Abstract
Purpose
The impact of quantitative parameters on the differentiation of primary colorectal tumours from normal colon tissue was assessed. Dynamic PET data (DPET) were acquired, and compartment and non-compartment modelling applied. The discriminant power of single parameters and the combination of PET parameters was assessed. All lesions were confirmed by histology.
Methods
FDG DPET studies were acquired in 22 patients with colorectal tumours prior to surgery. Five of these patients also had liver metastases at the time of the PET study. The SUV 56–60 min p.i. was included in the evaluation. A two-tissue compartment model was applied and the parameters k 1–k 4 as well as the fractional blood volume (V B) were obtained. The FDG influx was calculated from the compartment data. Non-compartment modelling was used to calculate the fractal dimension (FD) of the time-activity data.
Results
FD, SUV, influx and k 3 were the most important single parameters for lesion differentiation. The highest accuracy was achieved for FD (88.78%). The overall tracer uptake was mainly dependent on k 3 and not on k 1 or V B. The support vector machines (SVM) algorithm was used to predict the classification based on the combination of individual PET parameters. The overall accuracy was 97.3%, with only one false positive case and no false negative results. The analysis of the subgroup of five patients with primary tumours and synchronous metastases revealed no significant differences for the individual PET parameters. However, V B tended to be lower while k 1 and k 2 were higher in patients with synchronous metastases. The SVM classification analysis predicted the presence of metastases based on the PET data of the primary tumour in three of five patients.
Conclusion
Quantitative FDG PET studies provide very accurate data for the differentiation of primary colorectal tumours from normal tissue. The use of quantitative data has the advantage that the detection of a colorectal tumour is not primarily dependent on the individual assessment and experience of the physician evaluating the FDG PET data only visually. The results suggest that the presence of metastatic lesions may be predicted by analysis of the dynamic PET data of the corresponding primary tumour. Further studies are needed to assess this aspect in detail.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For a number of years, 18F-fluorodeoxyglucose (FDG) has been used as the tracer of choice for PET studies in patients with colorectal cancer. In a review, Gambhir et al. reported an average sensitivity of 94% and specificity of 87% [1]. Based on the recent developments of CT and MRI techniques, it is an open question whether PET with FDG should find use for the examination of patients with primary colorectal tumours [2, 3]. One possible limitation in most of the studies in primary colorectal cancer is the visual evaluation of PET studies. The detection of colorectal cancer, either visually or using quantitative data, is correlated with changes in the FDG kinetics in the primary tumour and the normal colon tissue. Tracer retention, as detected visually in the tumour region, is the result of several processes that contribute to the FDG accumulation. The activity of the glucose transport mechanisms, primarily due to the protein-facilitated transporters, determines the intracellular availability of FDG for further metabolism by the hexokinases. The hexokinases are important for temporary trapping of the tracer in the cells. It is generally assumed that the dephosphorylation rate for the metabolised FDG is low in tumours. Besides these parameters, FDG uptake is modulated by the fractional blood volume in the tumour, and the FDG activity in the blood contributes to a varying degree to the overall uptake noted in the normal PET images. Therefore, a combination of different processes contribute to the visibility and detectability of tumours in PET images.
However, limited information exists about the quantitative analysis of the parameters determining the FDG uptake and the time-dependent changes in FDG kinetics. Compartment analysis provides the possibility to assess dedicated aspects of the FDG kinetics, which are associated with molecular biological data. For example, the transport rate constant k 1 of the two-tissue compartment model is associated with glucose transporters and k 3 with the phosphorylation process, which is based on the hexokinase activity. By applying compartment analysis to dynamic PET data, quantitative information about biological parameters is obtained and can be evaluated with regard to tumour detection.
The aim of this study was to assess the contribution of quantitative data to the differentiation of histologically confirmed primary colorectal tumours from normal colon tissue. Furthermore, a strategy for predicting classification as tumour or normal colon tissue based on a new method for discriminant analysis was evaluated, the so-called support vector machines (SVM) algorithm.
Materials and methods
The study population included 22 patients with primary colorectal tumours, scheduled for surgery. All patients were examined for staging purposes within less than 2 days prior to surgery. PET with FDG was used to assess the FDG kinetics in the primary tumour and to detect possible metastases. Histological confirmation of the primary tumours and metastases was obtained via surgery.
The standard FDG PET protocol at our institution is based on an initial dynamic data acquisition focussed on the region of the primary tumour, followed by additional static acquisitions. Dynamic PET studies were performed for 60 min beginning with the intravenous administration of 250–370 MBq FDG, using a 28-frame protocol (ten frames of 30 s, five frames of 60 s, five frames of 120 s and eight frames of 300 s). Additional static images from the neck to the proximal legs were acquired by moving the table in the cranial and caudal directions in relation to the initial position. The gap used for each repositioning was 13.5 cm. A dedicated PET system (ECAT EXACT HR+, Siemens, Erlangen, Germany) with an axial field of view of 15.3 cm and operated in 2D mode was used for all patient studies. The system provides simultaneous acquisition of 63 transverse slices with a theoretical slice thickness of 2.4 mm. A transmission scan (10 min) was obtained prior to radionuclide administration for the attenuation correction of the acquired dynamic emission data. A transmission scan of 3 min followed by an emission scan of 7 min was performed for each static acquisition. All PET images were attenuation corrected and an image matrix of 256×256 pixels was used for iterative image reconstruction (voxel size 2.277×2.277×2.425 mm). The reconstructed uptake images were converted to SUV images based on the formula [4]: SUV (standardised uptake value)=tissue concentration (Bq/g)/(injected dose (Bq)/body weight (g)). The SUV images were used for further quantitative evaluations.
Evaluation of the dynamic PET data was based on the Pmod software (PMOD Technologies, Adliswil, Switzerland) [5, 6]. Visual analysis was performed by evaluating the hypermetabolic areas on transaxial, coronal and sagittal images and correlating with pre-existing CT images as well as clinical data (e.g. endoscopy). Basically, we compared the PET images with CT and associated the CT findings with enhanced FDG uptake regions on PET. Furthermore, parametric images were generally calculated in all patients from the dynamic PET series, reflecting the distribution volume and metabolism of the tracer. Especially the k 3-weighted images were helpful and were used to support the positioning of the volumes of interest (VOIs). In the few cases in which the FDG was low in the tumours, the VOI positioning was primarily based on the parametric images. Time-activity curves were created using VOIs. A VOI consists of multiple regions of interest (ROIs) over the target area. Irregular ROIs were drawn manually. To compensate for possible patient motion during the acquisition time, the original ROIs were visually repositioned, but not redrawn. The SUV obtained from the last frame of the dynamic acquisition (56–60 min post injection) was used for the basic analysis of the FDG uptake. Following surgery, the surgical report was evaluated to compare the location where the tissue specimen was obtained from the tumour and the reference area with the placement of the VOIs in the PET studies. If necessary, a correction of the VOIs was performed to achieve the highest anatomical correlation between PET and surgery.
A detailed quantitative evaluation of tracer kinetics requires the use of compartment modelling. A two-tissue-compartment model is the standard methodology for the quantification of dynamic FDG studies [7, 8]. One problem in patient studies is the accurate measurement of the input function, which theoretically requires arterial blood sampling. However, the input function can be retrieved from the image data with good accuracy [9]. For the input function the mean value of the VOI data obtained from a large arterial vessel like the descending aorta was used. A vessel VOI consisted of at least seven ROIs in sequential PET images. The descending aorta was preferentially used for this purpose, because the spill-over from other organs is very low and the descending aorta extends from the upper chest to the lower abdomen. The size of a lesion may have an impact on the uptake values owing to the partial volume effect. However, the recovery coefficient is 0.85 for a diameter of 8 mm and for the system described above. Because the diameter of all VOIs exceeded 8 mm, no partial volume correction was done. Noise in the input curve may have an impact on the parameter estimates. Therefore, we used a pre-processing tool, available in the PMod software, which allows a fit of the input curve, namely by a sum of up to three decaying exponentials to reduce noise. The constants k 1–k 4 were calculated using a two-compartment model implemented in the PMod software taking into account the fractional blood volume (V B) in a VOI as an additional variable. Details of the applied compartment model are described by Burger and Buck [5].
One major advantage of the PMod software is the graphical interface, which allows the interactive configuration of the kinetic model by the user. Furthermore, some pre-processing steps, e.g. setting up initial values and limits for the fit parameters, can be applied to the data. Following the compartment fitting procedure, visual evaluation of each plot was performed to check the quality of each fit. Each model curve was compared with the corresponding time-activity curve and the total X2 difference was used as the cost function, where the criterion was to minimise the summed squares (X2) of the differences between the measured and the model curve. The squared residuals (measured value minus estimated value) are multiplied by weights. In theory, the weight should be related to the standard error of a measurement. The distribution at each individual point is taken to be Gaussian, with a standard deviation to be specified. The residual covariance was dependent on the kinetic parameter and typically less than 10% for k 1. The model parameters were accepted when k 1–k 4 was less than one and the V B values exceeded zero. The unit for the rate constants is 1/min, while V B reflects the fraction of blood within the evaluated volume. Following compartment analysis, we calculated the global influx of FDG from the compartment data using the formula: \(\inf {\text{lux}} = {{\left( {{\text{k}}_{{\text{1}}} \times {\text{k}}_{{\text{3}}} } \right)}} \mathord{\left/ {\vphantom {{{\left( {{\text{k}}_{{\text{1}}} \times {\text{k}}_{{\text{3}}} } \right)}} {{\left( {{\text{k}}_{{\text{2}}} + {\text{k}}_{{\text{3}}} } \right)}}}} \right. \kern-\nulldelimiterspace} {{\left( {{\text{k}}_{{\text{2}}} + {\text{k}}_{{\text{3}}} } \right)}}\).
Besides the compartment analysis, a non-compartment model was used to calculate the fractal dimension. This model measures the complexity of a dimensional structure by calculating its fractal dimension based on the box counting method [10]. The idea is to subdivide the area into a number of square boxes and count the number of boxes containing some of the structure. The relative mesh size is defined as s, and the number of subdivisions for each axis is given by n = 2 (1/s). Specifying 1/s = 7 means, therefore, a subdivision into 27×27 = 128 × 128 boxes. The aim of the model was to calculate the fractal dimension of the time-activity data. Therefore, time-activity data were handled like images. The number of boxes [N(s)] which contained parts of the time-activity data was counted for each mesh size (s). This counting process was repeated while the number of intervals (the mesh size) was increased to a specified maximum given as a model parameter. Next, the data were plotted in a double-logarithmic way, namely log [N(s)] on the y-axis and log (1/s) on the x-axis. Fractal dimension is equivalent to the slope of a regression line of the plotted points. The fractal dimension is a parameter to assess the heterogeneity of the tracer kinetics and was calculated for each VOI using the time-activity data. The values of the fractal dimension vary from 0 to 2 showing the more deterministic or chaotic distribution of the tracer activity via time in a local volume defined by a VOI. No input function is needed for the fractal dimension model.
Parametric images were calculated based on the dynamic PET data and using two different methods. One procedure is based on a fit of a regression function to the voxel-based time-activity data. The slope reflects the trapping of FDG based primarily on the k 3 component of the FDG model, while the intercept is associated with the distribution volume of the tracer. The second procedure is based on the non-compartment approach and was performed to obtain parametric images of the fractal dimension. For this purpose the box plot procedure, as already used for VOIs to calculate the fractal dimension, was applied to each voxel.
The statistical evaluation of the data was performed using Stata/SE 9.2 (StataCorp, College Station, TX 77845, USA) on a notebook (MacBook Pro, 2.16 GHz, 2 GB RAM) using Mac OS X 10.4.7 (Apple, Cupertino, CA 95014 USA). The statistical evaluation was performed using the descriptive statistics, scatterplots and regression analysis. The classification analysis was performed using the GenePET software, which we developed as a module for Pmod. The software applies the SVM algorithm and provides a classification analysis by optimising a hyperplane between the target variables, e.g. the classification tumour versus normal tissue, using the PET parameters as input variables for the analysis [11, 12]. The algorithm for selection or elimination of variables, the feature ranking, can be based on different criteria, e.g. F-test, Mann-Whitney test or the SVM ranking feature elimination (SVM RFE) approach. The SVM RFE algorithm computes a multidimensional weight vector for the PET variables and the square of the vector is used to calculate the ranking criteria [13]. The type of the hyperplane used to discriminate groups is based on the kernel function. Different kernel types are provided to optimise the differences between the groups to be separated. The linear type of kernel function is comparable to the linear Fisher’s discriminant functions, while polynomial, radial basis (RBF) and sigmoid functions provide non-linear approaches for the generation of the hyperplane. We used the overall accuracy, which reflects the correct classification of both tumour and reference tissue in relation to the total number of samples, to assess the results of the SVM analysis. SVM has been applied in medicine for diagnostic purposes, especially if a classification has had to be made on the basis of a large data set. Oh et al. used SVM to diagnose early relapse in patients with ovarian cancer using proteomic profiling of the serum [14]. The authors were able to demonstrate that the SVM approach was superior to other classification methods.
Results
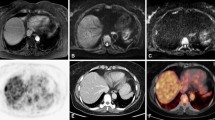
The visual evaluation demonstrated the colorectal tumours as FDG-accumulating lesions which were delineated with the highest contrast in the parametric images, reflecting the FDG phosphorylation (Fig. 1). In a few cases the delineation of the tumour was difficult on the normal FDG uptake images, while the parametric images clearly demonstrated the malignant lesion as a hypermetabolic area (Fig. 2). Overall, parametric imaging was helpful and supported the assessment of the PET studies. Generally, the images of the blood volume provided the delineation of the anatomy, while k 3-weighted images reflected the metabolised fraction of FDG.
Upper row: FDG PET examination of a patient with an adenocarcinoma of the colon. The SUV images reflect the tracer distribution 40–60 min following FDG administration. The images are scaled from 0 to 5 SUV. Middle row: the k 3-weighted images demonstrate trapping of FDG in the malignant lesion. The contrast is improved by parametric imaging. Lower row: images of the blood volume, which was not significantly enhanced in the tumour
Upper row: FDG PET images of a patient with an adenocarcinoma of the colon (right flexure). Low tracer uptake in the FDG images 40–60 min following FDG injection, which is nearly comparable to the tracer accumulation in the normal colon. Middle row: parametric images reflecting the trapping of FDG. The tumour is visible owing to the trapping of the tracer. Lower row: images of the distribution volume of the tracer. The distribution volume of FDG is equivalent or even higher in the normal colon as compared to the tumour; therefore detection of the tumour is hampered in the standard FDG uptake images
Quantitative evaluation using compartment modelling as well as histological verification of the target areas evaluated with PET was possible in 22 patients with primary colorectal tumours. Normal colon tissue served as reference and we were able to compare the PET data with the histologically verified reference areas (normal colon) in 15 patients. Therefore, the final evaluation included the quantitative PET data for the 22 tumour and 15 reference tissue samples.
The quantitative evaluation revealed increased FDG uptake in most of the malignant lesions (Table 1). However, the SUVs were highly variable in the tumours, with a range of 1.76–19.45. In contrast, the SUVs were less variable in the normal colon tissue, with a range of 0.88–2.60. The Mann-Whitney test was applied to the PET results to assess differences in the tracer kinetics for the tumours and the reference tissue. Significant differences were noted for the fractal dimension, SUV influx, and k 3 (order according to the significance level) based on an error level of p < 0.01 (Table 1). When we assessed the correlations of the SUV with the other parameters, significant correlations (p < 0.01) were found for k 3 (r = 0.5963), influx (r = 0.7626) and fractal dimension (r = 0.8268), while no significant correlations existed with V B, k 1, k 2 or k 4.
Discriminant analysis was applied to the data to assess the value of each PET parameter retrieved from the FDG kinetics as a single predictive parameter for the differentiation between tumours and normal colon tissues (Table 2). The highest accuracy, which includes both the correct prediction of tumours and reference tissues, was achieved when the fractal dimension was used for the classification (88.78%). The accuracy of the SUV, as well as that of influx and k 3, was about 10% lower as compared to that of the fractal dimension.
One major aspect of the quantitative assessment of the FDG kinetics by applying the modelling approach is the availability of multiple parameters for the differentiation of tumour and normal tissue. Therefore, we performed a discriminant analysis taking all available quantitative parameters into account (Table 3). The F-ratio served as a criterion to assess the impact of the individual PET variables on the overall classification accuracy. Based on the probability analysis with p < 0.01, a significant impact was noted for fractal dimension, SUV, influx, and k 3 among the group of available PET parameters. Again, the fractal dimension achieved the highest F-ratio, reflecting the most significant contribution to the classification of lesions.
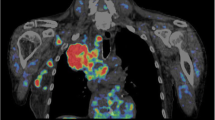
The effect of combining all kinetic PET parameters for the classification of lesions was evaluated with the SVM algorithm. Therefore, we applied the SVM to the PET data to achieve predictions of individual cases. All PET parameters were used to predict the probabilities of a tumour or normal colon tissue in each case. The SVM RFE algorithm was used for feature extraction and the RBF kernel was applied to optimise the hyperplane separations. A lesion was classified as a tumour if the probability was higher as compared to the probability for the normal colon tissue. The results revealed only one false positive result (Fig. 3). The overall accuracy was 97.3%.
The SVM analysis was focussed on the differentiation of tumour and normal colon tissue and a probability map was calculated from the PET parameters, demonstrating the probabilities for a tumour or normal colon tissue for each lesion. Red: tumours, n = 22; blue: normal colon tissue, n = 15. Only one misclassification (false positive) was observed, resulting in an overall accuracy of 97.3%
Five of the 22 patients had synchronous liver metastases of their primary colorectal tumour at the time of the PET examination. The metastases were confirmed by surgery and histology. To assess whether differences existed in the metabolism of the primary tumours, we performed a subgroup analysis of the PET parameters of the primary tumours, comparing patients with only a primary colorectal tumour to those with synchronous liver metastases (Table 4). There was a trend towards lower V B values and k 1and k 2 seemed to be higher in patients with tumours and metastases, but no statistically significant difference was achieved for the individual parameters when p < 0.01 was used. Furthermore, all other parameters, including SUV, appeared to be lower in the group with tumours and metastases, but no significance level was achieved with the single parameter analysis. The SVM analysis using FSCORE feature elimination and polynomial hyperplane generation combined the PET parameters and provided a correct identification of three of five patients with metastases, based on V B, k 1 and k 2 of the primary tumours (Fig. 4).
Probability map for patients with colorectal tumours (blue, n = 17) and patients with colorectal tumours and synchronous liver metastases (red, n = 5). The SVM algorithm was applied to the PET data of the primary tumours and provided a correct prediction of metastases in three of five patients with surgically and histologically confirmed metastatic lesions
Discussion
FDG has found widespread use in oncology for the detection of malignant lesions, based on the increased glucose metabolism in most tumour types. Even in the first publication on recurrent colorectal tumours and FDG PET, it was demonstrated that malignant lesions are usually detectable owing to the enhanced FDG accumulation [15]. The detection of recurrent lesions is generally facilitated because the target area is clearly defined owing to the previous surgery. Furthermore, FDG activity in the normal colon was only a minor problem in the aforementioned study because most of the patients had a tumour in the rectosigmoid region. Kantorová et al. evaluated FDG PET visually in 38 patients with colorectal cancer and reported a sensitivity of 95% [16]. Keogan et al. compared the FDG PET results in 13 patients with those of surgery and biopsy and noted FDG PET to have a sensitivity of 92.3% in the detection of recurrent colorectal tumours [17]. Comparable results were obtained using SUVs [17]. Gambhir and colleagues reviewed the literature and reported a sensitivity range of 29–100% for the staging of colorectal tumours, including possible metastatic sites [1]. The data show that PET can have a highly variable sensitivity if visual evaluation is primarily used for the assessment of malignant lesions.
Our results demonstrate, however, that quantitative parameters can also show wide variations in performance regarding the detection of tumours. The accuracy for the classification of lesions is primarily dependent on the parameters used to discriminate between tumour and normal colon tissue. The fractal dimension was the best single parameter, with an accuracy of 89% for the differentiation of tumour and normal colon (Table 2). This result is not surprising, because the fractal dimension reflects the regional heterogeneity of the changes in concentration of the tracer over time. The fractal dimension is a new parameter, which has found use in pathology for the assessment of irregularities [18]. We have applied the fractal dimension algorithm in several different tumour histologies to improve diagnostics and therapeutic management [19–22]. The results obtained with the fractal dimension in colorectal tumours are promising and support the use of this parameter for the further assessment of malignant lesions. The heterogeneity in the change in tracer distribution over time, as measured by the fractal dimension, appears to be a superior parameter as compared to the SUV, which reflects only the overall tracer uptake within one certain time range, summarising phosphorylated FDG and blood volume activity. Based on our results, a cut-off value of 1.2 for fractal dimension can be used for routine purposes to differentiate tumour and normal colon.
In contrast to non-compartment models like the fractal dimension, the application of a two-tissue compartment model demands the use of an input function. It has been demonstrated by Ohtake et al. that the input data can be retrieved with acceptable accuracy from dynamic PET images [9]. In a recent study, de Geus-Oei et al. evaluated 132 FDG PET scans and compared the image-derived and arterial input functions [23]. The metabolic rates showed a high correlation of r = 0.96 when image-derived data of the abdominal aorta were compared to the results obtained with arterial sampling [23]. Therefore, image-derived data are accurate for further tracer kinetic analysis. One limitation may be the use of filtered backprojection for image reconstruction. However, iterative reconstruction algorithms have found widespread use and provide a good image quality.
One limitation to the routine application of compartment modelling may be the complexity of dynamic data acquisition, which demands more time for the data acquisition and the use of sophisticated software. However, we have shown previously that at least some of the kinetic parameters can be retrieved using a shortened PET data acquisition protocol [24]. The protocol is based on a short dynamic acquisition, beginning with tracer injection for 10 min, and a static acquisition 56–60 min after tracer administration. V B, k 1 and the influx can be estimated from these data with high accuracy and correlation coefficients exceeding 0.9 [24]. Interestingly, it is even possible to use only the target VOIs without the input data for prediction if a correlation of 0.74–0.85 is considered acceptable [24].
The use of modelling provides several kinetic parameters which describe certain properties of the tracer distribution. Generally, the use of multiple parameters is superior to use of a single parameter. Again, the discriminant analysis demonstrated that fractal dimension, SUV, influx and k 3 are the most important parameters, making the major contributions to the correct classification (Table 3). If all available quantitative data were used, all tumours were correctly classified and only one false positive result was obtained. The SVM approach has previously been applied in several areas of biomedicine, e.g. it was found to be helpful for the classification of gene expression data obtained by micro-arrays [12, 13]. Wei et al. used SVM successfully for the automatic detection of clustered microcalcifications [25]. We applied the method to the kinetic PET parameters to assess the predictive value in colorectal tumours. One major advantage of SVM, in contrast to the classical linear Fisher’s discriminant function approach, is the use of a hyperplane to differentiate the groups (tumour, normal colon) instead of just linear functions. This non-linear approach provided a high accuracy for the classification of the lesions, with (as mentioned above) only one false positive classification, resulting in an accuracy of 97.3% (Fig. 3). The results obtained with SVM can be used for future PET studies to classify questionable colorectal tumours.
Limited information is available on the biological parameters determining the FDG uptake in colorectal tumours. Usually the facilitative glucose transporters (GLUTs) determine the transport of glucose into the cells [26]. Thirteen members of the GLUT family have been identified, but the majority of publications have focussed on GLUT1. Sakashita et al. evaluated the GLUT1 expression in T1 and T2 stage colorectal carcinomas and noted an association of GLUT1 expression with the depth of invasion and the histological differentiation [27]. No comparison was made with normal colon tissue. Haber et al. assessed the GLUT1 expression in 112 patients who underwent surgical resection of a colorectal tumour [28]. Surprisingly, only in 20 patients was high GLUT1 expression observed, which was significantly associated with higher mortality. These gene expression results demonstrate that the upregulation of glucose transport, which was observed in only 18% of the patients, cannot be the most important parameter for the FDG accumulation in these tumours. The results reported by Haber et al. are in agreement with our results, demonstrating only a small, statistically non-significant increase in the mean k 1 in most of the patients (Table 1). However, patients with metastases appeared more frequently to have higher k 1 and k 2 values as compared to patients without metastases (Table 4). The higher k 1 and k 2 values may be indicative of increased GLUT1 expression in this subgroup and of a more aggressive primary tumour.
In contrast to k 1, the SUV seemed to be lower in patients with tumour and metastases (Table 4). This result agrees with data reported by Burt et al., who showed that a short tumour doubling time was associated with a lower SUV [29]. Burt et al. assessed the FDG kinetics and accumulation in an animal model using five different human colorectal xenografts with different growth properties [29]. Interestingly, we were able to identify three of five patients with metastases using SVM analysis of the kinetic PET data derived from the primary tumour. Therefore, the kinetic data of the primary tumour may be helpful in predicting the likelihood of metastases from a primary colorectal tumour. However, the number of patients in this study is limited and further data are needed to assess the potential of a prediction based on the kinetic data of the primary tumour. We are continuing the study and aim to assess the possible dependency of metastases on the tracer kinetics of the primary tumour in more detail in a larger number of patients.
The overall uptake in the PET images following FDG injection is theoretically mainly dependent on the fractional blood volume of the tumour, the transport of FDG into the tumour cells via the different transporters, and the phosphorylation rate of the intracellular FDG, based on hexokinases. The SUV reflects the overall tracer uptake in the lesions and we noted a significant correlation with k 3 (r = 0.5963), but not with k 1, which is associated with the FDG transport. Furthermore, k 3, which is associated with the phosphorylation of FDG, but not k 1 was enhanced in the malignant lesions as compared to the normal colon tissue (Table 1). Burt et al. demonstrated with their studies of five colon tumour cell lines and different growth properties that hexokinase activity was inversely associated with the tumour doubling time and that the SUV was correlated with the hexokinase activity (r = 0.86) [29]. Interestingly, hexokinase activity is important not only for the growth rate of a tumour but also for the resistance via the mdr1-/P-170 pathway. Fanciulli et al. assessed the energy metabolism in cell lines with different sensitivity/resistance to doxorubicin and reported that the extent of alteration in energy metabolism was strictly correlated with the degree of resistance [30]. The authors demonstrated that hexokinase activity was correlated with the IC50 of doxorubicin [30]. All these data indicate that especially k 3 is of major importance for assessment of the growth behaviour of colorectal tumours and possible resistance to chemotherapy. Therefore, dynamic PET studies should find preferential use in these tumours.
Conclusion
FDG accumulation, as measured by the SUV, is primarily correlated with k 3, reflecting the phosphorylation of intracellular FDG. Quantitative evaluation of dynamic FDG PET studies in patients with primary colorectal tumours demonstrated that the heterogeneity of the tracer distribution, the tracer uptake as measured by the 56–60 min SUV or the influx, and the phosphorylation are the most important parameters for the differentiation of a colorectal tumour from normal colon tissue. The fractal dimension provided the highest accuracy, at 88.78%, if a single parameter was used for tumour diagnosis. The application of a classification analysis based on the SVM algorithm increased the overall accuracy to 97.3%, with only one false positive classification, when all PET parameters were combined for the analysis. Comparison of patients with primary tumours and those with tumours and synchronous liver metastases showed that V B, k 1 and k 2 of the primary tumour may be predictive for the presence of metastases.
References
Gambhir SS, Czernin J, Schwimmer J, Silverman DHS, Coleman RE, Phelps ME. A tabulated summary of the FDG PET literature. J Nucl Med 2001;42:1S–93S.
Koslin DB. Update on gastrointestinal imaging. Rev Gastroenterol Disord 2002;2:3–10.
Furukawa H, Ikuma H, Seki A, Yokoe K, Yuen S, Aramaki T, et al. Positron emission tomography scanning is not superior to whole-body multi-detector helical computed tomography in the preoperative staging of colorectal cancer. Gut 2005;55:1007–11.
Strauss LG, Conti PS. The applications of PET in clinical oncology. J Nucl Med 1991;32:623–48.
Burger C, Buck A. Requirements and implementations of a flexible kinetic modeling tool. J Nucl Med 1997;38:1818–23.
Mikolajczyk K, Szabatin M, Rudnicki P, Grodzki M, Burger C. A Java environment for medical image data analysis: initial application for brain PET quantitation. Med Inform 1998;23:207–14.
Miyazawa H, Osmont A, Petit-Taboué MC, Tillet I, Travère JM, Young AR, et al. Determination of 18F-fluoro-2-deoxy-D-glucose rate constants in the anesthetized baboon brain with dynamic positron tomography. J Neurosci Methods 1993;50:263–72.
Sokoloff L, Smith CB. Basic principles underlying radioisotopic methods for assay of biochemical processes in vivo. In: Greitz T, Ingvar DH, Widén L, editors. The metabolism of the human brain studied with positron emission tomography. New York: Raven Press; 1983; p. 123–48.
Ohtake T, Kosaka N, Watanabe T, Yokoyama I, Moritan T, Masuo M, et al. Noninvasive method to obtain input function for measuring glucose utilization of thoracic and abdominal organs. J Nucl Med 1991;32:1432–8.
Peitgen HO, Juergens H, Saupe D. Length, area and dimension: measuring complexity and scaling properties. In: Peitgen HO, Juergens H, Saupe D, editors. Chaos and fractals. New York: Springer; 1992; p. 192–219.
Chen PH, Lin CJ, Schölkopf B. A tutorial on v-support vector machines. Appl Stoch Models Bus Ind 2005;21:111–36.
Chu F, Wang L. Applications of support vector machines to cancer classification with microarray data. Int J Neural Syst 2005;15:475–84.
Guyon I, Weston J, Barnhill S, Vapnik V. Gene selection for cancer classification using support vector machines. Mach Learn 2002;46:389–422.
Oh JH, Nandi A, Gurnai P, Knowles L, Schorge J. Diagnosis of early relapse in ovarian cancer using serum proteomic profiling. Genome Inform 2005;16:195–204.
Strauss LG, Clorius JH, Schlag P, Lehner B, Kimmig B, Engenhart R, et al. Recurrence of colorectal tumours: PET evaluation. Radiology 1989;70:329–32.
Kantorová I, Lipská L, Bělohlávek O, Visokai V, Trubač M, Schneiderová M. Routine 18F-FDG PET preoperative staging of colorectal cancer: comparison with conventional staging and its impact on treatment decision making. J Nucl Med 2003;44:1784–8.
Keogan MT, Lowe VJ, Baker ME, McDermott VG, Lyerly HK, Coleman RE. Local recurrence of rectal cancer: evaluation with F-18 fluorodeoxyglucose PET imaging. Abdom Imaging 1997;22:332–7.
Dev P. Basic principles and applications of fractal geometry in pathology: a review. Anal Quant Cytol Histol 2005;27:284–90.
Strauss LG, Dimitrakopoulou-Strauss A, Koczan D, Bernd L, Haberkorn U, Ewerbeck V, et al. 18F-FDG kinetics and gene expression in giant cell tumours. J Nucl Med 2004;45:1528–35.
Koukouraki S, Strauss, LG, Georgoulias V, Schuhmacher J, Haberkorn U, Karkavitsas N, et al. Evaluation of the pharmacokinetics of 68Ga-DOTATOC in patients with metastatic neuroendocrine tumours scheduled for 90Y-DOTATOC therapy. Eur J Nucl Med Mol Imaging 2006;33:460–6.
Dimitrakopoulou-Strauss A, Strauss LG, Schwarzbach M, Burger C, Heichel T, Willeke F, et al. Dynamic PET 18F-FDG studies in patients with primary and recurrent soft-tissue sarcomas: impact on diagnosis and correlation with grading. J Nucl Med 2001;42:713–20.
Dimitrakopoulou-Strauss A, Georgoulias V, Eisenhut M, Koukouraki S, Mäcke HR, Haberkorn U, et al. Quantitative assessment of SSTR2 expression in patients with non-small cell lung cancer using 68Ga-DOTATOC PET and comparison with 18F-FDG PET. Eur J Nucl Med Mol Imaging 2006;33:823–30.
de Geus-Oei LF, Visser EP, Krabbe PFM, van Hoorn BA, Koenders EB, Willemsen AT, et al. Comparison of image-derived and arterial input functions for estimating the rate of glucose metabolism in therapy-monitoring 18F-FDG PET studies. J Nucl Med 2006;47:945–9.
Strauss LG, DImitrakopoulou-Strauss A, Haberkorn U. Shortened PET data acquisition protocol for the quantification of 18F-FDG kinetics. J Nucl Med 2003;44:1933–9.
Wei L, Yang Y, Nishikawa RM, Wenick MN, Edwards A. Relevance vector machine for automatic detection of clustered microcalcifications. IEEE Trans Med Imaging 2005;24:1278–85.
Macheda ML, Rogers S, Best JD. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol 2005;202:654–62.
Sakashita M, Aoyama N, Minami R, Maekawa S, Kuroda K, Shirasaka D, et al. Glut1 expression in T1 and T2 stage colorectal carcinomas: its relationship to clinicopathological features. Eur J Cancer 2001;37:204–9.
Haber RS, Rathan A, Weiser KR, Pritsker A, Itzkowitz SH, Bodian C, et al. GLUT1 glucose transporter expression in colorectal carcinoma: a marker for poor prognosis. Cancer 1998;83:34–40.
Burt BM, Humm JL, Kooby DA, Squire OD, Mastorides S, Larson SM, et al. Using positron emission tomography with [18F]FDG to predict tumour behavior in experimental colorectal cancer. Neoplasia 2001;3:189–95.
Fanciulli M, Bruno T, Giovannelli A, Gentile FP, Di Padova M, Rubiu O, et al. Energy metabolism of human LoVo colon carcinoma cells: correlation to drug resistance and influence of lonidamine. Clin Cancer Res 2000;6:1590–7.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Strauss, L.G., Klippel, S., Pan, L. et al. Assessment of quantitative FDG PET data in primary colorectal tumours: which parameters are important with respect to tumour detection?. Eur J Nucl Med Mol Imaging 34, 868–877 (2007). https://doi.org/10.1007/s00259-006-0319-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-006-0319-8