Abstract
FDG PET and PET/CT are now widely used in oncological imaging for tumor characterization, staging, restaging, and response evaluation. However, numerous benign etiologies may cause increased FDG uptake indistinguishable from that of malignancy. Multiple studies have shown that dual time-point imaging (DTPI) of FDG PET may be helpful in differentiating malignancy from benign processes. However, exceptions exist, and some studies have demonstrated significant overlap of FDG uptake patterns between benign and malignant lesions on delayed time-point images. In this review, we summarize our experience and opinions on the value of DTPI and delayed time-point imaging in oncology, with a review of the relevant literature. We believe that the major value of DTPI and delayed time-point imaging is the increased sensitivity due to continued clearance of background activity and continued FDG accumulation in malignant lesions, if the same diagnostic criteria (as in the initial standard single time-point imaging) are used. The specificity of DTPI and delayed time-point imaging depends on multiple factors, including the prevalence of malignancies, the patient population, and the cut-off values (either SUV or retention index) used to define a malignancy. Thus, DTPI and delayed time-point imaging would be more useful if performed for evaluation of lesions in regions with significant background activity clearance over time (such as the liver, the spleen, the mediastinum), and if used in the evaluation of the extent of tumor involvement rather than in the characterization of the nature of any specific lesion. Acute infectious and non-infectious inflammatory lesions remain as the major culprit for diminished diagnostic performance of these approaches (especially in tuberculosis-endemic regions). Tumor heterogeneity may also contribute to inconsistent performance of DTPI. The authors believe that selective use of DTPI and delayed time-point imaging will improve diagnostic accuracy and interpretation confidence in FDG PET imaging.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
[18F]FDG PET)/CT is now widely used as a powerful evaluation modality in clinical oncology for tumor characterization and staging, restaging, and therapy monitoring [1]. FDG PET imaging detects tumor glycolytic activity, which is an important marker of tumor biology and differentiation. However, FDG is not specific for malignancy. In recent years, multiple studies have shown that dual time-point imaging (DTPI) of FDG PET may be helpful in differentiating malignancy from benign processes, thus enhancing the diagnostic accuracy of FDG PET [2–10]. The underlying rationale is that FDG uptake and clearance depend on the time interval between intravenous FDG administration and imaging. On delayed time-point images, tissues with high glycolysis may have continuously increasing amounts of FDG trapped in cells in the form of FDG-6-phosphate, while tissues with high glucose-6-phosphatase activity (such as the liver [11]) will have an early peak followed by a gradual decrease in intracellular FDG retention. Increased cell proliferation rate [12] and enhanced expression of hexokinase type-II and glucose transporter-1 [13] may also contribute to increased FDG uptake in tumor cells on delayed time-point imaging. At the same time, a longer distribution time also allows improved blood pool and urinary tract clearance of FDG, and thus lower background activity.
At the same time, however, a number of other studies have demonstrated significant overlap of FDG uptake patterns between benign and malignant lesions even on delayed time-point images, indicating limited or no value of DTPI, especially for lung and mediastinal lesions [14–21]. Here, we summarize our experience and opinions on the value of DTPI and delayed time-point imaging in oncology, with a review of the relevant literature.
Variations among DTPI studies are significant
There exist significant differences in multiple aspects of DTPI studies, which may contribute to the non-uniform data and conflicting results reported in the literature.
Differences in image acquisition delay times
Current FDG PET procedure guidelines from the Society of Nuclear Medicine and Molecular Imaging recommends that imaging should commence after a delay of at least 45 min of radiotracer distribution time, but with no recommendation of a particular optimal delay time [22]. While many of DTPI studies are performed at 1 and 2 h after FDG injection, there is no generally accepted delay time for either initial time-point image acquisition or for the time interval between initial and delayed time points of image acquisition. For the majority of reported studies, there were significant variations in imaging delay times. Some studies had very short (30 min) intervals between initial and delayed imaging time points [2, 23–25], while others had quite long (120–140 min) intervals [15, 26–28]. Moreover, some studies had significant variations of the initial and delayed image acquisition time points such that there is overlap between the initial and delayed image acquisition time points from different reported studies [6, 16, 29, 30]. This variation could be one of the major problems leading to conflicting results.
Differences in reference standards
The reference standard used to define whether lesions are malignant or benign also vary significantly among the DTPI studies. Except for studies of breast cancer, only a few publications used pathology assessment as the sole method of confirmation of the presence or absence of malignancy within lesions studied [9, 14–17, 20, 31–34]. For the majority studies, the final diagnosis was based on combinations of pathology and imaging/clinical follow-up. While clinical follow-up is acceptable to determine the nature of a lesion for research purposes, it is difficult to standardize this approach to produce consistent results, especially since there exist significant variations in follow-up time, follow-up frequency, and follow-up examination methods between different studies. A few studies had a follow-up of more than 2 years [6, 35], but many had a nonspecified range of follow-up times [7, 10, 36]. Furthermore, some studies used shorter follow-up times such as 0.9 months [37], 5 months [38], or 6 months [30], potentially leading to errors in lesion classification as benign or malignant.
Differences in criteria to define malignancy on delayed time-point imaging and DTPI
Studies are prone to bias as the criteria used to define malignancy on DTPI have been quite variable. Criteria used on delayed imaging for the diagnosis of malignancy include: a maximum standardized uptake value (SUVmax) of more than 2.5, an increased SUVmax on the delayed time-point images (versus the initial images), or both. The relative change in SUVmax between initial and delayed time-point images, termed the retention index (RI), has been used as the preferred indicator of malignancy by some investigators, and is defined as: RI (%) = 100 % × (SUV[delayed] − SUV[initial])/SUV[initial] [13, 27]. However, the exact threshold value of RI used to define malignancy significantly varies among studies. RI >10 % is probably the most commonly used criterion as an indication of malignancy [6, 8, 29, 30, 35, 39], but other investigators have used RI >0 % [7, 27, 31]. The combination of a delayed time-point SUVmax >2.5 and RI >0 % has been reported by some investigators as the optimal means to define malignancy [24, 31, 40], whereas others have reported various adjusted threshold SUVmax on delayed time-point images as optimal parameters for differentiation between malignancy and benignity. For example, a delayed time-point SUVmax of >5.5 for pulmonary lesions [38], or the combined use of an initial time-point SUVmax of >3.0 or a delayed time-point SUVmax of >4.0 for lymph node lesions in patients with non-small-cell lung carcinoma [41].
Differences in experimental design
A few studies were prospective [5, 42–46], but the majority were retrospective in nature. Most recent publications are based on PET/CT imaging findings, while earlier studies were performed on PET-only machines. A few studies focused on the performance of DTPI and delayed time-point imaging in assessing residual/recurrent lesions after therapy [40, 47], although most studies were performed to assess them for purposes of diagnosis and staging. In addition, there are also significant differences in study sample size, subject characteristics such as age and gender composition, inclusion and exclusion criteria, as well as types of cancer assessed. In particular, a difference in the prevalence of granulomatous inflammation between studies is also a major determinant of the outcome of these research studies, for the reasons detailed below.
The consensus regarding DTPI and delayed time-point imaging in FDG PET
Despite the heterogeneity in research studies performed to assess DTPI, they do provide important information regarding its potential value. Some generally agreed consensuses from these studies are discussed in the following sections.
There is good agreement that background activity generally decreases on DTPI and delayed time-point imaging, leading to improved imaging quality
This phenomenon has been recognized since the early stages of FDG PET imaging [48, 49], years before the concept of DTPI was introduced. This was later confirmed through multiple DTPI studies [4, 23, 50–52]. In fact, decreased background levels is a critical feature and advantage of DTPI and delayed time-point imaging, because of the increased lesion-to-background ratios and image contrast. However, although the majority of tissue types have reduced levels of background activity, some tissues may have stable or even increased uptake on delayed time-point imaging [53], which has to be considered when using these approaches.
There is good agreement that SUVmax increases on DTPI and delayed time-point imaging in the majority of FDG-avid malignancies
Numerous studies have shown that FDG uptake in malignant lesions tends to increase on delayed time-point images [27, 50, 54–57], contributing to improved performance of DTPI. Beaulieu et al. demonstrated that in untreated breast cancer, FDG SUV values changed approximately linearly within the 27- to 75-min time interval examined, at a rate ranging from −0.02 to 0.15 per minute [58]. The peak of FDG uptake may not be reached before 4–5 h after tracer injection [59]. In fact, even in studies that did not show an improved diagnostic accuracy of DTPI, for many malignancies, SUVmax was still found to be higher on delayed images than on initial images [15, 16, 20, 21, 56]. Most studies have shown increased SUV on delayed time-point imaging in the range of 80–90 % for malignant lesions [4, 56, 60].
There is good agreement that diagnostic value is not compromised on DTPI and delayed time-point imaging
Many studies have shown that DTPI improves the diagnostic accuracy of FDG PET. Even in studies that showed no improvement in the diagnostic performance of DTPI, DTPI at least had a performance similar to that of single time-point FDG PET imaging (STPI).
Active infectious and non-infectious inflammatory lesions degrade the performance of DTPI
While the majority of benign lesions show a decrease in FDG uptake on DTPI, FDG uptake in some infectious and non-infectious inflammatory lesions may increase over time, as in malignant lesions. In fact, active infectious and non-infectious inflammatory etiologies remain a major problem in STPI in the oncological setting. There are numerous reports in the literature of false-positive findings on FDG PET imaging due to granulomas. Foreign body granulomatous lesions often mimic malignancy on FDG PET, for example from surgical sutures [61], from leakage of breast silicone injections [62], after gluteal injections [63], after Teflon injections [64], from spilled gallstones during cholecystectomy [65], and from intraperitoneal exposure to activated charcoal during surgery [66]. These granulomas may arise many years after the initial trauma or procedure in the form of tumor-like soft-tissue masses [67, 68]. Infection is another common cause of granulomatous lesions causing false-positive FDG PET findings, such as in xanthogranulomatous pyelonephritis [69], xanthogranulomatous cholecystitis [70], and most commonly in tuberculosis. In addition, granulomas from sarcoidosis are also a common cause of false-positive findings on FDG PET imaging [71–73].
Although DTPI was originally intended to differentiate inflammatory lesions from malignant lesions, it turned out that in many cases, it does not have the power to make this differentiation. FDG uptake in active granulomatous/infectious lesions remains a major obstacle for accurate interpretation, even on DTPI. Multiple studies have indicated that lesions of active inflammation and infection may have higher FDG activity on DTPI, similar to that in malignant lesions, thus resulting in suboptimal diagnostic performance of DTPI [15–19, 35, 74]. The negative impact of active granulomatous/infectious lesions on the value of DTPI remains to be investigated on a larger scale, but apparently depends on the prevalence of malignancy and of active granulomatous/infectious lesions in the study group of interest. This is especially important for studies conducted in populations from tuberculosis-endemic regions.
We have noted that acute infectious and non-infectious inflammatory lesions behave differently from chronic lesions on delayed time-point imaging. There are different patterns of FDG uptake and clearance in active (acute) versus inactive (chronic) inflammatory lesions, which we speculate is due to the different inflammatory cells involved. We examined dynamic FDG uptake and clearance among 30 patients with arthritic changes (degenerative changes in the shoulder and hip joints) on FDG PET imaging, and found that FDG activity in regions of chronic arthritic inflammation showed either no significant increase or a decrease over 3 h, which is significantly different from the uptake pattern that has been reported in active granulomatous lesions or malignancy (unpublished observation). Other investigators have reported similar results, i.e., that FDG uptake in active granulomatous lesions is typically higher than in inactive granulomas or in other benign lesions [17, 21, 75]. Although DTPI may not be able to differentiate acute infectious or non-infectious inflammatory lesions from malignancy in all cases, it is still potentially useful to differentiate malignancy from sites of inactive or chronic inflammation and other benign lesions.
Tumor heterogeneity may contribute to inconsistent performance of DTPI
It is well recognized that tumor heterogeneity exists not only among tumors with different histological subtypes, but also among different individuals with the same tumor subtype, or even among different lesions in the same patient (e.g., between primary and metastatic lesions). Tumor heterogeneity may arise from variations in tumor cells (tumor genotype, differentiation) or in the environment (stromal matrix and tumor location, tissue vascularity and tumor size, and immune status, etc.), causing variable phenotypes on FDG PET imaging [76, 77]. For example, imaging studies have demonstrated that variations in proliferation rate [78], tissue hypoxia [79], or differential amino acid uptake and blood flow [80] all contribute to tumor heterogeneity. In untreated breast cancer, tumor lesions with low FDG uptake tend to show less prominent changes in SUV over time, and can show a relatively stable SUV (or even a slight decrease in SUV) [58]. This is consistent with the finding that DTPI may be suboptimal in the differential diagnosis of minimally FDG-avid lung nodules [14, 35]. Similarly, it has been reported that pancreatic cancer with lower SUVs also have lower RIs [13], suggesting a more limited value of DTPI for the characterization of non-FDG-avid tumors. Although it has been reported that larger tumor lesions are more heterogeneous [81], the diagnosis of small lesions on imaging is much more challenging. For example, a small mediastinal node with only 1 % or even 10 % of tumor cells in a lung cancer patient will likely be missed and DTPI will not help much, as the lesion to background ratio will be low (the SUV will be the average from a small portion of tumor cells and a much larger portion of normal cell component, in addition to the partial volume effect). Indeed, small size (<8 mm) of metastatic lymph nodes has been reported to be the most common cause of false-negative findings on DTPI (minimal FDG avidity of the primary tumor was the second most common cause) [15]. These intertumor and intratumor heterogeneities will affect the performance of FDG PET in both STPI and DTPI, and remain to be investigated.
What can we conclude from available data on DTPI and delayed time-point imaging in FDG PET?
Increased sensitivity for lesion detection is a key strength of DTPI and delayed time-point imaging
Most normal tissues have decreased background activity and most malignant lesions (and some inflammatory lesions) have increased FDG uptake on delayed time-point images, leading to higher lesion-to-background ratios and thus higher sensitivity [5, 23, 29, 36, 46, 57, 75], especially if SUVmax 2.5 is still used as a diagnostic criterion [7]. (The diagnosis of brain tumors on DTPI and delayed time-point imaging is different: the increased sensitivity is due to increased tumor to normal gray matter ratios, but the underlying mechanisms are the same [45, 82–84].) Some authors have suggested that improvement in tumor image contrast with delayed imaging is primarily due to cumulative FDG uptake within tumor tissues [85], while others have found that reduced background activity is a major contributor [23, 50–52]. We have recently demonstrated that in the majority of normal tissues (the blood pool, liver, spleen, lungs, pancreas, inguinal nodes, and skeletal muscles), FDG uptake decreases significantly from 1 to 3 h after radiotracer injection, although SUV remains stable in some tissues (parotid gland, thyroid gland, and prostate gland) and increases in other tissues (myocardium and bone marrow) [53]. Background clearance is more consistent and predictable, whereas cumulative FDG uptake within tumor tissues may vary due to tumor heterogeneity. Although using the same criteria as used for initial imaging (SUVmax >2.5) increases the sensitivity, it has to be recognized that this will lead to compromised specificity and overall diagnostic accuracy [6–8].
Increased specificity of DTPI for lesion characterization depends on multiple factors
As FDG is not a specific marker for neoplasia, a high SUV or lesion-to-background ratio on delayed imaging by itself does not necessarily increase the specificity of FDG PET imaging. As stated above, active inflammation remains a major issue and leads to false-positive results. DTPI studies performed in tuberculosis-endemic regions or sarcoidosis-prone populations often reveal no additional value over STPI, especially for evaluation of the pulmonary or mediastinal lesions [14–21, 86]. However, for DTPI studies performed in a subject population with fewer cases of active granulomatous lesions, most benign lesions will have decreased FDG uptake on delayed time-point images, and such studies have shown improved specificity as well as improved sensitivity compared to STPI [3, 6, 7, 24, 27, 29–31, 33, 39, 50, 57, 75, 87–89]. Another factor that may affect the specificity of DTPI is tumor heterogeneity (including tumor FDG avidity), as stated above.
Increased FDG uptake on delayed time-point images should not be used as the sole criterion in interpreting FDG PET studies
As stated above, various criteria (including RI >10 % [6, 8, 29, 30, 35, 39], RI >0 % [7, 27, 31], or combination of SUVmax and RI [24, 31, 40]) have been suggested to define the presence of malignancy on delayed time-point FDG PET images. The optimal cut-off value of SUVmax on delayed FDG PET imaging remains to be determined and may be dependent on multiple factors (including tumor type, imaging time, and patient population). In general, the use of either SUVmax or RI alone will increase the sensitivity at the expense of specificity, whereas the use of a stricter criterion (e.g., SUVmax plus RI) may increase specificity (sometimes at the expense of sensitivity) [24, 31, 40]. Correlation with other imaging findings, as well as the patient’s history, should also be taken into consideration.
When to use DTPI and delayed time-point imaging in FDG PET
As a tool for tumor imaging, neither initial time-point nor delayed time-point FDG PET alone has the specificity to confirm the diagnosis of a malignancy. The major advantage of DTPI is its increased sensitivity, and as such should be utilized in clinical practice to optimize diagnostic performance. In most cases, DTPI and delayed time-point imaging are able to and will likely detect more lesions that are occult or barely visualized on STPI. In practice, DTPI and delayed time-point imaging will be most helpful in detecting suspected occult lesions (for example, an equivocal lymph node lesion in a patient with an FDG-avid cancer), and in defining the extent of malignancy after the diagnosis is established (i.e., for staging or restaging). However, they will generally not provide additional value if the diagnosis can be confidently established on initial imaging, such as in patients with very early stage (with no suspected metastases) or very late stage malignancy (with clear evidence of distant metastases) on initial imaging.
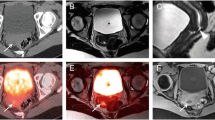
Both DTPI and delayed time-point imaging are also useful in other situations in which lesion-to-background ratio is likely compromised. For example, image quality is often poor in obese patients, or background level of FDG uptake is often high in patients with renal failure or poorly controlled diabetes. Delayed imaging may help to detect occult lesions in such patients. Similarly, delayed imaging may help to detect occult pleural lesions in patients with pleural effusion, or minor lesions in the radiation field that often has diffuse FDG uptake (Fig. 1).
A 56-year old man with non-small-cell lung cancer who underwent FDG PET/CT 3 months after radiation therapy. The initial (a–c) and delayed (d–f) images were obtained 64 and 131 min after tracer injection, respectively. The delayed FDG PET images show two foci of abnormal tracer uptake in the left upper lobe of the lung in a background of postradiation consolidation with diffuse FDG uptake, and a focal uptake in the left T4 vertebra. All are suspicious for residual malignancy but were poorly visualized on the initial images
The general guideline as described above essentially applies to most FDG-avid malignancies. However, DTPI and delayed time-point imaging are probably best performed to evaluate lesions in tissues with significant background clearance over time (such as the liver) [9, 23, 46, 52, 53, 90, 91], and are not or are less helpful for evaluating lesions in tissues with no background clearance over time (such as the thyroid) [92, 93]. The liver normally has relatively high and heterogeneous FDG uptake, making it difficult to visualize small hepatic lesions. However, the background liver activity decreases dramatically with time following radiotracer injection. From our own experience in patients with hepatic metastases detected on follow-up FDG PET studies, retrospective examination of the liver on earlier FDG PET studies often revealed very mild FDG uptake in the region of metastases, which was not possible to differentiate from normal liver uptake at the time of initial study interpretation (Fig. 2). DTPI and delayed time-point imaging may therefore be useful to either confirm or exclude the presence of hepatic malignancy with increased diagnostic performance and interpreter confidence. Similarly, the spleen, which also demonstrates continued background clearance over time, is likely another organ for which these approaches may be useful to detect and characterize malignant lesions.
A 62-year old man with a history of colon cancer with hepatic metastases. FDG PET/CT was performed after chemoradiation therapy. The initial (a–c) and delayed (d–f) images were obtained 65 and 150 min after tracer injection, respectively. Minimally increased FDG uptake is apparent on the initial images (a, c arrowheads) but is better visualized (and with an additional focal abnormality) on the delayed images (d, f arrowheads), corresponding to hypodense lesions on the CT images (b, e arrows) where malignancy was reported on pretherapy images
There is currently no consensus as to what time delay is optimal for DTPI and delayed time-point imaging, and what body parts should be imaged, which may depend on the tumor type present and the particular clinical scenario in which the imaging examination is to be performed. In our experience, the delay time for delayed time-point imaging should be at least 2 h, and ideally 3 h, after FDG injection. At present, we acquire delayed time-point images as part of DTPI only in selected patients and in selected bodily regions based on patient history and findings on initial time-point FDG PET. Another option is to perform delayed time-point imaging alone or whole-body DTPI in all patients. However, it is still not clear what percentage of patients will benefit from this approach as compared to initial time-point imaging alone or more limited DTPI. Further research is required to determine the optimal application of these approaches in various clinical settings.
Conclusion
The authors believe that selective use of DTPI and delayed time-point imaging will improve diagnostic accuracy and interpretation confidence in FDG PET imaging, although the additional value is somewhat limited in imaging patients from granuloma-endemic regions. Some of the most promising applications of these approaches include evaluation of the full extent of malignancy for staging or response assessment, evaluation of equivocal findings, and evaluation of tumor aggressiveness. They are also helpful in patients with high background uptake (for example, overweight or obese patients, patients with poorly controlled diabetes, or patients with poor renal function). Acute infectious and non-infectious inflammatory lesions remain the major culprit for diminished diagnostic performance of these approaches, as well as of STPI. Future prospective research studies are required to better understand the optimal role and workflow of DTPI and delayed time-point imaging in both oncological and non-oncological patient settings.
References
Cheng G, Alavi A, Zhuang H. Clinical application of FDG PET/CT in pediatric lymphoma patients. PET Clin. 2012;7:47–56.
Hustinx R, Smith RJ, Benard F, Rosenthal DI, Machtay M, Farber LA, et al. Dual time point fluorine-18 fluorodeoxyglucose positron emission tomography: a potential method to differentiate malignancy from inflammation and normal tissue in the head and neck. Eur J Nucl Med. 1999;26:1345–8.
Zhuang H, Pourdehnad M, Lambright ES, Yamamoto AJ, Lanuti M, Li P, et al. Dual time point 18F-FDG PET imaging for differentiating malignant from inflammatory processes. J Nucl Med. 2001;42:1412–7.
Kumar R, Loving VA, Chauhan A, Zhuang H, Mitchell S, Alavi A. Potential of dual-time-point imaging to improve breast cancer diagnosis with (18)F-FDG PET. J Nucl Med. 2005;46:1819–24.
Mavi A, Urhan M, Yu JQ, Zhuang H, Houseni M, Cermik TF, et al. Dual time point 18F-FDG PET imaging detects breast cancer with high sensitivity and correlates well with histologic subtypes. J Nucl Med. 2006;47:1440–6.
Xiu Y, Bhutani C, Dhurairaj T, Yu JQ, Dadparvar S, Reddy S, et al. Dual-time point FDG PET imaging in the evaluation of pulmonary nodules with minimally increased metabolic activity. Clin Nucl Med. 2007;32:101–5.
Alkhawaldeh K, Bural G, Kumar R, Alavi A. Impact of dual-time-point (18)F-FDG PET imaging and partial volume correction in the assessment of solitary pulmonary nodules. Eur J Nucl Med Mol Imaging. 2008;35:246–52.
Schillaci O, Travascio L, Bolacchi F, Calabria F, Bruni C, Ciccio C, et al. Accuracy of early and delayed FDG PET-CT and of contrast-enhanced CT in the evaluation of lung nodules: a preliminary study on 30 patients. Radiol Med. 2009;114:890–906.
Lee JW, Kim SK, Lee SM, Moon SH, Kim TS. Detection of hepatic metastases using dual-time-point FDG PET/CT scans in patients with colorectal cancer. Mol Imaging Biol. 2011;13:565–72.
Shinya T, Fujii S, Asakura S, Taniguchi T, Yoshio K, Alafate A, et al. Dual-time-point F-18 FDG PET/CT for evaluation in patients with malignant lymphoma. Ann Nucl Med. 2012;26:616–21.
Gallagher BM, Ansari A, Atkins H, Casella V, Christman DR, Fowler JS, et al. Radiopharmaceuticals XXVII. 18F-labeled 2-deoxy-2-fluoro-d-glucose as a radiopharmaceutical for measuring regional myocardial glucose metabolism in vivo: tissue distribution and imaging studies in animals. J Nucl Med. 1977;18:990–6.
Chang CC, Cho SF, Chen YW, Tu HP, Lin CY, Chang CS. SUV on dual-phase FDG PET/CT correlates with the Ki-67 proliferation index in patients with newly diagnosed non-Hodgkin lymphoma. Clin Nucl Med. 2012;37:e189–95.
Higashi T, Saga T, Nakamoto Y, Ishimori T, Mamede MH, Wada M, et al. Relationship between retention index in dual-phase (18)F-FDG PET, and hexokinase-II and glucose transporter-1 expression in pancreatic cancer. J Nucl Med. 2002;43:173–80.
Chen CJ, Lee BF, Yao WJ, Cheng L, Wu PS, Chu CL, et al. Dual-phase 18F-FDG PET in the diagnosis of pulmonary nodules with an initial standard uptake value less than 2.5. AJR Am J Roentgenol. 2008;191:475–9.
Yen R-F, Chen K-C, Lee J-M, Chang Y-C, Wang J, Cheng M-F, et al. 18F-FDG PET for the lymph node staging of non-small cell lung cancer in a tuberculosis-endemic country: is dual time point imaging worth the effort? Eur J Nucl Med Mol Imaging. 2008;35:1305–15.
Laffon E, de Clermont H, Begueret H, Vernejoux J-M, Thumerel M, Marthan R, et al. Assessment of dual-time-point 18F-FDG-PET imaging for pulmonary lesions. Nucl Med Commun. 2009;30:455–61.
Sathekge MM, Maes A, Pottel H, Stoltz A, van de Wiele C. Dual time-point FDG PET-CT for differentiating benign from malignant solitary pulmonary nodules in a TB endemic area. S Afr Med J. 2010;100:598–601.
Kim DW, Kim CG. Dual-time point positron emission tomography findings of benign mediastinal lymph nodes in a tuberculosis-endemic region. Jpn J Radiol. 2011;29:682–7.
Umeda Y, Demura Y, Morikawa M, Ameshima S, Tsuchida T, Fujibayashi Y, et al. Prognostic value of dual-time-point 18F-fluorodeoxyglucose positron emission tomography in patients with pulmonary sarcoidosis. Respirology. 2011;16:713–20.
Li M, Wu N, Liu Y, Zheng R, Liang Y, Zhang W, et al. Regional nodal staging with 18F-FDG PET-CT in non-small cell lung cancer: additional diagnostic value of CT attenuation and dual-time-point imaging. Eur J Radiol. 2012;81:1886–90.
Zheng Z, Pan Y, Guo F, Wei H, Wu S, Pan T, et al. Multimodality FDG PET/CT appearance of pulmonary tuberculoma mimicking lung cancer and pathologic correlation in a tuberculosis-endemic country. South Med J. 2011;104:440–5.
Delbeke D, Coleman RE, Guiberteau MJ, Brown ML, Royal HD, Siegel BA, et al. Procedure guideline for tumor imaging with 18F-FDG PET/CT 1.0. J Nucl Med. 2006;47:885–95.
Dirisamer A, Halpern BS, Schima W, Heinisch M, Wolf F, Beheshti M, et al. Dual-time-point FDG-PET/CT for the detection of hepatic metastases. Mol Imaging Biol. 2008;10:335–40.
Alkhawaldeh K, Biersack HJ, Henke A, Ezziddin S. Impact of dual-time-point F-18 FDG PET/CT in the assessment of pleural effusion in patients with non-small-cell lung cancer. Clin Nucl Med. 2011;36:423–8.
Hahn S, Hecktor J, Grabellus F, Hartung V, Poppel T, Kimmig R, et al. Diagnostic accuracy of dual-time-point 18F-FDG PET/CT for the detection of axillary lymph node metastases in breast cancer patients. Acta Radiol. 2012;53:518–23.
Ma SY, See LC, Lai CH, Chou HH, Tsai CS, Ng KK, et al. Delayed (18)F-FDG PET for detection of paraaortic lymph node metastases in cervical cancer patients. J Nucl Med. 2003;44:1775–83.
Demura Y, Tsuchida T, Ishizaki T, Mizuno S, Totani Y, Ameshima S, et al. 18F-FDG accumulation with PET for differentiation between benign and malignant lesions in the thorax. J Nucl Med. 2003;44:540–8.
Yen TC, Chang YC, Chan SC, Chang JT, Hsu CH, Lin KJ, et al. Are dual-phase 18F-FDG PET scans necessary in nasopharyngeal carcinoma to assess the primary tumour and loco-regional nodes? Eur J Nucl Med Mol Imaging. 2005;32:541–8.
Matthies A, Hickeson M, Cuchiara A, Alavi A. Dual time point 18F-FDG PET for the evaluation of pulmonary nodules. J Nucl Med. 2002;43:871–5.
Lan XL, Zhang YX, Wu ZJ, Jia Q, Wei H, Gao ZR. The value of dual time point (18)F-FDG PET imaging for the differentiation between malignant and benign lesions. Clin Radiol. 2008;63:756–64.
Nishiyama Y, Yamamoto Y, Kimura N, Ishikawa S, Sasakawa Y, Ohkawa M. Dual-time-point FDG-PET for evaluation of lymph node metastasis in patients with non-small-cell lung cancer. Ann Nucl Med. 2008;22:245–50.
Kim SJ, Kim YK, Kim IJ, Kim YD, Lee MK. Limited predictive value of dual-time-point F-18 FDG PET/CT for evaluation of pathologic N1 status in NSCLC patients. Clin Nucl Med. 2011;36:434–9.
Hu M, Han A, Xing L, Yang W, Fu Z, Huang C, et al. Value of dual-time-point FDG PET/CT for mediastinal nodal staging in non-small-cell lung cancer patients with lung comorbidity. Clin Nucl Med. 2011;36:429–33.
Nakamura S, Okochi K, Kurabayashi T. Dual-time-point fluorodeoxyglucose positron emission tomography for diagnosis of cervical lymph node metastases in patients with head and neck squamous cell carcinoma. J Comput Assist Tomogr. 2011;35:303–7.
Cloran FJ, Banks KP, Song WS, Kim Y, Bradley YC. Limitations of dual time point PET in the assessment of lung nodules with low FDG avidity. Lung Cancer. 2010;68:66–71.
Hu Q, Wang W, Zhong X, Yuan S, Fu Z, Guo H, et al. Dual-time-point FDG PET for the evaluation of locoregional lymph nodes in thoracic esophageal squamous cell cancer. Eur J Radiol. 2009;70:320–4.
Kim SJ, Kim YK, Kim IJ, Kim YD, Lee MK. Limited prognostic value of dual time point F-18 FDG PET/CT in patients with early stage (stage I & II) non-small cell lung cancer (NSCLC). Radiother Oncol. 2011;98:105–8.
Suga K, Kawakami Y, Hiyama A, Sugi K, Okabe K, Matsumoto T, et al. Dual-time point 18F-FDG PET/CT scan for differentiation between 18F-FDG-avid non-small cell lung cancer and benign lesions. Ann Nucl Med. 2009;23:427–35.
Macdonald K, Searle J, Lyburn I. The role of dual time point FDG PET imaging in the evaluation of solitary pulmonary nodules with an initial standard uptake value less than 2.5. Clin Radiol. 2011;66:244–50.
Suga K, Kawakami Y, Hiyama A, Matsunaga N. Differentiation of FDG-avid loco-regional recurrent and compromised benign lesions after surgery for breast cancer with dual-time point F-18-fluorodeoxy-glucose PET/CT scan. Ann Nucl Med. 2009;23:399–407.
Suga K, Kawakami Y, Hiyama A, Sugi K, Okabe K, Matsumoto T, et al. Differential diagnosis between (18)F-FDG-avid metastatic lymph nodes in non-small cell lung cancer and benign nodes on dual-time point PET/CT scan. Ann Nucl Med. 2009;23:523–31.
Sanghera B, Wong WL, Lodge MA, Hain S, Stott D, Lowe J, et al. Potential novel application of dual time point SUV measurements as a predictor of survival in head and neck cancer. Nucl Med Commun. 2005;26:861–7.
Basu S, Mavi A, Cermik T, Houseni M, Alavi A. Implications of standardized uptake value measurements of the primary lesions in proven cases of breast carcinoma with different degree of disease burden at diagnosis: does 2-deoxy-2-[F-18]fluoro-D-glucose-positron emission tomography predict tumor biology? Mol Imaging Biol. 2008;10:62–6.
Tian R, Su M, Tian Y, Li F, Li L, Kuang A, et al. Dual-time point PET/CT with F-18 FDG for the differentiation of malignant and benign bone lesions. Skeletal Radiol. 2009;38:451–8.
Kim DW, Jung SA, Kim CG, Park SA. The efficacy of dual time point F-18 FDG PET imaging for grading of brain tumors. Clin Nucl Med. 2010;35:400–3.
Fuster D, Lafuente S, Setoain X, Navales I, Perissinotti A, Pavia J, et al. Dual-time point images of the liver with (18)F-FDG PET/CT in suspected recurrence from colorectal cancer. Rev Esp Med Nucl. 2012;31:111–6.
Kubota K, Yokoyama J, Yamaguchi K, Ono S, Qureshy A, Itoh M, et al. FDG-PET delayed imaging for the detection of head and neck cancer recurrence after radio-chemotherapy: comparison with MRI/CT. Eur J Nucl Med Mol Imaging. 2004;31:590–5.
Fischman AJ, Alpert NM. FDG-PET in oncology: there’s more to it than looking at pictures [editorial]. J Nucl Med. 1993;34:6–11.
Lowe VJ, DeLong DM, Hoffman JM, Coleman RE. Optimum scanning protocol for FDG-PET evaluation of pulmonary malignancy. J Nucl Med. 1995;36:883–7.
Kubota K, Itoh M, Ozaki K, Ono S, Tashiro M, Yamaguchi K, et al. Advantage of delayed whole-body FDG-PET imaging for tumour detection. Eur J Nucl Med. 2001;28:696–703.
Nishiyama Y, Yamamoto Y, Fukunaga K, Kimura N, Miki A, Sasakawa Y, et al. Dual-time-point 18F-FDG PET for the evaluation of gallbladder carcinoma. J Nucl Med. 2006;47:633–8.
Arena V, Skanjeti A, Casoni R, Douroukas A, Pelosi E. Dual-phase FDG-PET: delayed acquisition improves hepatic detectability of pathological uptake. Radiol Med. 2008;113:875–86.
Cheng G, Alavi A, Lim E, Werner TJ, Del Bello CV, Akers SR. Dynamic changes of FDG uptake and clearance in normal tissues. Mol Imaging Biol. 2012. doi:10.1007/s11307-012-0600-0.
Gupta N, Gill H, Graeber G, Bishop H, Hurst J, Stephens T. Dynamic positron emission tomography with F-18 fluorodeoxyglucose imaging in differentiation of benign from malignant lung/mediastinal lesions. Chest. 1998;114:1105–11.
Nishiyama Y, Yamamoto Y, Monden T, Sasakawa Y, Tsutsui K, Wakabayashi H, et al. Evaluation of delayed additional FDG PET imaging in patients with pancreatic tumour. Nucl Med Commun. 2005;26:895–901.
Chen YM, Huang G, Sun XG, Liu JJ, Chen T, Shi YP, et al. Optimizing delayed scan time for FDG PET: comparison of the early and late delayed scan. Nucl Med Commun. 2008;29:425–30.
Shinya T, Rai K, Okumura Y, Fujiwara K, Matsuo K, Yonei T, et al. Dual-time-point F-18 FDG PET/CT for evaluation of intrathoracic lymph nodes in patients with non-small cell lung cancer. Clin Nucl Med. 2009;34:216–21.
Beaulieu S, Kinahan P, Tseng J, Dunnwald LK, Schubert EK, Pham P, et al. SUV varies with time after injection in (18)F-FDG PET of breast cancer: characterization and method to adjust for time differences. J Nucl Med. 2003;44:1044–50.
Hamberg LM, Hunter GJ, Alpert NM, Choi NC, Babich JW, Fischman AJ. The dose uptake ratio as an index of glucose metabolism: useful parameter or oversimplification? J Nucl Med. 1994;35:1308–12.
Zytoon AA, Murakami K, El-Kholy MR, El-Shorbagy E. Dual time point FDG-PET/CT imaging… Potential tool for diagnosis of breast cancer. Clin Radiol. 2008;63:1213–27.
Kikuchi M, Nakamoto Y, Shinohara S, Fujiwara K, Tona Y, Yamazaki H, et al. Suture granuloma showing false-positive finding on PET/CT after head and neck cancer surgery. Auris Nasus Larynx. 2012;39:94–7.
Ho L, Wassef H, Seto J. FDG PET/CT imaging in granulomatous changes secondary to breast silicone injection. Clin Radiol. 2010;65:659–61.
Prosch H, Mirzaei S, Oschatz E, Strasser G, Huber M, Mostbeck G. Case report: gluteal injection site granulomas: false positive finding on FDG-PET in patients with non-small cell lung cancer. Br J Radiol. 2005;78:758–61.
Kirsch CFE, Suh JD, Lufkin RB, Canalis RF. False-positive positron-emission tomography-CT of a Teflon granuloma in the parapharyngeal space occurring after treatment for a patulous eustachian tube. AJNR Am J Neuroradiol. 2007;28:1371–2.
Morishita K, Otomo Y, Sasaki H, Yamashiro T, Okubo K. Multiple abdominal granuloma caused by spilled gallstones with imaging findings that mimic malignancy. Am J Surg. 2010;199:e23–4.
Lim ST, Jeong H-J, Kim DW, Yim C-Y, Sohn M-H. F-18 FDG PET-CT findings of intraperitoneal carbon particles-induced granulomas mimicking peritoneal carcinomatosis. Clin Nucl Med. 2008;33:321–4.
Tenconi S, Luzzi L, Paladini P, Voltolini L, Gallazzi MS, Granato F, et al. Pleural granuloma mimicking malignancy 42 years after slurry talc injection for primary spontaneous pneumothorax. Eur Surg Res. 2010;44:201–3.
Miyake KK, Nakamoto Y, Mikami Y, Ishizu K, Saga T, Higashi T, et al. F-18 FDG PET of foreign body granuloma: pathologic correlation with imaging features in 3 cases. Clin Nucl Med. 2010;35:853–7.
Cheng G, Torigian DA, Alavi A. FDG PET/CT and MRI findings in a patient with focal xanthogranulomatous pyelonephritis mimicking cystic renal malignancy. Clin Nephrol. 2011;76:484–6.
Makino I, Yamaguchi T, Sato N, Yasui T, Kita I. Xanthogranulomatous cholecystitis mimicking gallbladder carcinoma with a false-positive result on fluorodeoxyglucose PET. World J Gastroenterol. 2009;15:3691–3.
Li Y-J, Zhang Y, Gao S, Bai R-J. Cervical and axillary lymph node sarcoidosis misdiagnosed as lymphoma on F-18 FDG PET-CT. Clin Nucl Med. 2007;32:262–4.
Cheng C-Y, Huang W-S, Shen DH, Fan Y-M, Hsu H-H, Cherng S-C, et al. FDG PET/CT demonstrated rapid progression of mediastinal lymphadenopathy in sarcoidosis. Clin Nucl Med. 2007;32:117–21.
Prabhakar HB, Rabinowitz CB, Gibbons FK, O’Donnell WJ, Shepard J-AO, Aquino SL. Imaging features of sarcoidosis on MDCT, FDG PET, and PET/CT. AJR Am J Roentgenol. 2008;190:S1–6.
Razak HR, Geso M, Abdul Rahim N, Nordin AJ. Imaging characteristics of extrapulmonary tuberculosis lesions on dual time point imaging (DTPI) of FDG PET/CT. J Med Imaging Radiat Oncol. 2011;55:556–62.
Nunez R, Kalapparambath A, Varela J. Improvement in sensitivity with delayed imaging of pulmonary lesions with FDG-PET. Rev Esp Med Nucl. 2007;26:196–207.
Basu S, Kwee TC, Gatenby R, Saboury B, Torigian DA, Alavi A. Evolving role of molecular imaging with PET in detecting and characterizing heterogeneity of cancer tissue at the primary and metastatic sites, a plausible explanation for failed attempts to cure malignant disorders. Eur J Nucl Med Mol Imaging. 2011;38:987–91.
Asselin M-C, O’Connor JPB, Boellaard R, Thacker NA, Jackson A. Quantifying heterogeneity in human tumours using MRI and PET. Eur J Cancer. 2012;48:447–55.
Beresford M, Sanghera B, Wong W-L, Makris A. Imaging of primary breast cancer with 18F-fluorodeoxythymidine PET-CT reveals heterogeneity of proliferation throughout the tumour. Eur J Nucl Med Mol Imaging. 2006;33:624.
Sorensen M, Horsman MR, Cumming P, Munk OL, Keiding S. Effect of intratumoral heterogeneity in oxygenation status on FMISO PET, autoradiography, and electrode PO2 measurements in murine tumors. Int J Radiat Oncol Biol Phys. 2005;62:854–61.
Wyss MT, Hofer S, Hefti M, Bartschi E, Uhlmann C, Treyer V, et al. Spatial heterogeneity of low-grade gliomas at the capillary level: a PET study on tumor blood flow and amino acid uptake. J Nucl Med. 2007;48:1047–52.
Hatt M, Cheze-le Rest C, van Baardwijk A, Lambin P, Pradier O, Visvikis D. Impact of tumor size and tracer uptake heterogeneity in (18)F-FDG PET and CT non-small cell lung cancer tumor delineation. J Nucl Med. 2011;52:1690–7.
Spence AM, Muzi M, Mankoff DA, O’Sullivan SF, Link JM, Lewellen TK, et al. 18F-FDG PET of gliomas at delayed intervals: improved distinction between tumor and normal gray matter. J Nucl Med. 2004;45:1653–9.
Prieto E, Marti-Climent JM, Dominguez-Prado I, Garrastachu P, Diez-Valle R, Tejada S, et al. Voxel-based analysis of dual-time-point 18F-FDG PET images for brain tumor identification and delineation. J Nucl Med. 2011;52:865–72.
Horky LL, Hsiao EM, Weiss SE, Drappatz J, Gerbaudo VH. Dual phase FDG-PET imaging of brain metastases provides superior assessment of recurrence versus post-treatment necrosis. J Neurooncol. 2011;103:137–46.
Chin BB, Green ED, Turkington TG, Hawk TC, Coleman RE. Increasing uptake time in FDG-PET: standardized uptake values in normal tissues at 1 versus 3 h. Mol Imaging Biol. 2009;11:118–22.
Deppen S, Putnam Jr JB, Andrade G, Speroff T, Nesbitt JC, Lambright ES, et al. Accuracy of FDG-PET to diagnose lung cancer in a region of endemic granulomatous disease. Ann Thorac Surg. 2011;92:428–32.
Alavi A, Gupta N, Alberini J-L, Hickeson M, Adam L-E, Bhargava P, et al. Positron emission tomography imaging in nonmalignant thoracic disorders. Semin Nucl Med. 2002;32:293–321.
Conrad GR, Sinha P. Narrow time-window dual-point 18F-FDG PET for the diagnosis of thoracic malignancy. Nucl Med Commun. 2003;24:1129–37.
Uesaka D, Demura Y, Ishizaki T, Ameshima S, Miyamori I, Sasaki M, et al. Evaluation of dual-time-point 18F-FDG PET for staging in patients with lung cancer. J Nucl Med. 2008;49:1606–12.
Lin W-Y, Tsai S-C, Hung G-U. Value of delayed 18F-FDG-PET imaging in the detection of hepatocellular carcinoma. Nucl Med Commun. 2005;26:315–21.
Nishiyama Y, Yamamoto Y, Kimura N, Miki A, Sasakawa Y, Wakabayashi H, et al. Comparison of early and delayed FDG PET for evaluation of biliary stricture. Nucl Med Commun. 2007;28:914–9.
Kim SJ, Kim BH, Jeon YK, Kim SS, Kim IJ. Limited diagnostic and predictive values of dual-time-point 18F FDG PET/CT for differentiation of incidentally detected thyroid nodules. Ann Nucl Med. 2011;25:347–53.
Hsiao YC, Wu PS, Chiu NT, Yao WJ, Lee BF, Peng SL. The use of dual-phase 18F-FDG PET in characterizing thyroid incidentalomas. Clin Radiol. 2011;66:1197–202.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheng, G., Torigian, D.A., Zhuang, H. et al. When should we recommend use of dual time-point and delayed time-point imaging techniques in FDG PET?. Eur J Nucl Med Mol Imaging 40, 779–787 (2013). https://doi.org/10.1007/s00259-013-2343-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-013-2343-9