors, oncologic follow-up investigations are usually performed at 6-month intervals and include CT of the lungs and of the original tumor site, while in high-grade tumors follow-up visits are scheduled every 3 months after surgery. However, not only grade III tumor patients but also high-risk patients as identified by combined histopathologic tumor grading and SUVmax may benefit from tightened follow-up regimens at 3-month intervals.
In summary, the results of this study show that in chondrosarcoma patients, pretherapeutic tumor SUVmax obtained by quantitative FDG PET imaging is a useful parameter for tumor grading and for predicting patient outcome in terms of local relapse or metastatic disease. The combination of histopathologic tumor grade and SUVmax improved prediction of outcome substantially as compared with each of these measures as a single parameter.
References
Evans HL, Ayala AG, Romsdahl MM. Prognostic factors in chondrosarcoma of bone: a clinicopathologic analysis with emphasis on histologic grading. Cancer 1977; 40:818–831.
Arsos G, Venizelos I, Karatzas N, Koukoulidis A, Karakatsanis C. Low-grade chondrosarcomas: a difficult target for radionuclide imaging. Case report and review of the literature. Eur J Radiol 2002; 43:66–72.
Aoki J, Watanabe H, Shinozaki T, et al. FDG PET of primary benign and malignant bone tumors: standardized uptake value in 52 lesions. Radiology 2001; 219:774–777.
Eary JF, O’Sullivan F, Powitan Y, et al. Sarcoma tumor FDG uptake measured by PET and patient outcome: a retrospective analysis. Eur J Nucl Med 2002; 29:1149–1154.
Eary JF, Conrad EU, Bruckner JD, et al. Quantitative [F-18]fluorodeoxyglucose positron emission tomography in pretreatment and grading of sarcoma. Clin Cancer Res 1998; 4:1215–1220.
Folpe AL, Lyles RH, Sprouse JT, Conrad EU, Eary JF. (F-18) fluorodeoxyglucose positron emission tomography as a predictor of pathologic grade and other prognostic variables in bone and soft tissue sarcoma. Clin Cancer Res 2000; 6:1279–1287.
Garcia R, Kim EE, Wong FC, et al. Comparison of fluorine-18-FDG PET and technetium-99m-MIBI SPECT in evaluation of musculoskeletal sarcomas. J Nucl Med 1996; 37:1476–1479.
Schulte M, Brecht-Krauss D, Heymer B, et al. Grading of tumors and tumorlike lesions of bone: evaluation by FDG PET. J Nucl Med 2000; 41:1695–1701.
Watanabe H, Shinozaki T, Yanagawa T, et al. Glucose metabolic analysis of musculoskeletal tumours using 18fluorine-FDG PET as an aid to preoperative planning. J Bone Joint Surg Br 2000; 82:760–767.
Aoki J, Watanabe H, Shinozaki T, et al. FDG-PET in differential diagnosis and grading of chondrosarcomas. J Comput Assist Tomogr 1999; 23:603–608.
DeGrado TR, Turkington TG, Williams JJ, et al. Performance characteristics of a whole-body PET scanner. J Nucl Med 1994; 35:1398–1406.
Lewellen TK, Kohlmeyer S, Miyaoka R, Schubert S, Stearns C. Investigation of the count rate performance of the General Electric Advance positron emission tomograph. IEEE Trans Nucl Sci 1995; 42:1051–1057.
Bovee JVMG, Hogendoorn PCW. Cartilage-forming tumours of bone and soft tissue and their differential diagnosis. Curr Diagn Pathol 2001; 7:223–234.
Meis-Kindblom JM, Bergh P, Gunterberg B, Kindblom LG. Extraskeletal myxoid chondrosarcoma: a reappraisal of its morphologic spectrum and prognostic factors based on 117 cases. Am J Surg Pathol 1999; 26:636–650.
Rubin BP, Fletcher JA. Skeletal and extraskeletal myxoid chondrosarcoma: related or distinct tumors? Adv Anat Pathol 1999; 6:204–212.
Fiorenza F, Abudu A, Grimer RJ, et al. Risk factors for survival and local control in chondrosarcoma of bone. J Bone Joint Surg Br 2002; 84:93–99.
Allal AS, Dulguerov P, Allaoua M, et al. Standardized uptake value of 2-[(18)F] fluoro-2-deoxy-d-glucose in predicting outcome in head and neck carcinomas treated by radiotherapy with or without chemotherapy. J Clin Oncol 2002; 20:1398–1404.
Graham MM, Peterson LM, Hayward RM. Comparison of simplified quantitative analyses of FDG uptake. Nucl Med Biol 2000; 27:647–655.
Higashi K, Ueda Y, Ayabe K, et al. FDG PET in the evaluation of the aggressiveness of pulmonary adenocarcinoma: correlation with histopathological features. Nucl Med Commun 2000; 21:707–714.
Dhital K, Saunders CA, Seed PT, O’Doherty MJ, Dussek J. [(18)F]Fluorodeoxyglucose positron emission tomography and its prognostic value in lung cancer. Eur J Cardiothorac Surg 2000; 18:425–428.
Acknowledgements
This work was supported by NIH grant R01-CA65537.
We would like to thank Cheryl Vernon for performing the blood sampling and plasma counting in all our patients and for preparing the patients and PET data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brenner, W., Conrad, E.U. & Eary, J.F. FDG PET imaging for grading and prediction of outcome in chondrosarcoma patients. Eur J Nucl Med Mol Imaging 31, 189–195 (2004). https://doi.org/10.1007/s00259-003-1353-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-003-1353-4