Abstract
Purpose
Recurrent prostate cancer is usually treated by combining radiotherapy and androgen deprivation therapy. To stage the cancer, choline positron emission tomography (PET)/CT can be performed. It is generally thought that androgen deprivation therapy does not influence choline PET/CT. In this article we focus on the molecular backgrounds of choline and androgens, and the results of preclinical and clinical studies performed using PET/CT.
Methods
Using PubMed, we looked for the relevant articles about androgen deprivation therapy and choline PET/CT.
Results
During ADT, a tendency of decreased uptake of choline in prostate cancer was observed, in particular in hormone-naïve patients.
Conclusion
We conclude that in order to prevent false-negative choline PET/CT scans androgen deprivation should be withheld prior to scanning, especially in hormone-naïve patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer is the most common malignancy among men in Europe and the USA. Depending on the stage and Gleason grade, radical prostatectomy, radiotherapy, androgen deprivation (hormonal) treatment or a combination is available as a treatment option. Distinguishing organ-confined from locally advanced and/or metastatic prostate cancer is an important aspect. Organ-confined disease allows for curative treatment using a single treatment modality. Locally advanced disease is very often treated by combined modalities using radiotherapy and adjuvant androgen deprivation treatment (ADT). Current imaging techniques for prostate cancer are moderately sensitive and specific. Bone scintigraphy, [11C] and [18F]choline positron emission tomography (PET) and magnetic resonance imaging (MRI) are used to determine the extent of prostate cancer both in the primary tumour and in lymph nodes and distant metastases [1]. However, [11C] and [18F]choline PET/CT is not (yet) recognized in broad clinical use or guidelines in (re)staging prostate cancer [2, 3].
In metastatic prostate cancer ADT is the first-line treatment, followed by chemotherapy and new hormonal agents or vaccines [1]. Androgens stimulate growth, function and proliferation in prostate cells and are the driving force behind prostate cancer growth [4]. Regulated by the hypothalamic-pituitary-gonadal axis, almost all of the androgens are produced in the testes, with a small portion being produced in the adrenal glands. In short, hypothalamic luteinizing hormone-releasing hormone (LHRH) causes the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) by the anterior pituitary gland. In turn, LH stimulates Leydig cells, located in the testis, to release testosterone. Testosterone is then converted into dihydrotestosterone (DHT) by 5α-reductase. DHT binds to the androgen receptor (AR) and is transported to the cell’s nucleus, where transcription for effector proteins starts.
When deprived of androgens, (cancerous) cells in the prostate become apoptotic. Achieving an androgen deprivation state plays an important part in the treatment of metastatic prostate cancer. Castration or bilateral orchidectomy offers an immediate decline of testosterone levels, with castration levels of testosterone as soon as 12 h. This is however irreversible and can have negative psychological effects.
Using LHRH agonists most patients reach castration levels within 2–4 weeks [5]. The drawback is that during this overstimulation of the anterior pituitary gland a subsequent rise of FSH and LH occurs, which in turn raises serum testosterone levels. This flare-up can aggravate prostate cancer-related symptoms in patients. In 2–4 weeks, downregulation of LHRH receptors occurs. The flare-up effect can be avoided by using LHRH antagonists binding immediately and competitively to LHRH receptors. On a gonadal level, antiandrogens that directly compete with androgens on a receptor level can be administered.
ADT can be administered in an intermittent and continuous regimen. Continuous ADT is currently standard, with intermittent therapy being suitable for patients with prostate cancer in various clinical settings. The advantage of intermittent therapy is better toleration and sometimes improved sexual function. It makes however no difference in overall survival compared to continuous ADT. The optimal threshold for withdrawing ADT however is not clearly known and usually done empirically [6]. Androgen blocking can also be done minimally to maintain normal serum levels of testosterone in order to sustain sexual function and quality of life.
Hormonal therapy is indicated when a patient is unfit for treatment with curative intent or in need of palliation (for relief of symptoms). In the surgical treatment of prostate cancer, neoadjuvant treatment does not have a significant advantage for overall survival [7–9]. Adjuvant ADT after radical prostatectomy is still controversial [10]. In cases of extensive extracapsular extension ADT is indicated in symptomatic patients with high prostate-specific antigen (PSA) levels or a PSA doubling of less than 1 year. ADT alone is not recommended in patients fit enough for radiotherapy or in those with advanced asymptomatic prostate cancer [11]. It has been shown that the combination of adjuvant and simultaneous ADT with external beam radiation can improve overall survival and is therefore standard in management of high-risk prostate cancer [12]. In cases of nodular involvement, ADT (as mono-therapy) is only recommended in those unfit for local therapy. When fit, ADT is standard adjuvant therapy in cases of more than two positive nodes.
At present it remains unclear if ADT could influence the clinical detection rates in prostate cancer patients. We describe the background of choline uptake in prostate cancer, the role of the AR in prostate cancer treatment and the (pre)clinical results on androgen deprivation and choline PET from the literature. Due to the absence of a direct relationship between the choline pathway and the AR, it is hypothesized that effects on choline uptake during ADT are not expected.
Molecular backgrounds
Choline, a quaternary ammonium cation, is an essential nutrient for humans and is mostly derived from the diet and via recycling in the liver [13, 14]. Through enzymatic processes described by Kennedy as early as 1956, choline is mainly synthesized into phosphatidylcholine (see Fig. 1) [15]. The Kennedy pathway, in short, is comprised of three steps. The enzyme choline kinase converts choline into phosphocholine, an ATP-dependent process. In turn, phosphocholine is synthesized into cytidine diphosphate (CDP) choline using phosphocholine cytidylyltransferase. Then, cholinephosphotransferase catalyses CDP choline into phosphatidylcholine. Phosphatidylcholine is a phospholipid and a major component of biological membrane and therefore over-represented in the human body. A small part consists of acetylcholine, choline plasmalogens, cytidine diphosphocholine (CDP choline), free choline, glycerophosphocholine, lysophosphatidylcholine, phosphocholine and sphingomyelin [14].
In mammals, three isoforms of creatine kinase (CK) are encoded by two separates genes (CKα and CKβ) [16]. CKα encodes both choline and ethanolamine kinase, whereas CKβ predominantly encodes ethanolamine kinase [17, 18]. The CKα-β heterodimer has intermediate specificity. In prostate cancer, CKα expression is increased compared to normal prostate tissue [19], which results in an increase of CK [20]. This change in gene expression leading to aberrant choline levels was also shown by Bertilsson et al., who performed high-resolution magic angle spinning in 133 prostate cancer samples of 41 patients [21]. All these patients were hormone naïve. Chemicals inhibiting CKα led to reduction of tumour growth and inhibition of proliferative capacity [22, 23].
Phosphocholine cytidylyltransferase produces CDP choline using phosphocholine and phosphoethanolamine cytidylyltransferase (CTP). Pyrophosphate is the by-product. This second step in the Kennedy pathway seems to be the rate-limiting step [24]. As a result, build-up of the first step, phosphocholine occurs.
The final step in the Kennedy pathway is the conversion of CDP choline into phosphatidylcholine using CDP cholinephosphotransferase using either diacylglycerol or alkyl-acylglycerol, both lipid anchors. The by-product is cytidine monophosphate.
Breakdown of phosphatidylcholine takes place mainly through phospholipases A1, A2 and D. The latter produces phosphatidic acid and choline, whereas phospholipases A1 and A2 produce free fatty acids and glycerophosphocholine, which in turn gets broken down into glycerol 3-phosphate and choline by a phosphodiesterase [14]. A significant covariance was seen between increased choline and PLA2G7 and cholinephosphotransferase. PLA2G7 is the gene that encodes the phospholipase A2 enzyme lipoprotein-associated phospholipase A2 [25]. Vainio et al. showed its overexpression in prostate cancer [26] which may lead to an increase in choline metabolism via breakdown.
The prostate is an androgen-dependent organ, requiring testosterone for growth. In benign prostatic hyperplasia and malignant transformation this androgen-dependent growth continues. Circulating testosterone is transported into the cell where it is converted to DHT by 5α-reductase. The DHT in turn binds to the AR, releasing heat shock proteins. This dissociation in the newly formed homodimer induces a conformational change in the AR, which in turn facilitates binding to promotor regions in target genes, after being transported into the cell’s nucleus. Activation of the AR leads to binding of co-activators, which starts gene transcription (Fig. 2).
AR activation. Testosterone is, upon entering the cell, converted into DHT by 5α-reductase. DHT binds to the AR, releasing HSPs. Then, the AR-DHT complex is transported into the cell nucleus where target genes are transcribed. T testosterone, 5-AR 5α-reductase, DHT dihydrotestosterone, AR androgen receptor, HSP heat shock protein, EP effective protein
Currently, there are no studies that link the expression of AR under ADT with the activity of CK.
Preclinical studies
Hara et al. investigated the effect of androgens on the uptake of choline under aerobic and anoxic conditions in androgen-dependent LNCaP cells and androgen-independent PC3 cells. Androgen depletion resulted in a marked decrease in the uptake of choline in the androgen-sensitive LNCaP cells but not in PC3 cells [27]. A recently published study evaluated the impact of androgen ablation therapy in different prostate cancer cell lines, reflecting different stages of the disease [28]. Uptake of choline in androgen-supplemented cell cultures was compared to the uptake in cells grown in the absence of androgens. Androgens significantly influenced the uptake of choline in the androgen-independent, AR-expressing cell line 22Rv1 and induced a time-dependent stimulation in choline uptake in androgen-sensitive PC346C cells. However, in another androgen-sensitive cell line (LNCaP) no influence in uptake of choline was seen. The authors speculated that androgens could have stimulated choline uptake in the PC346C cell line, whereas a reverse or reduced effect could have occurred in LNCaP cells. In agreement with the finding of Hara et al., androgens did not modulate the uptake of choline in the androgen-independent, AR-negative cell lines PC3 and PC346DCC. The authors concluded that androgens may interfere with the uptake of choline, depending on differences in AR-induced cell signalling among prostate cell lines.
To our knowledge, only one animal study was published. In this study conducted by Jadvar et al., castrated (n = 9) and non-castrated (n = 9) athymic male mice were implanted with androgen-dependent (CWR22) and androgen-independent (PC3) human prostate cancer cells. After injection of 5 μCi [14C]choline, the mice were sacrificed and prepared for quantitative autoradiography. The uptake time interval and castration did not significantly affect the level of choline uptake by the human prostate cancer xenograft [29].
Clinical studies
In patient studies, one should clearly separate between hormone-naïve and castrate-resistant prostate cancer. In hormone-naïve prostate cancer patients, most studies postulate an inhibitory effect of ADT on the uptake of choline, generally based on heterogeneous, retrospective patient cohorts. A 60 % decrease in standardized uptake value (SUV) after 2 months of ADT as measured by SUV using [18F]choline in primary prostate cancer was first described by DeGrado et al. [30]; another case report using [11C]choline did not visualize uptake in prostate and lymph nodes (initially positive) after 6 months of ADT [31]. Beheshti et al. showed that reduced uptake of [18F]choline is seen when comparing pre- and post-therapeutic studies in prostate cancer patients who respond to hormonal therapy [2]. Almost all of the [18F]choline-negative (sclerotic) bone lesions were detected in patients under hormonal therapy, thereby raising the possibility that lesions might no longer be viable or active [32]. Giovacchini et al. assessed the dependence of [11C]choline uptake on antiandrogenic hormonal therapy in six patients (scans before and after a median treatment of 4 months). Prostate uptake of choline decreased significantly (SUVmax 6.4 ± 4.6) compared to baseline (11.8 ± 5.3), with a mean decrease of 45 % in primary prostate cancer [33]. However, no relationship between duration of therapy and percentage changes in choline uptake could be shown. Fuccio et al. retrospectively studied 14 recurrent prostate cancer patients during follow-up after radical prostatectomy with rising PSA levels with two [11C]choline PET scans: the first scan before start of ADT and the second scan 6 months after ADT administration. Before starting ADT, 13 of 14 patients showed increased uptake of choline in lesions on choline PET, while after 6 months of ADT 9 of 14 patients did not show uptake on choline PET. The authors concluded that ADT significantly reduces choline uptake in androgen-sensitive prostate cancer patients [34]. The decrease in choline uptake after ADT could be explained by the effect ADT has on the tumour size. In the opinion of the authors of these papers, the choline PET scan should be performed either before starting ADT or the treatment should be interrupted for a certain time before the scan. In another study by Price et al. no significant differences in the uptake of [18F]choline was measured in patients with androgen-sensitive or androgen-independent prostate cancer [35]. Hara et al. showed that in a state of androgen dependence during ADT choline uptake is reduced. When the transition to androgen independence has occurred, this reduction is no longer seen [27]. This was supported by Giovacchini et al. [36]. Unfortunately there are no studies available comparing choline uptake in patients with hormone-naïve prostate cancer later converting to a castration-resistant state.
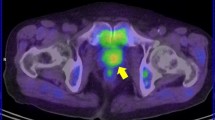
Patients who develop biochemical failure and progressive prostate cancer are difficult to interpret for the effects of androgen status and choline PET results. Studies on these patients looking directly at the influence of ADT on choline uptake are scarce, population groups studied are often inhomogeneous and data on the use of androgen deprivation are not always presented. A good example of a dramatic treatment response resulting from ADT is shown in Fig. 3 [34].
a Maximum intensity projection (MIP) image (left) and fused PET/CT image (right) of [11C]choline PET/CT scan performed after discontinuation of ADT (initial PSA 12.1 ng/ml). Increased [11C]choline uptake in multiple lymph nodes is observed in the MIP image: a large and hot lymph node (SUVmax = 8) is evident in the left iliac chain. b MIP image (left) and fused PET/CT image (right) of [11C]choline PET/CT scan performed 6 months after ADT administration. A complete response is evident. PSA dropped down to 0.01 ng/ml. Reproduced with permission [34]
Table 1 is an overview of studies using [11C] or [18F]choline PET/CT, showing sensitivities with or without ADT in patients with biochemical recurrence after radical treatment. Most recently, Henninger et al. performed [18F]choline PET in 13 patients under androgen deprivation and in 22 patients who had no hormonal treatment. In patients using androgen deprivation the choline PET was true-positive in 8 of 10 patients (overall sensitivity 80 %). Of the patients treated with radical prostatectomy only, 10 of 20 turned out to be true-positive, resulting in a sensitivity of 50 %. So, in patients with biochemical recurrence during ADT after radical prostatectomy, choline PET can yield true-positive findings and withdrawal of ADT is not necessary according to the authors [37]. Most other studies in Table 1 do not show significant differences in sensitivity on PET; these studies have small and heterogeneous patient populations and mostly do not identify whether scans were performed before or during ADT. Furthermore, these studies did not specifically investigate the influence of ADT on choline uptake. It remains unclear if differences in choline uptake can be contributed to an effect caused by the therapeutic effect of ADT, e.g. the reduced tumour volume or changes in metabolism. In a study by Mueller-Lisse et al. a decline in observable metabolites, including choline, throughout different intervals of ADT using MRI and 3-D magnetic resonance spectroscopic imaging was measured [38]. Long-term ADT led to complete loss of all prostatic metabolites in 25 % of the patients, However, strong individual variations were noticed. The overall tendency is that ADT negatively influences uptake of choline in prostate cancer, in particular in hormone-naïve patients. This is confirmed in small patient populations studied with choline PET/CT under ADT.
Conclusion
Based on known biochemical pathways as well as the presented preclinical and clinical studies, the hypothesis that ADT does not influence choline PET/CT has not been overruled. However, the clinical studies are limited in number and were not designed to prospectively assess the effect of androgen deprivation on choline uptake of prostate cancer. We therefore recommend withholding androgen deprivation prior to choline PET/CT to prevent false-negative scans due to treatment effects, especially in restaging of recurrent prostate cancer in hormone-naïve patients. However, since there is limited evidence for withdrawal of ADT in choline PET/CT, further studies should be done to measure any differences in effects in time of hormonal manipulation on the uptake of choline in both hormone-naïve prostate cancer and castration-resistant prostate cancer in a preclinical model. If choline uptake is reduced shortly after starting ADT, before an antitumoural effect is to be expected, an interaction between AR manipulation and choline uptake would be supported.
References
Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, Mason MD, et al. Guidelines on prostate cancer. European Association of Urology. 2012. http://www.uroweb.org/gls/pdf/08%20Prostate%20Cancer_LR%20March%2013th%202012.pdf.
Bauman G, Belhocine T, Kovacs M, Ward A, Beheshti M, Rachinsky I. 18F-fluorocholine for prostate cancer imaging: a systematic review of the literature. Prostate Cancer Prostatic Dis 2012;15(1):45–55.
Souvatzoglou M, Weirich G, Schwarzenboeck S, Maurer T, Schuster T, Bundschuh RA, et al. The sensitivity of [11C]choline PET/CT to localize prostate cancer depends on the tumor configuration. Clin Cancer Res 2011;17(11):3751–9.
Walsh PC. Physiologic basis for hormonal therapy in carcinoma of the prostate. Urol Clin North Am 1975;2(1):125–40.
Limonta P, Montagnani Marelli M, Moretti RM. LHRH analogues as anticancer agents: pituitary and extrapituitary sites of action. Expert Opin Investig Drugs 2001;10(4):709–20.
Abrahamsson PA. Potential benefits of intermittent androgen suppression therapy in the treatment of prostate cancer: a systematic review of the literature. Eur Urol 2010;57(1):49–59.
Shelley MD, Kumar S, Coles B, Wilt T, Staffurth J, Mason MD. Adjuvant hormone therapy for localised and locally advanced prostate carcinoma: a systematic review and meta-analysis of randomised trials. Cancer Treat Rev 2009;35(7):540–6.
McLeod DG, Iversen P, See WA, Morris T, Armstrong J, Wirth MP, et al. Bicalutamide 150 mg plus standard care vs standard care alone for early prostate cancer. BJU Int 2006;97(2):247–54.
Shelley MD, Kumar S, Wilt T, Staffurth J, Coles B, Mason MD. A systematic review and meta-analysis of randomised trials of neo-adjuvant hormone therapy for localised and locally advanced prostate carcinoma. Cancer Treat Rev 2009;35:9–17.
Kumar S, Shelley M, Harrison C, Coles B, Wilt TJ, Mason MD. Neo-adjuvant and adjuvant hormone therapy for localised and locally advanced prostate cancer. Cochrane Database Syst Rev 2006;4:CD006019.
van der Kwast TH, Lopes C, Santonja C, Pihl CG, Neetens I, Martikainen P, et al. Guidelines for processing and reporting of prostatic needle biopsies. J Clin Pathol 2003;56(5):336–40.
Bolla M, de Reijke TM, Van Tienhoven G, Van den Bergh AC, Oddens J, Poortmans PM, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med 2009;360(24):2516–27.
Bathen TF, Sitter B, Sjøbakk TE, Tessem MB, Gribbestad IS. Magnetic resonance metabolomics of intact tissue: a biotechnological tool in cancer diagnostics and treatment evaluation. Cancer Res 2010;70:6692–6.
Li Z, Vance DE. Phosphatidylcholine and choline homeostasis. J Lipid Res 2008;49(6):1187–94.
Kennedy EP, Weiss SB. The function of cytidine coenzymes in the biosynthesis of phospholipides. J Biol Chem 1956;222(1):193–214.
Aoyama C, Liao H, Ishidate K. Structure and function of choline kinase isoforms in mammalian cells. Prog Lipid Res 2004;43(3):266–81.
Gallego-Ortega D, Ramirez de Molina A, Ramos MA, Valdes-Mora F, Barderas MG, Sarmentero-Estrada J, et al. Differential role of human choline kinase alpha and beta enzymes in lipid metabolism: implications in cancer onset and treatment. PLoS One 2009;4(11):e7819.
Aoyama C, Ohtani A, Ishidate K. Expression and characterization of the active molecular forms of choline/ethanolamine kinase-alpha and -beta in mouse tissues, including carbon tetrachloride-induced liver. Biochem J 2002;363(Pt 3):777–84.
Ramírez de Molina A, Rodríguez-González A, Gutiérrez R, Martínez-Piñeiro L, Sánchez J, Bonilla F, et al. Overexpression of choline kinase is a frequent feature in human tumor-derived cell lines and in lung, prostate, and colorectal human cancers. Biochem Biophys Res Commun 2002;296(3):580–3.
Hara T, Bansal A, DeGrado TR. Choline transporter as a novel target for molecular imaging of cancer. Mol Imaging 2006;5(4):498–509.
Bertilsson H, Tessem MB, Flatberg A, Viset T, Gribbestad I, Angelsen A, et al. Changes in gene transcription underlying the aberrant citrate and choline metabolism in human prostate cancer samples. Clin Cancer Res 2012;18:3261–9.
Rodríguez-González A, Ramírez de Molina A, Fernández F, Ramos MA, del Carmen Núñez M, Campos J, et al. Inhibition of choline kinase as a specific cytotoxic strategy in oncogene-transformed cells. Oncogene 2003;22(55):8803–12.
Hernández-Alcoceba R, Saniger L, Campos J, Núñez MC, Khaless F, Gallo MA, et al. Choline kinase inhibitors as a novel approach for antiproliferative drug design. Oncogene 1997;15(19):2289–301.
Katz-Brull R, Degani H. Kinetics of choline transport and phosphorylation in human breast cancer cells; NMR application of the zero trans method. Anticancer Res 1996;16(3B):1375–80.
Tew DG, Southan C, Rice SQ, Lawrence MP, Li H, Boyd HF, et al. Purification, properties, sequencing, and cloning of a lipoprotein-associated, serine-dependent phospholipase involved in the oxidative modification of low-density lipoproteins. Arterioscler Thromb Vasc Biol 1996;16(4):591–9.
Vainio P, Gupta S, Ketola K, Mirtti T, Mpindi JP, Kohonen P, et al. Arachidonic acid pathway members PLA2G7, HPGD, EPHX2, and CYP4F8 identified as putative novel therapeutic targets in prostate cancer. Am J Pathol 2011;178:525–36.
Hara T, Bansal A, DeGrado T. Effect on hypoxia on the uptake of [methyl-3H]choline, [1-14C]acetate and [18F]FDG in cultured prostate cancer cells. Nucl Med Biol 2006;33:977–84.
Emonds KM, Swinnen JV, van Weerden WM, Vanderhoydonc F, Nuyts J, Mortelmans L, et al. Do androgens control the uptake of 18F-FDG, 11C-choline and 11C-acetate in human prostate cancer cell lines? Eur J Nucl Med Mol Imaging 2011;38:1842–53.
Jadvar H, Gurbuz A, Li X, Shahinian A, Conti PS. Choline autoradiography of human prostate cancer xenograft: effect of castration. Mol Imaging 2008;7:147–52.
DeGrado TR, Coleman RE, Wang S, Baldwin SW, Orr MD, Robertson CN, et al. Synthesis and evaluation of 18F-labeled choline as an oncologic tracer for positron emission tomography: initial findings in prostate cancer. Cancer Res 2001;61:110–7.
De Waele A, Van Binnebeek S, Mottaghy FM. Response assessment of hormonal therapy in prostate cancer by [11C]choline PET/CT. Clin Nucl Med 2010;35:701–3.
Beheshti M, Vali R, Waldenberger P, Fitz F, Nader M, Hammer J, et al. The use of F-18 choline PET in the assessment of bone metastases in prostate cancer: correlation with morphological changes on CT. Mol Imaging Biol 2009;11:446–54.
Giovacchini G, Picchio M, Coradeschi E, Scattoni V, Bettinardi V, Cozzarini C, et al. [(11)C]Choline uptake with PET/CT for the initial diagnosis of prostate cancer: relation to PSA levels, tumour stage and anti-androgenic therapy. Eur J Nucl Med Mol Imaging 2008;35:1065–73.
Fuccio C, Schiavina R, Castellucci P, Rubello D, Martorana G, Celli M, et al. Androgen deprivation therapy influences the uptake of 11C-choline in patients with recurrent prostate cancer: the preliminary results of a sequential PET/CT study. Eur J Nucl Med Mol Imaging 2011;38:1985–9.
Price DT, Coleman E, Liao RP, Robertson CN, Polascik TJ, DeGrado TR. Comparison of [18F]fluorocholine and [18F]fluorodeoxyglucose for positron emission tomography of androgen dependent and androgen independent prostate cancer. J Urol 2002;168:273–80.
Giovacchini G, Picchio M, Coradeschi E, Bettinardi V, Gianolli L, Scattoni V, et al. Predictive factors of [(11)C]choline PET/CT in patients with biochemical failure after radical prostatectomy. Eur J Nucl Med Mol Imaging 2010;37(2):301–9.
Henninger B, Vesco P, Putzer D, Kendler D, Loizides A, Bale RJ, et al. [18F]choline positron emission tomography in prostate cancer patients with biochemical recurrence after radical prostatectomy: influence of antiandrogen therapy – a preliminary study. Nucl Med Commun 2012;33:889–94.
Mueller-Lisse UG, Swanson MG, Vigneron DB, Hricak H, Bessette A, Males RG, et al. Time-dependent effects of hormone-deprivation therapy on prostate metabolism as detected by combined magnetic resonance imaging and 3D magnetic resonance spectroscopic imaging. Magn Reson Med 2001;46(1):49–57.
Rinnab L, Mottaghy FM, Blumstein NM, Reske SN, Hautmann RE, Hohl K, et al. Evaluation of [11C]-choline positron-emission/computed tomography in patients with increasing prostate-specific antigen levels after primary treatment for prostate cancer. BJU Int 2007;100(4):786–93.
Reske SN, Blumstein NM, Glatting G. [11C]choline PET/CT imaging in occult local relapse of prostate cancer after radical prostatectomy. Eur J Nucl Med Mol Imaging 2008;35(1):9–17.
Krause BJ, Souvatzoglou M, Tuncel M, Herrmann K, Buck AK, Praus C, et al. The detection rate of [11C]choline-PET/CT depends on the serum PSA-value in patients with biochemical recurrence of prostate cancer. Eur J Nucl Med Mol Imaging 2008;35(1):18–23.
Cimitan M, Bortolus R, Morassut S, Canzonieri V, Garbeglio A, Baresic T, et al. [18F]fluorocholine PET/CT imaging for the detection of recurrent prostate cancer at PSA relapse: experience in 100 consecutive patients. Eur J Nucl Med Mol Imaging 2006;33(12):1387–98.
Heinisch M, Dirisamer A, Loidl W, Stoiber F, Gruy B, Haim S, et al. Positron emission tomography/computed tomography with F-18-fluorocholine for restaging of prostate cancer patients: meaningful at PSA < 5 ng/ml? Mol Imaging Biol 2006;8(1):43–8.
Husarik DB, Miralbell R, Dubs M, John H, Giger OT, Gelet A, et al. Evaluation of [(18)F]-choline PET/CT for staging and restaging of prostate cancer. Eur J Nucl Med Mol Imaging 2008;35(2):253–63.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dost, R.J., Glaudemans, A.W.J.M., Breeuwsma, A.J. et al. Influence of androgen deprivation therapy on choline PET/CT in recurrent prostate cancer. Eur J Nucl Med Mol Imaging 40 (Suppl 1), 41–47 (2013). https://doi.org/10.1007/s00259-013-2398-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-013-2398-7