Abstract
Purpose
The purpose of the present study was to evaluate whether 2-fluoro[fluorine-18]-2-deoxy-d-glucose (F-18 FDG) positron emission tomography (PET) could differentiate malignant and benign bone lesions and whether obtaining delayed F-18 FDG PET images could improve the accuracy of the technique.
Methods
In a prospective study, 67 patients with bone lesions detected by computed tomography (CT) or magnetic resonance imaging were included. Whole body PET/CT imaging was performed at 1 h (early) after the F-18 FDG injection and delayed imaging at 2 h post injection was performed only in the abnormal region. Semiquantitative analysis was performed using maximum standardized uptake value (SUVmax), obtained from early and delayed images (SUVmaxE and SUVmaxD, respectively). The retention index (RI) was calculated according to the equation: RI = (SUVmaxD − SUVmaxE) × 100/SUVmaxE. Histopathology of surgical specimens and follow-up data were used as reference criteria. The SUVmaxE and RI were compared between benign and malignant lesions.
Results
The final diagnoses revealed 53 malignant bone lesions in 37 patients and 45 benign lesions in 30 patients. There were statistically significant differences in the SUVmaxE between the malignant and benign lesions (P = 0.03). The mean SUVmaxE was 6.8 ± 4.7 for malignant lesions and 4.5 ± 3.3 for benign lesions. However, a considerable overlap in the SUVmaxE was observed between some benign and malignant tumors. With a cutoff value of 2.5 for the SUVmaxE, the sensitivity, specificity, and accuracy were 96.0%, 44.0%, and 72.4%, respectively. The positive predictive value (PPV) and negative predictive value (NPV) were 67.1% and 90.9%, respectively. There were significant differences in the RI between the malignant and benign lesions (P = 0.004). But there was overlap between the two groups. The mean RI was 7 ± 11 for the benign lesions and 18 ± 11 for the malignant lesions. When an RI of 10 was used as the cutoff point, the sensitivity, specificity, and accuracy were 90.6%, 76.0%, and 83.7.0%, respectively. The PPV and NPV were 81.4% and 87.1%, respectively.
Conclusions
The results of this study indicate that dual-time point F-18 FDG PET may provide more help in the differentiation of malignant tumors from benign ones.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The differentiation of benign and malignant intraosseous lesions can often be accomplished by means of radiographs, computed tomography (CT), and magnetic resonance imaging (MRI). All of these methods of morphologic imaging are very important. Radiographs including CT and X-ray provide important information about the appearance, intraosseous extent, and internal characteristics of bone tumors. MRI is highly sensitive for the detection of bone marrow abnormalities, cortical destruction, or soft tissue tumors adjacent to or infiltrating neighboring bone [1]. However, the morphologic appearance of many lesions is non-specific. With some entities, the diagnosis can be difficult with radiographic imaging or MRI.
Positron emission tomography (PET) with 2-fluoro[fluorine-18]-2-deoxy-d-glucose (F-18 FDG) has been used to differentiate malignant tumors from benign lesions [2, 3]. In the musculoskeletal system, some reports suggested a good correlation between glucose consumption measured by F-18 FDG and the aggressiveness of musculoskeletal tumors [4–7]. Dehdashti et al. [8] performed F-18 FDG PET studies in a series of 20 intraosseous lesions and emphasized that 14 of 15 malignant tumors and four of five benign lesions were correctly diagnosed, using a cutoff value of 2.0 for the SUVmax. However, 13 of the 15 malignant tumors were metastatic carcinomas. Aoki et al. reported a statistically significant difference in the SUVmax between benign and malignant bone tumors in 52 bone lesions. However, they observed a significant overlap of the SUVmax among some of the benign and malignant bone tumors, and a high accumulation of F-18 FDG was reported in some benign bone tumors [9]. Other reports [10, 11] raised questions about a clear correlation between the F-18 FDG accumulation and the malignant potential of bone tumors. The value of F-18 FDG in the musculoskeletal system is questioned.
F-18 FDG PET usually is performed 1 h after F-18 FDG administration. Some studies have shown that delayed PET (2–3 h post injection) might help in differentiating malignant lesions from benign ones [12–17]. But to our knowledge, there are only a few reports of the quantitative analysis of delayed PET imaging in differentiating malignant bone lesions from benign ones. Sahlmann et al. [18] investigated glucose metabolism in 17 patients with chronic bacterial osteomyelitis and four patients with malignant bone lesions by using a dual time point F-18 FDG PET (30 and 90 min after injection). They concluded that dual time point F-18 FDG PET may be of value in the differentiation between chronic bacterial osteomyelitis and malignant bone lesions. However, the patient number in the study was small and only one kind of benign bone lesion was investigated.
The purpose of the present study was to evaluate whether the early time point of F-18 FDG PET imaging (1 h post injection) can differentiate malignant from benign bone lesions and to determine whether delayed F-18 FDG PET imaging can improve the accuracy of the technique.
Materials and methods
Patients
In a current prospective study initiated in March 2007, 67 patients (46 males, 21 females; age range, 9–76 years; median age, 46 ± 18 years) with suspected bone tumors or tumor-like lesions based upon their X-ray, CT, or MRI studies were examined by F-18 FDG PET. In those patients, 5 had X-ray images, 48 had diagnostic CT, 7 had MRI, and 7 had both CT and MRI before the PET/CT study. Each research subject gave his or her written informed consent before participating in the study.
F-18 FDG PET image acquisition and reconstruction
The study protocol was approved by our institutional ethics committee and informed consent was obtained in all cases. Patients were fasted for at least 4–6 h before intravenous administration of 185–370 MBq of F-18 FDG (4 MBq/kg of body weight). A serum glucose concentration was obtained before the injection and the blood glucose levels were less than 200 mg/dl in all patients. The patients were at rest in a quiet room after the injection and the PET/CT scans were performed at 1 h (early) and 2 h (delayed) post injection with a PET/CT unit (Gemini GXL; Philips Medical Systems, The Netherlands). The CT image acquisition was performed by spiral CT at 0.75 s per rotation with 40 mAs and 120 kVp, a section thickness of 4 mm, and a 4-mm interval. No intravenous contrast agent was administered. The PET emission images (early images) were acquired from the proximal thigh to the mid cranium, typically requiring six to seven bed positions with a 2-min acquisition in each position. Delayed PET emission images of the abnormal areas were acquired at 2 h after the administration of F-18 FDG, using two or three bed positions with a 2-min acquisition in each position. All PET images were reconstructed using an LOR algorithm, with CT-based attenuation correction applied. Integrated images were obtained by Syntegra software.
PET image interpretation and calculation of related parameters
Early and delayed PET images were reviewed on a computer monitor in the transaxial, coronal, and sagittal planes along with maximum-intensity-projection images. Two experienced nuclear medicine physicians independently evaluated the F-18 FDG uptake semiquantitatively. The evaluating physicians were unaware of the clinical histories or the results of CT or MRI. The PET images were compared with the corresponding CT or MR images for accurate anatomic identification of the tumor. Any difference of opinion was resolved by consensus.
For semiquantitative analysis, a circular region of interest (ROI) was placed over the identified bone lesion using the transverse PET image. For lesions visualized on PET, the ROIs were placed over the entire F-18 FDG-avid lesion, including the largest amount of radioactivity. When little or no lesion-related radioactivity was visually discernible, the ROI was placed at the position of the lesion on the CT or MR images. The standardized uptake value (SUV) was calculated using the following formula: SUV = tissue concentration (MBq/g) / [injected dose (MBq) / body weight (g)]. The maximal SUV (SUVmax) in the lesion ROI was calculated for each ROI. Furthermore, we evaluated the change in the uptake in the lesions as the retention index (RI), computed as follows: RI = (SUVmaxD − SUVmaxE) × 100 / SUVmaxE.
Final diagnosis
The final diagnosis was made by histological examination or by following up for at least 1 year with CT, MRI, or PET/CT imaging. When a tissue-based diagnosis was not available, lesion was considered malignant if an increase in size or change of character (lytic to sclerotic) was confirmed with follow-up imaging, including CT, magnetic resonance (MR), or PET/CT, with or without exacerbation of clinical symptoms; whereas, lesion was considered benign if the size and character of the lesion remained unchanged without treatment over at least 1 year [19].
Statistical analysis
Differences in semiquantitative parameters were analyzed by the Student’s t test. All semiquantitative data were expressed as mean ± SD. For all analyses, P values of less than 0.05 were considered statistically significant.
Receiver-operating-characteristic (ROC) curves for the SUVmaxE and the RI were derived and evaluated by comparing the areas under the curves. The sensitivity, specificity, PPV, and NPV for the SUVmaxE and RI were determined. Significance was assumed if the probability of a first-degree error was less than 0.05 (x 2 > 3.841).
Results
The final diagnoses were confirmed by histologic examination in 60 patients (91 lesions) and by clinical and imaging follow-up ranging between 14 and 18 months (mean 15.5 months) in 7 patients (7 lesions). Of these 98 lesions, 53 (39 primary and 14 metastatic, all based on histological verification) were malignant in 37 patients and 45 were benign (38 based on histological verification) in 30 patients. The malignant lesions were comprised of 6 osteosarcomas, 4 Ewing’s sarcomas, 3 chondrosarcomas, 13 myelomas, 11 primary bone non-Hodgkin’s lymphomas, 2 neuroblastomas, and 14 bone metastases. Among the metastatic bone lesions were eight lung adenocarcinomas, two esophageal carcinomas, two renal carcinomas, one hepatic cell cancer, and one thyroid cancer. The benign lesions were comprised of six giant cell tumors, seven tuberculosis, three osteomyelitis, ten fibrous dysplasia, five osteoblastomas, four bone hemangiomas, three fractures, three eosinophilic granulomas, two osteitis, and two enchondromas. The histological subtypes are shown in Table 1.
There were statistically significant differences in the SUVmaxE between the malignant and benign lesions (P = 0.03). The SUVmaxE in the malignant group was higher than that in the benign group. The mean SUVmaxE was 6.8 ± 4.7 (range, 1.7–18.9) for malignant lesions and 4.5 ± 3.3 (range, 1.5–13.9) for benign lesions. However, a considerable overlap in the SUVmaxE was observed between some benign and malignant tumors (Fig. 1). In the malignant group, most of lesions showed a high uptake of F-18 FDG. A particularly high uptake of F-18 FDG was seen in all 11 malignant lymphomas, 12 of 14 metastatic lesions, 7 of 13 myelomas, and all 6 osteosarcomas, whereas glucose consumption was lower in the other neoplasms, such as was seen in 6 of 13 myelomas, 2 metastatic lesions from hepatic cell cancer and thyroid cancer, and in 2 chondrosarcomas. The benign lesions that showed a high accumulation of F-18 FDG included all of the giant cell tumors (Fig. 2), tuberculosis, Langerhans cell histiocytosis, osteomyelitis, osteoblastoma (Fig. 3), and some of the fibrous dysplasia, whereas the glucose consumption was substantially lower in the other benign lesions. The characteristics of the benign lesions showing an increasing FDG uptake over time are shown in Table 2.
Box and whisker plot of SUVmaxE for benign and malignant bone lesions. The top of the boxes represent the mean and error bars represent the range of SUVmaxE. Although there is a statistically significant difference between the SUVmaxE of the benign and malignant bone lesions (p < 0.05), there are significant overlaps between the malignant and the benign lesions (p < 0.05)
With a cutoff value of 2.5 for the SUVmaxE, 51 of the 53 malignant lesions and 20 of the 45 benign lesions were characterized correctly, and the sensitivity, specificity, and accuracy of the F-18 FDG PET for the differentiation of benign from malignant intraosseous lesions were 96.0%, 44.0%, and 72.4%, respectively. The positive and negative predictive values were 67.1% and 90.9%, respectively.
There were significant differences in the RI between the malignant and benign lesions (P = 0.004). The RIs in the malignant group were higher than those in the benign group. But there was still overlap between the two groups (Fig. 4). The mean RI was 7 ± 11 (range, −7 to 23) for the benign lesions and 18 ± 11 (range, −7 to 46) for the malignant lesions (Fig. 5). When an RI of 10 was used as the cutoff point,48 of the 53 malignant lesions and 34 of the 45 benign lesions were characterized correctly, and the sensitivity, specificity, and accuracy of the F-18 FDG PET for differentiation of benign from malignant intraosseous lesions were 90.6% and 76.0%, and 83.7.0% respectively. The positive and negative predictive values were 81.4% and 87.1%, respectively. Three of 16 myeloma lesions and two of 14 metastatic lesions (from thyroid cancer and hepatic cell carcinoma) showed decreased uptakes of F-18 FDG in the delayed image. In the benign group, a particularly high RI was observed in TB and chronic osteomyelitis. The diagnostic value of F-18 FDG PET using semiquantitative analysis for the differentiation of benign and malignant bone lesions is shown in Table 3.
Box and whisker plot of RI for benign and malignant lesions. The top of the boxes represent the mean and error bars represent the range of RI. Although there are overlaps between the malignant and benign lesions, there is a statistically significant difference between the RI of the benign and malignant bone lesions (p < 0.05)
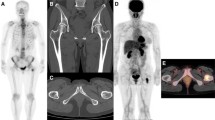
Biopsy-proved chondrosarcoma in a 39-year-old woman with paralysis in both lower limbs. a Early fused image shows increased uptake of F-18 FDG at her vertebra (SUVmaxE = 5.81; arrow). b The delayed fused image shows more uptake at her vertebra (SUVmaxD = 7.28, RI = 25; arrow) than does the early image. c CT imaging showed bone destruction in both the right aspect of the vertebral body and the posterior elements, and faint calcification was seen in the soft-tissue mass
ROC analyses were performed to compare the roles of the SUVmaxE and RI in the differentiation of malignant from benign lesions. The areas under curves (AUCs) for the SUVmaxE and RI were 0.597 (95%CI 0.511–0.707) and 0.757 (95%CI 0.622–0.816), respectively. The AUCs for RI were statistically higher than those for the SUVmaxE (P = 0.03).
Discussion
The usefulness of F-18 FDG has been reported in the distinction of benign from malignant bone tumors [6, 7, 20]. However, other reports [10] raised the question of a clear correlation between F-18 FDG accumulation and the malignant potential of bone tumors. A high accumulation of F-18 FDG has been reported in some benign bone diseases [9].
The current study showed that although the SUVmaxE in the malignant bone lesions was statistically higher than that in the benign lesions in total, a considerable overlap of SUVmaxE was observed between the two groups. This indicated that early F-18 FDG PET imaging (1 h post injection) might not give more information for the differentiation of benign from malignant intraosseous lesions. This result corresponds with the findings from Kole et al. [10, 11]. Kole et al. investigated the glucose metabolism in 19 malignant and 7 benign bone tumors with F-18 FDG PET. They found that there was a large overlap between the rate of glucose consumption (MRglc) of benign and malignant lesions and thus concluded that it was not possible to differentiate between benign and malignant bone tumors with F-18 FDG PET. However, other reports showed a clear distinction in the uptake of F-18 FDG between benign and malignant intraosseous lesions. Dehdashti et al. [8] studied 20 patients with intraosseous lesions and reported that the SUV was helpful in the diagnosis of benign and malignant intraosseous lesions. However, they investigated only 3 primary bone tumors and 12 metastatic lesions, so their patient group was not representative of patients with primary bone tumors. Shin et al. [20] analyzed the characteristics and amount of F-18 FDG uptake in 91 patients with soft tissue and bone tumors, and investigated the ability of F-18 FDG PET/CT to differentiate malignant from benign tumors. They found that F-18 FDG PET/CT reliably differentiated malignant soft tissue and bone tumors from benign ones using a cutoff level of 3.4 for SUVmax, although there were many false-positive and false-negative lesions. However, this was a retrospective study. Schulte et al. reported 202 histologically verified bone lesions and found a sensitivity of 93% and a specificity of 66.7% using a cutoff level of 3.0 for the tumor-to-background ratio. They concluded that F-18 FDG PET provides a promising tool for estimating the biologic activity of skeletal lesions. But, they also found that aggressive benign lesions could not be distinguished from sarcomas, and their data showed that the sensitivity and specificity depended on the variety of histologic subtypes included in a study and on the cutoff level defined by the investigators.
The different histologic subtypes of lesions included in various studies and the different number of cases in these reports may be the cause of the varying opinions on the value of early F-18 FDG PET. The known false-positive lesions include giant cell tumor, osteoid osteoma, histiocytosis-X, chondroblastoma, enchondroma, and non-ossifying fibroma in benign tumors. The false-negative malignant tumors were chondrosarcomas [9, 21, 22]. In the current study, benign lesions such as giant cell tumors, tuberculosis, Langerhans cell histiocytosis, osteomyelitis, osteoblastoma, fibrous dysplasia, and fracture showed a particularly high uptake of F-18 FDG. Some of these benign lesions, such as giant cell tumors, Langerhans cell histiocytosis, and osteoblastoma can be categorized as “histiocytic or giant-cell-containing lesions” and are known to show locally aggressive features in conventional radiological imaging, such as ill-defined margins, interrupted periosteal reactions, and extensive surrounding edema [21]. Histiocytes and giant cells in tissues are in the monocyte–macrophage lineage [23]. Macrophages play a central role in the host response to injury and infection, and their energy is predominantly supplied by means of intracellular glucose metabolism. Although it is still controversial whether histiocytic cells and giant cells in primary bone tumors are reactive or neoplastic, these cells might partially contribute to the high uptake of F-18 FDG in the benign bone lesions [9]. Increased F-18 FDG uptake might similarly reflect the local aggressiveness of these benign bone lesions, but the results revealed no additional information helpful in their differentiation from malignant tumors. The high uptake of F-18 FDG in fibrous dysplasia lesions may be attributed to fibroblasts, which are the predominant proliferating cells of fibrous dysplasia lesions and are known to show a relatively high accumulation of F-18 FDG [24]. Our study indicated that the SUVmaxE in some fibrous dysplasia lesions were less than 2.5. The difference in the SUVmaxE among fibrous dysplasia lesions may be due to a difference in the amount of actively proliferating fibroblasts [21]. Active granulomatous processes, such as tuberculosis and eosinophilic granuloma, have been reported to accumulate F-18 FDG. Granulomatous lesions are characterized by cellular infiltrates, granuloma formation, and macrophage proliferation. Activated inflammatory cells have markedly increased glycolysis and the hexose monophosphate shunt is stimulated by phagocytosis, with increases of 20–30 times baseline values common in these stimulated cells [25–27]. In our study, one of three fracture lesions showed a high uptake of F-18 FDG (SUVmaxE = 3.6). This was proven to be an acute benign fracture. The reason why acute benign fractures have a high uptake of F-18 FDG may be related to the inflammatory infiltrate associated with the acute healing process. Our results are similar to other reports. Bredella et al. [28] reported the usefulness of F-18 FDG PET for the differentiation of benign and malignant fracture. Their study showed that the range of SUVmaxE for benign fracture was 0.7 to 4.9.
This study also indicated that dual-time point F-18 FDG PET may provide more help in differentiating malignant bone tumors from benign ones. The results in this study demonstrated that there was a difference in the RI between benign and malignant lesions and the areas under curves (AUCs) for RI were statistically higher than those for the SUVmaxE. This suggests that dual-time point imaging may provide more information than early imaging in the differentiation of benign and malignant bone lesions.
To our knowledge, there are only a few reports of the quantitative analysis of delayed PET imaging in differentiating malignant bone lesions from benign ones. Sahlmann et al. [18] investigated glucose metabolism in 17 patients with chronic bacterial osteomyelitis and 4 patients with malignant bone lesions by using a dual-time point F-18 FDG PET. They concluded that dual-time point F-18 FDG PET may be of value in the differentiation between chronic bacterial osteomyelitis and malignant bone lesions. However, the patient number in the study was small and they only investigated one kind of benign bone lesion.
Our findings are consistent with other results of the role of dual-time point F-18 FDG PET for the differentiation of malignant lesions from benign ones. Xiu et al. analyzed the retention index (RI) of 46 patients with pulmonary nodules with borderline levels of increased F-18 FDG activity on the initial PET scan. They found that the dual-time point imaging yielded the most accurate result when an RI threshold of 10% was used [17]. Mavi et al. investigated 152 patients with newly diagnosed breast cancer who underwent dual-time point imaging for reoperative staging. They found that the sensitivity and accuracy was improved in dual-time point imaging [16].
In head and neck cancer patients, a 12% change of the SUVmax between two PET scans was noted in malignant cancer, whereas benign inflammatory lesions and physiologic F-18 FDG uptake lesions showed a stable pattern over time, or a slight decline [29]. There are also other reports which claimed that dual-time point F-18 FDG PET might have the capability to differentiate malignant lesions from benign ones [15, 30].
Although numerous studies demonstrated the value of dual-time point F-18 FDG PET in the differentiation of malignant lesions from benign ones, there are other reports which claimed that dual-time point F-18 FDG PET might have a limited capability to differentiate malignant lesions from benign ones. Hamada et al. [31] found that there was no significant difference in the RI between malignant and benign soft-tissue lesions and indicated that the delayed F-18 FDG PET scan might not help to differentiate malignant soft-tissue tumors from benign ones. However, there was bias in their case selection.
Although the results in our study demonstrated that there was a difference in the RI between benign and malignant lesions, there was overlap between the two groups. Three of 16 myeloma lesions and 2 of 14 metastatic lesions showed decreased uptakes of F-18 FDG in the delayed image. In the benign group, a particularly high RI was observed in TB and chronic osteomyelitis. The reasons for the overlap are not clear.
Our study has limitations. In particular, the number of histologic subtypes of lesions in this study is not sufficient to analyze according to subgroups. In a future study, the inclusion of a large number of lesions in each subtype is recommended. Besides, in this study, CT was used only as attenuation correction, although its additional value in the differential diagnosis of benign vs. malignant primary bone lesions with F-18 FDG PET/CT has been reported recently [32].
Conclusions
Dual-time point F-18 FDG PET is practical in the daily work routine. This study indicated that dual-time point F-18 FDG PET may provide more help in differentiating malignant tumors from benign ones. According to the results of this study, dual-time point F-18 FDG PET was recommended for the evaluation of unclear bone lesions. The inclusion of a larger number of cases, including those with different histologic subtypes, is recommended for future studies.
References
Erlemann R. MRI is highly sensitive for the detection of bone marrow abnormalities, cortical destruction or soft tissue tumors adjacent or infiltrating neighboring bones. Eur J Radiol 2006; 58(1): 48–67.
Hillner BE, Siegel BA, Shields AF, et al. Relationship between cancer type and impact of PET and PET/CT on intended management: findings of the National Oncologic PET Registry. J Nucl Med 2008; 49(12): 1928–1935.
Kim SK, Allen-Auerbach M, Goldin J, et al. Accuracy of PET/CT in characterization of solitary pulmonary lesions. J Nucl Med 2007; 48(2): 214–220.
Dimitrakopoulou-Strauss ASL, Heichel T, Wu H, Burger C, Bernd L, Ewerbeck V. The role of quantitative 18F-FDG PET studies for the differentiation of malignant and benign bone lesions. J Nucl Med 2002; 43(4): 510–518.
Kern KA, Brunetti A, Norton JA, et al. Metabolic imaging of human extremity musculoskeletal tumors by PET. J Nucl Med 1988; 29(2): 181–186.
Adler LP, Blair HF, Makley JT, et al. Noninvasive grading of musculoskeletal tumors using PET. J Nucl Med 1991; 32(8): 1508–1512.
Griffeth LK, Dehdashti F, McGuire AH, et al. PET evaluation of soft-tissue masses with fluorine-18 fluoro-2-deoxy-d- glucose. Radiology 1992; 182(1): 185–194.
Dehdashti FSB, Griffeth LK, Fusselman MJ, Trask DD, McGuire AH. Benign versus malignant intraosseous lesions: discrimination by means of PET with 2-[F-18]fluoro-2-deoxy-d-glucose. Radiology 1996; 200(1): 243–247.
Aoki J, Watanabe H, Shinozaki T, et al. FDG PET of primary benign and malignant bone tumors: standardized uptake value in 52 lesions. Radiology 2001; 219(3): 774–777.
Kole AC, Nieweg OE, Hoekstra HJ, van Horn JR, Koops HS, Vaalburg W. Fluorine-18-fluorodeoxyglucose assessment of glucose metabolism in bone tumors. J Nucl Med 1998; 39(5): 810–815.
Schulte M, Brecht-Krauss D, Heymer B, et al. Grading of tumors and tumorlike lesions of bone: evaluation by FDG PET. J Nucl Med 2000; 41(10): 1695–1701.
Zhuang H, Pourdehnad M, Lambright ES, et al. Dual time point 18F-FDG PET imaging for differentiating malignant from inflammatory processes. J Nucl Med 2001; 42(9): 1412–1417.
Lan XL, Zhang YX, Wu ZJ, Jia Q, Wei H, Gao ZR. The value of dual time point 18F-FDG PET imaging for the differentiation between malignant and benign lesions. Clinical radiology 2008; 63(7): 756–764.
Matthies A, Hickeson M, Cuchiara A, Alavi A. Dual time point 18F-FDG PET for the evaluation of pulmonary nodules. J Nucl Med 2002; 43(7): 871–875.
Ma S-Y, See L-C, Lai C-H, et al. Delayed 18F-FDG PET for detection of paraaortic lymph node metastases in cervical cancer patients. J Nucl Med 2003; 44(11): 1775–1783.
Mavi A, Urhan M, Yu JQ, et al. Dual time point 18F-FDG PET imaging detects breast cancer with high sensitivity and correlates well with histologic subtypes. J Nucl Med 2006; 47(9): 1440–1446.
Xiu Y, Bhutani C, Dhurairaj T, et al. Dual-time point FDG PET imaging in the evaluation of pulmonary nodules with minimally increased metabolic activity. Clin Nucl Med 2007; 32(2): 101–105.
Sahlmann CO, Siefker U, Lehmann K, Meller J. Dual time point 2-[18F]fluoro-2′-deoxyglucose positron emission tomography in chronic bacterial osteomyelitis. Nucl Med Commun 2004; 25(8): 819–823.
Daisuke U, Shinya S, Masanori I, et al. Added value of SPECT/CT fusion in assessing suspected bone metastasis: comparison with scintigraphy alone and nonfused scintigraphy and CT. Radiology 2005; 238(11): 264–271.
Shin DS, Shon OJ, Han DS, Choi JH, Chun KA, Cho IH. The clinical efficacy of 18F-FDG-PET/CT in benign and malignant musculoskeletal tumors. Ann Nucl Med 2008; 22(7): 603–609.
Aoki J, Endo K, Watanabe H, Shinozaki T, Yanagawa T, Ahmed AR, et al. FDG-PET for evaluating musculoskeletal tumors. J Orthop Sci 2003; 8(3): 435–441.
Feldman F, Heertum RV, Manus C. 18FDG PET scanning of benign and malignant musculoskeletal lesions. Skeletal Radiol 2003; 32: 201–208.
Meszaros K, Lang CH, Bagby GJ, Spitzer JJ. Contribution of different organs to increased glucose consumption after endotoxin administration. J Biol Chem 1987; 262(23): 10965–10970.
Strobel K, Bode B, Lardinois D, Exner U. PET-positive fibrous dysplasia—a potentially misleading incidental finding in a patient with intimal sarcoma of the pulmonary artery. Skeletal Radiology 2007; 36: 24–28.
Conill C, Setoain X, Colomo L, et al. Diagnostic efficacy of bone scintigraphy, magnetic resonance imaging, and positron emission tomography in bone metastases of myxoid liposarcoma. J Magn Reson Imaging 2008; 27(3): 625–628.
Goo JM, Im J-G, Do K-H, et al. Pulmonary tuberculoma evaluated by means of FDG PET: findings in 10 cases. Radiology 2000; 216(1): 117–121.
Knight SB, Delbeke D, Stewart JR, Sandler MP. Evaluation of pulmonary lesions with FDG-PET: comparison of findings in patients with and without a history of prior malignancy. Chest 1996; 109(4): 982–988.
Bredella MA, Essary B, Torriani M, Ouellette HA, Palmer WE. Use of FDG-PET in differentiating benign from malignant compression fractures. Skeletal Radiol 2008; 37(5): 405–413.
Hustinx R, Smith RJ, Benard F, Rosenthal DI, Machtay M, Farber LA, et al. Dual time point fluorine-18 fluorodeoxyglucose positron emission tomography: a potential method to differentiate malignancy from inflammation and normal tissue in the head and neck. Eur J Nucl Med 1999; 26(10): 1345–1348.
Yoshihiro N, Yuka Y, Naruhide K, Shinya I, Yasuhiro S, Ohkawa M. Dual-time-point FDG-PET for evaluation of lymph node metastasis in patients with non-small-cell lung cancer. Ann Nucl Med 2008; 22: 245–250.
Hamada K, Tomita Y, Ueda T, et al. Evaluation of delayed 18F-FDG PET in differential diagnosis for malignant soft-tissue tumors. Ann Nucl Med 2006; 20(10): 671–675.
Strobel K, Exner UE, Stumpe KD, Hany TF, Bode B, Mende K, et al. The additional value of CT images interpretation in the differential diagnosis of benign vs. malignant primary bone lesions with 18F-FDG-PET/CT. Eur J Nucl Med Mol Imaging 2008; 35(11): 2000–2008.
Acknowledgments
We thank BioMed Proofreading for assistance in editing the manuscript. We also thank Luyi Zhou, Ph.D., for biostatistical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tian, R., Su, M., Tian, Y. et al. Dual-time point PET/CT with F-18 FDG for the differentiation of malignant and benign bone lesions. Skeletal Radiol 38, 451–458 (2009). https://doi.org/10.1007/s00256-008-0643-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-008-0643-0