Abstract
Objective
The aim of this prospective study is to evaluate the combined use of fluorine-18 fluorodeoxyglucose (18 F-FDG) and fluorine-18 sodium fluoride (18 F-NaF) PET/CT in the skeletal assessment of patients with multiple myeloma (MM) and to compare the efficacy of these two PET tracers regarding detection of myeloma-indicative osseous lesions.
Patients and methods
The study includes 60 patients with multiple myeloma (MM) diagnosed according to standard criteria. All patients underwent dynamic (dPET/CT) scanning of the pelvis as well as whole body PET/CT studies with both tracers. The interval between the two exams was one day. Sites of focal increased 18 F-FDG uptake were considered as highly suspicious of myelomatous involvement. The lesions detected on the 18 F-NaF PET/CT scans were then correlated with those detected on 18 F-FDG PET/CT, which served as a reference. Moreover, the 18 F-FDG PET/CT results were also correlated with the low-dose CT findings. The evaluation of dPET/CT studies was based on qualitative evaluation, SUV calculation, and quantitative analysis based on a 2-tissue compartment model and a non-compartmental approach.
Results
Whole body 18 F-FDG PET/CT revealed approximately 343 focal lesions while 18 F-NaF PET/CT revealed 135 MM-indicative lesions (39 % correlation). CT demonstrated 150 lesions that correlated with those in 18 F-FDG PET/CT (44 % correlation). Six patients demonstrated a diffuse pattern of disease with 18 F-FDG, while 15 of them had a mixed (diffuse and focal) pattern of skeletal 18 F-FDG uptake. A high number of degenerative, traumatic and arthritic disease lesions were detected with 18 F-NaF PET/CT. In three patients with multiple focal 18 F-FDG-uptake, 18 F-NaF PET/CT failed to demonstrate any bone lesion. The dPET/CT scanning of the pelvic area with 18 F-FDG and 18 F-NaF revealed 77 and 24 MM-indicative lesions, respectively. Kinetic analysis of 18 F-FDG revealed the following mean values: SUVaver = 5.1, k1 = 0.37 (1/min), k3 = 0.10 (1/min), VB = 0.06, influx = 0.04 (1/min), FD = 1.28; the respective values for 18 F-NaF were SUVaverage = 10.7, k1 = 0.25 (1/min), k3 = 0.34 (1/min), VB = 0.02, influx = 0.10 (1/min), FD = 1.37. Apart from the correlation between VB of 18 F-FDG and k1 of 18 F-NaF (r = 0.54), no other significant correlation was observed between the two tracers’ kinetic parameters. We found a significant correlation between FD and SUVaverage (r = 0.93), FD and SUVmax (r = 0.80), FD and influx ( r = 0.85), as well as between influx and SUVaverage (r = 0.74) for 18 F-FDG. In 18 F-NaF we observed the most significant correlations between FD and SUVaverage (r = 0.97), FD and SUVmax (r = 0.87), and between influx and k1 (r = 0.72).
Conclusion
The combined use of 18 F-FDG PET/CT and 18 F-NaF PET/CT provides different molecular information regarding the biological processes that take place in a MM osseous lesion. 18 F-FDG PET/CT proved to be a more specific biomarker than 18 F-NaF PET/CT in multiple myeloma skeletal assessment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Multiple myeloma (MM) is a neoplastic plasma cell disorder, characterized by clonal proliferation of plasma cells in the bone marrow. The diagnosis of MM is based on certain criteria, which comprise the detection of M-protein in the serum and/or urine, bone marrow infiltration by plasma cells and myeloma-related organ or tissue impairment [1]. It is the major plasma cell neoplasm, accounting for approximately 1 % of neoplastic diseases and 13 % of all hematologic malignancies. The skeleton is invaded in almost 80 % of patients resulting in lytic bone lesions [2]. Therefore, imaging assessment of the skeleton is pivotal. The hallmark radiographic lesion of the disease is the focal osteolytic bone lesion [3]. However, an osteolytic lesion occurs after a 30 % involvement of the trabecular bone structure has taken place. Therefore, the application of other imaging modalities that render possible the early detection of tumor burden before irreversible osseous changes take place is needed.
PET and PET/CT technology is becoming more widely available and has nowadays a great impact on tumour diagnostics and management. Although its role is more clarified in solid tumours, PET is gradually becoming more widely acceptable in the evaluation of MM. Dynamic PET (dPET) and PET/CT (dPET/CT) are a modalities that, after application of compartment modelling, enables the acquisition of pharmacokinetic information; this leads to the extraction of values of tracer kinetic parameters, which depict specific molecular processes. Moreover, a non-compartmental model can be applied leading to the estimation of fractal dimension (FD) for the time-activity data, a parameter reflecting heterogeneity. This information is unattainable when the classical whole body PET and PET/CT protocols are performed.
18 F-FDG, the workhorse of PET imaging, is a biomarker of intracellular glucose metabolism. The basic concept in its application in MM is that malignant plasma cells, that infiltrate the bone marrow, consume much more glucose than normal cells. 18 F-FDG, as a glucose analogue, is taken up by the neoplastic cells, undergoes phosphorylation and then gets trapped intracellularly, since FDG is not a substrate for further metabolic processing by either phosphohexose isomerase or glucose-6-phosphate dehydrogenase [4]. A number of studies have highlighted the value of 18 F-FDG PET in the assessment of MM [5–8]. Moreover, the recently updated Durie/Salmon plus staging system integrates 18 F-FDG PET/CT in a MM diagnostic approach [9].
18 F-NaF is another PET tracer (positron emitter), early recognized as an excellent radiopharmaceutical for skeletal imaging [10, 11]. However, this agent was replaced during the three previous decades by 99mTc-MDP bone scintigraphy, performed with the conventional nuclear medicine gamma-cameras. Nevertheless, during recent years the role of 18 F-NaF has been revalidated as a highly sensitive and reliable biomarker of bone reconstruction, with potential indications in a wide range of bone diseases [12]. The reason for this re-emergence is the wide availability nowadays of PET and PET/CT scanners and the capability to quantify tracer kinetics. The mechanism of uptake of 18 F-NaF is totally different than that of 18 F-FDG. The uptake of the tracer in bone occurs by exchange of fluoride with hydroxyl groups in the hydroxyapatite resulting in formation of fluoroapatite [13]. The tracer accumulates in both osteoblastic and osteolytic lesions, reflecting regional blood flow and bone turnover [14]. Little is yet known about the efficiency of 18 F-NaF PET/CT in the evaluation of MM. Nishiyama et al., compared the sensitivity of 18 F-NaF PET/CT with that of 99mTc-MDP bone scintigraphy in detecting myeloma lesions in seven MM pts. Their results suggested that the detectability of MM lesions on 18 F-NaF PET/CT was promising, but nonspecific [15].
The molecular processes described before, which refer to these two different PET tracers, have been studied and compartmental models have been developed for description of their kinetics. In general, a two-tissue compartmental model can be used for the evaluation of both tracers [14, 16]. These models lead to the extraction of kinetic parameters that describe specific molecular processes, different for each tracer, which will be discussed below. Moreover, a non-compartmental model based on the fractal dimension can be applied for the evaluation of dPET or dPET/CT data, resulting in the extraction of a parameter of heterogeneity (fractal dimension, FD) [17–19].
In the present study, we performed 18 F-FDG PET/CT and 18 F-NaF PET/CT scanning in patients suffering from MM. The aim of our study was to evaluate the combined use of 18 F-FDG PET/CT and 18 F-NaF PET/CT in the skeletal assessment of patients with MM and to compare the efficacy of these tracers in detecting MM-indicative lesions. A comparison between the 18 F-FDG PET/CT and low-dose CT findings was also performed. Furthermore, we aimed to evaluate, by means of dynamic PET/CT, the pharmacokinetics of these two different PET tracers and detect potential statistically significant correlation between them as well as between parameters of each tracer.
Materials and methods
Patients
The evaluation included 60 patients (37 male, 23 female) confirmed to suffer from MM based on the criteria established by the International Myeloma Working Group [1]. According to the Durie/Salmon staging system, 14 patients were characterized as stage I, five of them as stage II and 41 as stage III of the disease. Their mean age was 59.9 years. The included patients were not diabetic. Forty-six of the patients had primary disease and had never received chemotherapy. Fourteen patients (three in stage II, 11 in stage III) demonstrated recurrent disease; however, none of them had undergone chemotherapy the past six months prior to the dPET/CT studies. Patients gave written informed consent to participate in the study and to have their medical records released. The study was approved by the Ethical Committee of the University of Heidelberg and the Bundesamt für Strahlenschutz.
Data acquisition
The double-tracer study in each patient was completed in two consecutive days. The patients were intravenously administered with a maximum of 250 MBq 18 F-FDG on the first day and, respectively, a maximum of 250 MBq 18 F-NaF on the second day. Data acquisition consisted of two parts for each tracer: the dynamic part (dPET/CT studies) and the static part (whole body PET/CT). The dPET/CT studies were performed over the pelvic area for 60 minutes using a 28-frame protocol (ten frames of 30 seconds, five frames of 60 seconds, five frames of 120 seconds and eight frames of 300 seconds). The use of lower lumbar spine and pelvic entry for the dynamic series is justified by the fact that this anatomic area is regularly used for diagnostic bone marrow biopsies. Additional whole body static images were acquired in all patients with an image duration of 2 min per bed position for the emission scans. A dedicated PET/CT system (Biograph mCT, 128 S, Siemens Co., Erlangen, Germany) with an axial field of view of 21.6 cm with TruePoint and TrueV, operated in a three-dimensional mode, was used for patient studies. A low-dose attenuation CT (80 kV, 30 mA) was used for the attenuation correction of the dynamic emission PET data and for image fusion. A second low-dose CT (80 kV, 30 mA) was performed after the end of the dynamic series covering the area from the skull to the knees in order to avoid patient movement after the dynamic series. The last images (55–60 minutes post-injection) were used for semi-quantitative analysis. All PET images were attenuation-corrected and an image matrix of 400 x 400 pixels was used for iterative image reconstruction. Iterative images reconstruction was based on the ordered subset expectation maximization algorithm (OSEM) with six iterations and 12 subsets. The reconstructed images were converted to SUV images based on the formula [20]: SUV = tissue concentration (Bq/g)/(injected dose (Bq)/body weight (g)). The SUVs 55 to 60 minutes postinjection served for the quantification of tracer data.
Data analysis
Data analysis and evaluation was based on: visual analysis of the PET/CT scans, semi-quantitative evaluation based on SUV calculations, and quantitative analysis. Qualitative analysis was based on visual assessment of the PET/CT scans. Two nuclear medicine physicians (CS, ADS) evaluated the hypermetabolic areas on transaxial, coronal, and sagittal images. In particular, skeletal foci presenting with significantly enhanced 18 F-FDG uptake, for which another benign aetiology (trauma, inflammation, degenerative changes, arthritic disease, etc.) was excluded, were considered indicative for myeloma. For the minimization of possible false-positive results, the patients’ history was studied thoroughly; moreover, the focal 18 F-FDG avid lesions were correlated to the fused low-dose CT findings and a comparison between the findings of the two modalities was performed. In particular, we focussed on the evaluation of clearly delineated osteolytic lesions in CT (>8 mm) and compared them with both tracers. Furthermore, patients who demonstrated on maximum intensity projection (MIP) images an intense, homogenous, diffuse increase of bone marrow 18 F-FDG uptake were considered as positive for MM involvement (diffuse pattern); the same applied to those patients with a mixed (focal/diffuse) pattern of 18 F-FDG uptake. Afterwards, the results of 18 F-NaF PET/CT (performed one day later) were correlated to those of 18 F-FDG PET/CT, which served as a reference. The basic concept regarding 18 F-NaF PET/CT evaluation was that only lesions that correlated with respective lesions on 18 F-FDG PET/CT were considered as MM-indicative.
Semi-quantitative evaluation was based on volumes of interest (VOIs), drawn with a 50 % isocontour, placed over foci of increased tracer uptake in the selected area and on subsequent calculation of SUV.
Quantitative evaluation of the dynamic PET/CT data was performed using a dedicated software [21, 22]. Like for SUV calculations, quantitative evaluation was based on irregular VOIs, drawn with a 50 % isocontour, placed over foci of increased radiotracer uptake in the selected osseous area. Time activity curves (TACs) were created using VOIs. It has to be remembered that only lesions located in the anatomic area where dPET/CT was performed (lower lumbar spine and pelvis) were evaluated quantitatively. A detailed quantitative evaluation of tracer kinetics was performed based on a two-tissue compartment model [16, 23, 24]. The input function was retrieved from the image data according to methods already reported in the literature and performed previously by our group [25, 26]. One problem in patients is the accurate measurement of input function. This theoretically requires arterial blood sampling. It has been shown, however, that input function can be accurately enough retrieved from image data [25]. For the input function, the mean value of the VOI data from a large arterial vessel of the pelvic area (iliac artery) was used. A vessel VOI consisted of at least seven ROIs in sequential PET/CT images. The recovery coefficient was 0.85 for a diameter of 8 mm. Partial volume correction was performed for small vessels (diameter less than 8 mm) based on phantom measurements of the recovery function using dedicated software [21]. The two-tissue compartment model we applied is a modification of the one proposed by Sokoloff et al., which did not take into account the parameters k4 and VB [16]. This lack of k4 and VB however leads to different values of the parameters k1 and k3, since k1 is dependent on VB and k3 on k4. In this study, the constants k1 to k4 were calculated taking into account the vascular fraction (VB) in a VOI as an additional variable. The model parameters were accepted when k1-k4 were less than 1 and VB exceeded zero. The unit for the rate constants k1 to k4 is 1/min, while VB reflects the fraction of blood within the VOI. Following compartment analysis, we calculated the global influx from the compartment data using the formula: influx = (k1 x k3)/(k2 + k3). In addition to creating VOIs over MM lesions, we also created reference VOIs at the osseous structures of this area (5th lumber vertebra, os ilium) that didn’t demonstrate any focal or intense, diffuse radiotracer accumulation. These areas were considered as negative for myelomatous involvement and served as a reference. The values extracted from these reference areas were then compared to the respective values of MM lesions for potential statistically significant differences.
Besides the compartment analysis, a non-compartment model was used in order to calculate the fractal dimension (FD) for the time-activity data. FD is a parameter of heterogeneity based on the box counting procedure of chaos theory and was calculated for the time activity data in each individual voxel of a VOI. The values of the FD vary from 0 to 2 showing the deterministic or chaotic distribution of the tracer activity. A subdivision of 7 x 7 and a maximal SUV of 20 were applied for the calculation of FD [17].
Data were statistically evaluated using the STATA/SE 12.1 (StataCorp) software on a Intel Core (2 · 3.06 GHz, 4 GB RAM) running with Mac OS X 10.8.4 (Apple Inc., Cupertino, CA, USA). The statistical evaluation was performed using the descriptive statistics, box plots, Wilcoxon rank-sum test and Spearman´s rank correlation analysis. The results were considered significant for p less than 0.05 (p < 0.05).
Results
Whole body PET/CT studies
Areas of focal enhanced 18 F-FDG uptake were considered to be indicative of myelomatous involvement (MM lesions). As expected, the number of lesions increased with disease stage. In total, approximately 343 focal 18 F-FDG avid skeletal lesions were detected; the 14 stage I pts had six lesions, the five stage II pts had 47 lesions, and the 41 stage III pts demonstrated 290 focal lesions. A correlation between the 18 F-FDG and the low-dose CT findings was performed. The comparison revealed 150 circumscribed osteolytic lesions in CT that correlated with the 18 F-FDG PET lesions (44 % correlation). In three patients the extent of skeletal disease was very large, rendering the exact calculation of MM lesions practically impossible (innumerable focal lesions). In six patients (n = 4 stage I, n = 2 stage III) we observed an intense, diffuse, homogenous increase in 18 F-FDG uptake in the bone marrow of the spine, in the os femur, and/or the pelvic bones; 15 patients (n = 1 stage I, n = 2 stage II, n = 12 stage III) demonstrated a mixed (focal/diffuse) pattern of 18 F-FDG skeletal uptake. In order to interpret these (diffuse and mixed) patterns of 18 F-FDG uptake, we searched the patients’ history in order to exclude a reactive phenomenon (e.g., post-chemotherapy reaction); only one patient with diffuse uptake and two with a mixed pattern of 18 F-FDG uptake had received chemotherapy in the past. However, none of them had undergone treatment at least six months prior to scanning. Moreover, bone marrow biopsy in patients with diffuse and mixed pattern of 18 F-FDG uptake revealed a mean value of 37.8 % and a median value of 43 % regarding plasma cell infiltration rate.
18 F-NaF PET/CT demonstrated a generally increased bone uptake. Due to the high sensitivity of the tracer in detecting bone reconstruction, it is difficult to differentiate malignant from benign bone lesions like degenerative, arthritic, traumatic bone disease, etc. Therefore, and as already mentioned, the focal lesions detected on 18 F-FDG PET/CT scans were used as reference. Foci of enhanced 18 F-NaF uptake were correlated to low-dose CT findings in order to enhance the diagnostic certainty regarding their benign or malignant origin. In general, 18 F-NaF PET/CTs demonstrated fewer myeloma-indicative lesions than 18 F-FDG PET/CTs. Totally, 135 focal MM lesions were detected with whole body 18 F-NaF PET/CT (39 % correlation with 18 F-FDG PET/CT). In those MM patients with multiple focal bone lesions in 18 F-FDG PET/CT, 18 F-NaF PET/CT demonstrated multiple focal lesions in different anatomic areas, not all of which necessarily correlated to 18 F-FDG. In three patients with myelomatous skeletal involvement, 18 F-NaF PET/CT failed to demonstrate any bone lesion; in 11 of them the number of lesions detected with 18 F-NaF was significantly lower than that detected with 18 F-FDG.
Interestingly, seven patients with stage III disease didn’t demonstrate any focal, diffuse or mixed pattern of increased18F-FDG uptake. However, CT was also negative for myelomatous lesions in this subgroup. On the other hand, in 15 patients low-dose CT revealed multiple small osteolytic lesions (diffuse involvement) in the axial skeleton mainly, while PET studies with both tracers were negative; in particular, three patients had multiple small cranial osteolytic lesions, six patients had lesions along the spinal column, four of them in both the skull and the spinal column, one in the humerus, while one in the spine, sternum and pelvis. The results referring to whole body evaluation with both tracers and according to disease stage are presented in Table 1.
Figure 1 demonstrates a characteristic example of a stage III MM patient, who had more skeletal lesions on 18 F-FDG than on 18 F-NaF PET/CT. 18 F-NaF PET/CT detected only three of the multiple osseous MM-indicative lesions that were demonstrated in 18 F-FDG PET/CT.
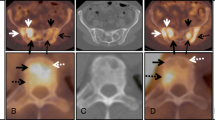
a: A 69-year-old female stage III MM patient referred to our department for evaluation of extent of skeletal disease with 18 F-FDG and 18 F-NaF PET/CT. Maximum intensity projection (MIP) revealed multiple hypermetabolic 18 F-FDG foci indicative of myelomatous involvement. The respective MIP of 18 F-NaF demonstrated much fewer myelomatous indicative lesions. Notice the high number of degenerative lesions depicted on 18 F-NaF PET/CT. b: CT image of the same patient at the level of the seventh thoracic vertebra, where no osteolysis is depicted (upper row). Corresponding 18 F-FDG PET at the same level, where a site of focal increase tracer uptake is depicted in the seventh thoracic vertebral body (middle row). 18 F-NaF PET performed 24 hours later demonstrates no pathological radiotracer accumulation (lower row). Example of 18 F-FDG and 18 F-NaF “mismatch”. c: A sternal lesion of the same patient showing soft tissue infiltration, clearly depicted on CT and 18 F-FDG PET, but not demonstrated with 18 F-NaF PET (18 F-FDG and 18 F-NaF “mismatch”)
Figure 2 shows a stage III MM patient, first diagnosed five years ago, who had undergone in the past chemotherapy treatment. The patient had no symptoms at the time of the study. No signs of active myelomatous skeletal involvement are depicted on 18 F-FDG PET/CT. 18 F-NaF PET/CT reveals several positive findings. These represent old fractures, which demonstrate some osteoblastic activity, as well as findings attributed to degenerative and traumatic aetiology. The lesion in the left humerus as well as the lesions in the basis of the skull did not demonstrate any respective correlative findings in the low dose CT.
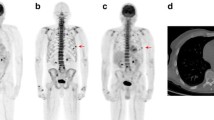
a: A 48-year-old male stage III MM patient referred to our department for imaging evaluation of the skeleton with 18 F-FDG and 18 F-NaF PET/CT. The patient was first diagnosed with MM six years ago and had already undergone multiple chemotherapies in the past. At the time of the study he had no signs of active disease, which was consistent with his clinical status. 18 F-FDG PET/CT MIP shows no lesions indicative of active myelomatous disease. 18 F-NaF PET/CT MIP demonstrates multiple old rib fractures, and other findings due to degenerative changes. b: Lesion in the 10th rib due to an old fracture (patient of Fig. 2a). CT shows an osteoblastic lesion due to bone remodelling (upper row). No corresponding radiotracer accumulation depicted on 18 F-FDG PET (middle row). 18 F-NaF PET demonstrates tracer accumulation indicating the osteoblastic activity (lower row)
Figure 3 depicts a stage I MM patient with a diffuse pattern of disease on 18 F-FDG PET/CT. The patient demonstrates a homogenous,diffuse, intense tracer uptake along the spinal column and both femurs.
Dynamic PET/CT studies of the pelvis
In total, 77 MM pelvic lesions were detected with 18 F-FDG dPET/CT and 24 with 18 F-NaF dPET/CT. Table 2 demonstrates the values of the SUVs and kinetic parameters’ acquired from the MM lesions with both tracers (mean ± SD values). All quantitative data were obtained from VOIs over the focal lesions. The mean values of the MM-lesions’ kinetic parameters derived from kinetic analysis, based on 18 F-FDG dPET/CT acquisition, were the following: SUVaver = 5.1, SUVmax = 7.9, k1 = 0.37 (1/min), k3 = 0.10 (1/min), VB = 0.06, influx = 0.04 (1/min), FD = 1.28 In comparison, the respective mean values of MM lesions as detected with 18 F-NaF dPET/CT were: SUVaverage = 10.7, SUVmax = 21.7, k1 = 0.25 (1/min), k3 = 0.34 (1/min), VB = 0.02, influx = 0.10 (1/min), FD = 1.37. According to these results, SUVs for 18 F-FDG were significantly lower than for 18 F-NaF. Two-tissue compartment analysis revealed that the parameters k1 and VB were significantly lower for 18 F-NaF than for 18 F-FDG; on the in contrary, k3 and influx were significantly higher for 18 F-NaF. The results concerning the between tracers’ differences were considered significant for p less than 0.05 (p < 0.05). Box plots of k1, k3, VB and influx for both tracers in myelomatous lesions are depicted in Fig. 4. It has to be noted that small MM lesions (8 < mm) were not taken into consideration for the dPET/CT evaluations.
Fractal dimension (FD) was applied for further characterization of 18 F-NaF and 18 F-FDG kinetics in MM-indicative foci. The mean FD value was higher for 18 F-NaF, implying a higher degree of heterogeneity of tracer uptake.
We performed a Spearman´s rank correlation analysis between 18 F-FDG and 18 F-NaF kinetic parameters: apart from the correlation between VB of 18 F-FDG and k1 of 18 F-NaF (r = 0.54), no other significant correlation was observed between the two tracers’ kinetic parameters. Correlation analysis was also performed between SUVs and kinetic parameters for each tracer separately. In the case of 18 F-FDG, the most significant correlations (p < 0.05) were found between FD and SUVaverage (r = 0.93), FD and SUVmax (r = 0.80), FD and influx (r = 0.85), as well as between influx and SUVaverage (r = 0.74) (Table 3). In 18 F-NaF we observed the most significant correlations between FD and SUVaverage (r = 0.97), FD and SUVmax (r = 0.87), and between influx and k1 (r = 0.72) (Table 4).
We also assessed both tracers’ SUVs and kinetic parameters in MM-indicative lesions, in comparison with the respective values of the osseous structures of the pelvis that served as reference. (os ilium, 5th lumbar vertebrae). The comparison was performed after application of the Wilcoxon rank-sum test. This analysis revealed that in the case of 18 F-FDG, all important semi-quantitative and quantitative parameters were significantly higher in MM-lesions than in the reference skeleton (Table 2). On the other hand, in 18 F-NaF, only SUVaverage and SUVmax were significantly higher in myelomatous lesions than in the reference skeleton. All other 18 F-NaF parameters (k1, k3, VB, influx and FD) demonstrated higher mean values in MM lesions than reference areas, but their differences were not statistically significant (p < 0.05) (Table 2).
Discussion
Although the routine use of 18 F-FDG PET imaging in MM is still under discussion, it is an undisputable fact that the modality is becoming more widespread and acceptable in diagnostic evaluation and work-up of this malignancy [27]. Moreover, it has been demonstrated that the sensitivity of the modality in detecting bone lesions is higher than the standard whole body radiologic skeletal survey [28]. On the other hand, little is known about the contribution of 18 F-NaF PET/CT in the diagnostic approach of the disease. In our study, we tried to evaluate the complementary role of 18 F-NaF PET/CT in diagnostic imaging of MM patients. The study was comprised of two parts: the whole body PET/CT evaluation with the use of the two different PET tracers 18 F-FDG and 18 F-NaF, and the dPET/CT part in which we compared the kinetics of 18 F-FDG with the kinetics of 18 F-NaF. As already mentioned, the MM-indicative lesions detected on 18 F-FDG PET/CT were the reference on which the lesions detected with 18 F-NaF were correlated and compared. However, all lesions detected on 18 F-NaF PET/CT were studied thoroughly. A comparison between the 18 F-FDG PET and low-dose CT findings was also performed.
Whole body PET/CT imaging with these two PET tracers leads us to the conclusion that in general terms 18 F-FDG is a more reliable biomarker than 18 F-NaF in the evaluation of MM: The number of MM lesions detected with 18 F-FDG was significantly higher than that detected with 18 F-NaF. In addition, although 18 F-FDG is a non-specific tracer, its number of false-positive findings (based on low dose CT and patients’ history) was much smaller than the false-positive findings detected with 18 F-NaF. The reason for this, is the accumulation of 18 F-NaF in every site of newly mineralizing bone, irrelevant of its aetiology; this means that any cause of bone reconstruction (e.g., traumatic, degenerative bone lesions) will lead to 18 F-NaF accumulation. It is actually impossible to differentiate a malignant from a benign bone lesion with 18 F-NaF PET alone. The correlation with other imaging modalities like CT or MRI, as well as a detailed patient’s history are required, as realized in our study. In particular, most positive rib findings in 18 F-NaF, which did not have any 18 F-FDG correlation were attributed – after correlation with CT - to traumatic aetiology (old fractures) or may represent old myelomatous lesions that still accumulated 18 F-NaF due to persistent increased osteoblastic activity; furthermore, degenerative changes like osteophytes in the spinal column or arthritis (primarily in the joints of the knees and hips) could be easily interpreted as “non-myelomatous lesions”. Our results demonstrated that 18 F-NaF PET/CT didn’t actually aid in MM diagnostic evaluation. Its main contribution was that it gave a general view of the bone remodelling of the whole skeleton. As already mentioned, 18 F-FDG PET/CT did not demonstrate any enhanced accumulation (focal, diffuse or mixed) in seven patients suffering from stage III MM. However, this shouldn’t be considered as a limitation of the modality in detecting MM skeletal lesions, since other myeloma-related organ or tissue impairment (such as renal insufficiency, anaemia, hypercalcemia, etc.) could lead to patient classification in a high disease stage. This is also supported by the fact that these patients also didn’t demonstrate any osteolytic lesions in the low-dose CT.
The second part of our study investigated the kinetics of 18 F-FDG in MM osseous lesions and compared them with the kinetics of 18 F-NaF in respective lesions, as these were derived from dPET/CT studies of the pelvic skeleton.
A two-tissue compartment model was used for the evaluation of dPET/CT studies with both tracers. The use of the two-tissue compartment model is a generally accepted approach for the assessment of 18 F-FDG kinetics; the application of this model leads to the extraction of the kinetic parameters k1 – k4, influx (Ki) and fractional blood volume (VB), also called vessel density, which correlates to blood volume in the VOI. Briefly, in the case of 18 F-FDG k1 reflects the influx while k2 reflects the efflux of the radiopharmaceutical, and k3 represents the phosphorylation rate while k4 the dephosphorylation rate of the glucose analogue. Influx (Ki) is derived from the equation = (k1 x k3)/(k 2 + k3). The application of the two-tissue compartment model in 18 F-NaF leads also to the extraction of kinetic parameters [14]. However, these parameters reflect different molecular mechanisms than 18 F-FDG. In the case of 18 F-NaF, rate constants k1 and k2 describe the fluoride ions exchange with hydroxyl groups of hydroxyapatite crystal of the bone and the reverse, while k3 and k4 represent the formation of fluoroapatite and the opposite [24]. Influx Ki is related to Ca2+ influx and bone apposition rate and, presumably, represents bone remodelling rate [29]. We compared the kinetic parameters (k1–k4, VB, influx) and SUVs for 18 F-FDG and 18 F-NaF using multivariate analysis; with the exception of the correlation between VB of 18 F-FDG and k1 of 18 F-NaF (r = 0.54), no other significant correlation was found for k1–k4 and SUVs between the two tracers. The data indicate that the rate constants k1–k4 are tracer specific and provide different molecular information. Correlation analysis performed for each tracer separately demonstrated the very high correlation between FD and SUVaverage and SUVmax in both tracers. Furthermore, we found a highly significant correlation (r = 0.74) between the influx and SUVaver in 18 F-FDG. This finding is in accordance with respective results derived from kinetic 18 F-FDG PET studies in other tumors. Strauss et al., reported a correlation of 0.76 (p < 0.01) between SUVaver and the influx in primary colorectal cancers [26]. We also noted a siginificant but lower correlation (r = 0.65) between SUVaverage and the nflux in 18 F-NaF.
Our group has previously shown that the application of dynamic quantitative 18 F-FDG PET has a high accuracy in differentiation of malignant and benign bone lesions. In particular, that study showed that all kinetic parameters (with the exception of k2 and k4) and SUVs were higher in malignant bone tumors than in benign ones. Moreover the t test revealed significantly higher mean values of SUV, VB, influx and FD in malignant than benign tumors (p < 0.05) [30]. In the present study, we compared the SUVs and kinetic parameters of both 18 F-FDG and 18 F-NaF in multiple myeloma lesions with the respective parameters of reference osseous tissue. The results of our study showed that in the case of 18 F-FDG, the MM-indicative bone lesions demonstrated SUVs and kinetic parameters’ values that ranged in significantly higher levels than the reference skeleton. In contradiction to the results of 18 F-FDG kinetic analysis, in the case of 18 F-NaF only SUVaverage and SUVmax were statistically significantly higher in MM-indicative lesions than the reference skeleton; all other parameters (k1, k3, VB, influx and FD) didn’t reveal significant differences between lesions and reference tissue. These data support our basic conclusion that 18 F-NaF is not a specific tracer for malignancy detection in MM patients. Due to its very high sensitivity for bone reconstruction detection, it is difficult to differentiate between malignant and benign lesions only based on the 18 F-NaF PET results, even after the application of quantitative analysis. Moreover, it is possible for an initially positive lesion in both tracers to become 18 F-FDG negative following chemotherapy, but to demonstrate a prolonged 18 F-NaF uptake, due to persistent surrounding osteoblastic activity.
In addition to applying a two-tissue compartment model for evaluation of tracers’ kinetics, a non-compartmental approach was also performed leading to the extraction of another index that is representative of tissue heterogeneity, FD. This parameter is based on the box counting procedure of the chaos theory for the analysis of dPET data [17]. The basic concept is that an increased FD is indicative of a more chaotic tracer distribution, correlating to a higher tissue heterogeneity. In the present study, the FD values in MM-lesions were comparable with a median of 1.28 for 18 F-FDG and 1.37 for 18 F-NaF. Furthermore, as already mentioned, FD demonstrated a highly significant correlation with SUVaverage both in 18 F-FDG (r = 0.93) as well as in 18 F-NaF studies (r = 0.97). This is in accordance with previous studies showing that both FD for the time-activity data and SUV´s are enhanced in malignancies [17]. It is our belief that the application of this non-compartment derived parameter can be useful in evaluation of complicated oncological differential diagnosis issues.
A limitation of this study is the lack of histological confirmation for myelomatous osseous lesions. In clinical practice, however, it is not possible to confirm by histology every MM-indicative lesion. In fact, only the correlation to other imaging modalities, like CT and MRI, and the patient’s clinical outcome serve as a reference. Another limitation is the use of low dose CT, which was performed for the anatomical assignment of the lesions and for attenuation correction.
Conclusion
In recent years, the role of PET/CT with 18 F-FDG in diagnostic evaluation of MM has been upgraded. 18 F-NaF is another PET tracer, which serves as a highly sensitive and reliable biomarker of bone reconstruction and with potential indications in a wide range of bone diseases. We aimed to study the combined use of these two PET tracers in the diagnostic work-up of patients with MM. The results of our study imply that the additional PET/CT scanning with 18 F-NaF doesn’t add significant information to the diagnostic approach of MM patients, who have already gone through 18 F-FDG PET/CT. However, 18 F-NaF provides general information regarding bone remodelling and the patient´s skeletal history.
References
International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121:749–57.
Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–60.
Walker RC, Brown TL, Jones-Jackson LB, De Blanche L, Bartel T. Imaging of multiple myeloma and related plasma cell dyscrasias. J Nucl Med. 2012;53:1091–101.
Mason NS, Lin EC. Basics of fluorodeoxyglucose radiochemistry and biology. In: Lin EC, Alavi A (eds). PET and PET/CT: a clinical guide. Thieme medical publisher, Inc 2005; p.15-20.
Durie BG, Waxman AD, D’Agnolo A, Williams CM. Whole-body (18)F-FDG PET identifies high-risk myeloma. J Nucl Med. 2002;43:1457–63.
Schirrmeister H, Bommer M, Buck AK, Müller S, Messer P, Bunjes D, et al. Initial results in the assessment of multiple myeloma using 18 F-FDG PET. Eur J Nucl Med Mol Imaging. 2002;29:361–6.
Bredella MA, Steinbach L, Caputo G, Segall G, Hawkins R. Value of FDG PET in the assessment of patients with multiple myeloma. AJR Am J Roentgenol. 2005;184:1199–204.
Fonti R, Larobina M, Del Vecchio S, De Luca S, Fabbricini R, Catalano L, et al. Metabolic tumor volume assessed by 18 F-FDG PET/CT for the prediction of outcome in patients with multiple myeloma. J Nucl Med. 2012;53:1829–35.
Durie BG. The role of anatomic and functional staging in myeloma: description of Durie/Salmon plus staging system. Eur J Cancer. 2006;42:1539–43.
Blau M, Ganatra R, Bender MA. 18 F-Fluoride for bone imaging. Semin Nucl Med. 1972;2:31–7.
Grant FD, Fahey FH, Packard AB, Davis RT, Alavi A, Treves ST. Skeletal PET with 18 F-fluoride: applying new technology to an old tracer. J Nucl Med. 2008;49:68–78.
Segall G, Delbeke D, Stabin MG, Even-Sapir E, Fair J, Sajdak R, et al. SNM. SNM practice guideline for sodium 18 F-fluoride PET/CT bone scans 1.0. J Nucl Med. 2010;51:1813–20.
Schiepers C, Nuyts J, Bormans G, Dequeker J, Bouillon R, Mortelmans L, et al. Fluoride kinetics of the axial skeleton measured in vivo with fluorine-18-fluoride PET. J Nucl Med. 1997;38:1970–6.
Hawkins RA, Choi Y, Huang SC, Hoh CK, Dahlbom M, Schiepers C, et al. Evaluation of the skeletal kinetics of fluorine-18-fluoride ion with PET. J Nucl Med. 1992;33:633–42.
Nishiyama Y, Tateishi U, Shizukuishi K, Shishikura A, Yamazaki E, Shibata H, et al. Role of 18 F-fluoride PET/CT in the assessment of multiple myeloma: initial experience. Ann Nucl Med. 2013;27:78–83.
Sokoloff L, Smith CB. Basic principles underlying radioisotopic methods for assay of biochemical processes in vivo. In: Greitz T, Ingvar DH, Widén L, editors. The metabolism of the human brain studied with positron emission tomography. New York: Raven Press; 1983. p. 123–48.
Dimitrakopoulou-Strauss A, Strauss LG, Burger C, Mikolajczyk K, Lehnert T, Bernd L, et al. On the fractal nature of positron emission tomography (PET) studies. World J Nucl Med. 2003;4:306–13.
Dimitrakopoulou-Strauss A, Pan L, Strauss LG. Quantitative approaches of dynamic FDG-PET and PET/CT studies (dPET/CT) for the evaluation of oncological patients. Cancer Imaging. 2012;12:283–9.
Pan L, Mikolajczyk K, Strauss L, Haberkorn U, Dimitrakopoulou-Srrauss A. Machine learning based parameter imaging and kinetic modeling of PET data. J Nucl Med. 2007;48:158p.
Strauss LG, Conti PS. The applications of PET in clinical oncology. J Nucl Med. 1991;32:623–48.
Burger C, Buck A. Requirements and implementations of a flexible kinetic modeling tool. J Nucl Med. 1997;38:1818–23.
Mikolajczyk K, Szabatin M, Rudnicki P, Grodzki M, Burger C. A Java environment for medical image data analysis: initial application for brain PET quantitation. Med Inform. 1998;23:207–14.
Miyazawa H, Osmont A, Petit-Taboué MC, Tillet I, Travère JM, Young AR, et al. Determination of 18 F-fluoro-2-deoxy-D-glucose rate constants in the anesthetized baboon brain with dynamic positron tomography. J Neurosci Methods. 1993;50:263–72.
Cheng C, Alt V, Dimitrakopoulou-Strauss A, Pan L, Thormann U, Schnettler R, et al. Evaluation of new bone formation in normal and osteoporotic rats with a 3-mm femur defect: functional assessment with dynamic PET-CT (dPET-CT) using a 2-deoxy-2 [(18)F] fluoro-D-glucose (18 F-FDG) and 18 F-fluoride. Mol Imaging Biol. 2013;15:336–44.
Ohtake T, Kosaka N, Watanabe T, Yokoyama I, Moritan T, Masuo M, et al. Noninvasive method to obtain input function for measuring tissue glucose utilization of thoracic and abdominal organs. J Nucl Med. 1991;32:1432–8.
Strauss LG, Klippel S, Pan L, Schönleben K, Haberkorn U, Dimitrakopoulou-Strauss A. Assessment of quantitative FDG PET data in primary colorectal tumours: which parameters are important with respect to tumour detection? Eur J Nucl Med Mol Imaging. 2007;34:868–77.
Bird JM, Owen RG, D’Sa S, Snowden JA, Pratt G, Ashcroft J, et al. Guidelines for the diagnosis and management of multiple myeloma 2011. Br J Haematol. 2011;154:32–75.
van Lammeren-Venema D, Regelink JC, Riphagen II, Zweegman S, Hoekstra OS, Zijlstra JM. 18 F-fluoro-deoxyglucose positron emission tomography in assessment of myeloma-related bone disease: a systematic review. Cancer. 2012;15(118):1971–81.
Czernin J, Satyamurthy N, Schiepers C. Molecular mechanisms of bone 18 F-NaF deposition. J Nucl Med. 2010;51:1826–9.
Dimitrakopoulou-Strauss A, Strauss LG, Heichel T, Wu H. Burger et al. The role of quantitative (18)F-FDG PET studies for the differentiation of malignant and benign bone lesions. J Nucl Med. 2002;43:510–8.
Acknowledgements
This study is part of the Sonderforschungsbereich-Transregio 79 (SFB-TRR 79) and was financially supported by the Deutsche Forschungsgemeinschaft (German Research Foundation, DFG).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sachpekidis, C., Goldschmidt, H., Hose, D. et al. PET/CT studies of multiple myeloma using 18 F-FDG and 18 F-NaF: comparison of distribution patterns and tracers’ pharmacokinetics. Eur J Nucl Med Mol Imaging 41, 1343–1353 (2014). https://doi.org/10.1007/s00259-014-2721-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-014-2721-y