Abstract
Objective:
The management of non-contrast-enhancing brain tumours largely depends on biopsy, which allows a differentiation of low-grade gliomas (LGG) from high-grade gliomas (HGG). The aim of this study was to compare positron emission tomography using 2-[18F]-fluoro-2-deoxy-d-glucose (FDG-PET) and O-(2-[18F]-fluoroethyl)-l-tyrosine (FET-PET) in terms of providing target regions for biopsies.
Materials and methods:
Fifteen consecutive patients with newly diagnosed brain tumours (n = 11) or suspected recurrence of a known LGG (n = 4), in whom MRI demonstrated no contrast enhancement, were studied by both FET-PET and FDG-PET. FET-PET, FDG-PET and MRI data were fused, and then transferred to the neurosurgical navigation system, prior to neurosurgical interventions.
Results:
Histology showed HGG (WHO grade III) in 6/15 and LGG (WHO grade II) in 9/15 patients. FET-PET revealed an increased intratumoural tracer uptake in 8/9 LGG and in 5/6 HGG. FDG-PET depicted hypermetabolic spots in 2/9 LGG and in 4/6 HGG. In 6 patients we observed an increased intratumoural uptake of both tracers. In 4 of them, the area of highest FET accumulation in the tumour corresponded to the focus of increased FDG uptake.
Conclusions:
FET-PET appears to be superior to FDG-PET for biopsy planning in non-contrast-enhancing brain tumours. FDG-PET does not provide any additional information in this issue.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The prognosis and further treatment of patients with primary brain tumours depends on the grade of malignancy. Because gliomas are heterogeneous and frequently contain both anaplastic and low-grade parts, the correct estimation of tumour grade by stereotactic biopsy can be challenging. As the most malignant portion determines the prognosis, it is fairly important not to miss a biopsy in this part of the tumour. Final diagnosis and therapeutic strategy is often misled when biopsy is taken from the low-grade area [1, 2]. The current practice in glioma surgery is that certain parts of the tumour are vaporized by ultrasound aspirators. Thus, the histological diagnosis is based on the investigation of specimens, selected by the neurosurgeon, so that even in extensive tumours, only small tissue fractions are available for neuropathological review. Magnetic resonance imaging (MRI) using enhancement with gadolinium-DTPA has evolved as the standard diagnostic tool for assessing brain neoplasms due to its excellent soft tissue contrast [3]. However, MRI is of limited value for tumour grading, for selection of a representative biopsy site and for planning of tumour resection [1, 4, 5]. From the clinical point of view, planning of neurosurgical interventions is challenging, especially in those patients who present with non-enhancing brain masses in the MRI, taking into consideration that 40 to 45% of these tumours contain anaplastic areas [6–9].
Positron emission tomography using 2-[18F]-fluoro-2-deoxy-d-glucose (FDG-PET) was proposed as an alternative imaging method for targeting stereotactic biopsy [10–13]. FDG-PET was shown to be useful in estimating tumour grade and prognosis of gliomas, but has some important limitations. The detection of hypo- or isometabolic tumours is often hampered by the high glucose metabolism in the cerebral cortex [14, 15]. Measures of increasing the contrast between tumours and grey matter, like delayed imaging [16] or scanning under hyperglycaemia [17], were proposed to improve the detectability of gliomas by FDG-PET. However, the lack of FDG accumulation in low-grade gliomas represents a principal limitation of this tracer [14]. Moreover, our previous study showed that 30% of malignant gliomas fail to show an increased FDG uptake and some oligodendrogliomas (WHO grade II) present with glucose hypermetabolism [18]. PET using amino acid tracer 11С-methionine (MET) was shown to be more useful than FDG-PET for biopsy targeting of gliomas [14, 19–22]. The 11C-methionine, however, is of limited availability due to the short physical half-life (20 min) of the 11C isotope and can therefore be used only in a few PET centres, equipped with a cyclotron. A synthetic amino acid tracer O-(2-[18F]-fluoroethyl)-l-tyrosine (FET), which is labelled with 18F (half-life, 110 min), represents a reasonable alternative to 11C-MET [23, 24]. Although FET is not incorporated into proteins, and its accumulation only reflects amino acid transport, the uptake of FET has been shown to be closely correlated with that of 11C-methionine [25]. A recent study by Pauleit et al. showed a superior sensitivity of FET as compared to FDG for providing biopsy targets in brain tumours [26]. From the clinical point of view, planning of neurosurgical interventions is especially challenging in those patients who present with non-enhancing tumours in the MRI. Therefore, in this prospective investigation we focused on the comparative usefulness of FDG-PET and FET-PET for biopsy guidance in these cases.
Material and methods
Patients
Included were 15 consecutive patients (nine females, six males; mean age 44 years, range 26–65 years) with newly diagnosed brain tumours (n = 11) or suspected recurrence of a known low-grade glioma after gross total resection (n = 3) or partial resection and radiotherapy (n = 1), inwhom MRI demonstrated no contrast enhancement. The inclusion criterion was a Karnofsky index ≥70 (patients who at least care for themselves, but are unable to perform normal activity or active work). None of the patients received steroid medication prior to surgery or had epileptic seizures within 7 days prior to FDG-PET. Patient characteristics and pretreatment data are given in Table 1. The institutional ethics committee approved this study. Written informed consent for the PET examinations was obtained from all patients.
Methods
MR imaging
MR imaging of the skull was performed with the use of a head coil at a 1.5-T system (1.5 T Signa, General Electric, Milwaukee, USA, or 1.5 T Philips Gyroscan ACS NT, Philips, Best, Holland) or a high-field MR system at 3.0 T using the quadratic head coil (3.0 T Signa, General Electric, Milwaukee, USA). T2-weighted spin-echo sequences as well as T1-weighted magnetization-prepared rapid-acquired gradient echoes (MPRAGE) and fluid-attenuated inversion-recovery sequences (FLAIR) were acquired. MPRAGE images were obtained without and after intravenous application of gadolinium-DTPA (Magnevist®, Schering AG, Berlin) at a dose of 0.1 mmol/kg of body weight.
PET imaging
FDG- and FET-PET were performed on separate days. The time interval between the FET-PET and FDG-PET was less than 4 weeks (less than 1 week in 8/15 cases). The patients were on a low-protein diet for 12 h prior to FET-PET investigation and fasted 12 h prior to FDG-PET. Blood glucose levels on the day of FDG-PET were less than 5.6 mmol/L in all cases. FDG-PET started after an uptake phase of 60 min; FET-PET was performed 10 min after tracer injection. For acquisition, the patients were positioned in a dedicated positioning device for the head with an additional cushion and bandages for fixation.
Five out of 15 FDG-PET investigations and 6/15 FET-PET investigations were performed by using an ECAT-EXACT 47 PET system (Siemens, Erlangen, Germany). The administered activities of FDG and FET for mono-PET measurements were 370 MBq and 250 MBq, respectively. For attenuation correction, “hot” transmission data were measured for 10 min using a 68Ge/68 Ga rod source. Thereafter, two-dimentional static emission data were acquired for 30 min. PET images were reconstructed by filtered back projection using a Hanning filter (cut-off 0.4) into a 128 × 128 matrix using a zoom of 2. All subsequent PET data were obtained in three-dimensional mode using a hybrid PET/CT system consisting of a multislice CT and a full-ring PET system (Biograph 16 Siemens, Erlangen, Germany); the crystal and block dimensions were equal to those of the ECAT-EXACT detectors, but different detector materials were used (LSO vs. BGO). Hence, intrinsic spatial resolution can be expected to be comparable. The administered activities of FDG and FET for PET/CT measurements were 300 MBq and 200 MBq, respectively. An unenhanced CT image (detector collimation 16 × 1.5 mm; tube current 100 mAs; tube voltage 120 kV; gantry rotation time 0.8 s) covering the entire head was performed for attenuation correction. Static PET data were acquired in two bed positions (10 min per bed position) from the base of the skull to the vertex. PET emission data were reconstructed iteratively (OSEM algorithm, 6 iterations, 16 subsets) using a 128 × 128 matrix and a zoom of 2. For both PET systems, reconstructed images were resliced to a pixel slice of 2 mm and a plane separation of 2 mm.
Image fusion and data analysis
First, the 3D MRI data of each patient were transferred to a Siemens LEONARDO workstation and fused with FET-PET and FDG-PET data using the “3D volume viewer”. The automatic fusion of PET and MRI data implemented in this software package is based on rigid transformation using normalized mutual information. The data analysis and definition of biopsy target was performed by using fused FET-PET, FDG-PET and MRI data. The region with maximum FET and FDG uptake, the differences in FET and FDG uptake, and relation of PET findings to signal alterations in the MRI were estimated visually by an experienced nuclear medicine physician (MP). In the case of FET-PET, areas of abnormal FET uptake that was higher than the surrounding non-tumour-affected brain tissue were used for target selection. In the case of FDG-PET, biopsy target was defined as the area of abnormal FDG uptake that was higher than the surrounding white matter or, in cortical or subcortical lesions, higher than the adjacent grey matter. FET-PET was used for target selection when there was no increased intratumoural FDG uptake or when FDG uptake was not distinguishable from the metabolic activity in the grey matter.
Stereotactic and neuronavigated biopsy
All surgery was performed by the same neurosurgeon (FS). The target setting for biopsy took place in a corporate setting (FS and MP). Tumour resection was not indicated in five patients, so that serial stereotactic biopsies from the area of maximum FET and/or FDG uptake were performed by using the Riechert–Mundinger head ring. For biopsy planning the MPRAGE and PET/(PET/CT) data were transferred to the multiplanar trajectory planning software and were fused with the CT or MRI image (Stryker Stereotaxy, Kalamazoo, MI). The frame-based stereotactic technique as used in our department provides a high precision of less than 2-mm deviation [27] resulting in a diagnostic accuracy of about 96% [28, 29].
Ten patients were candidates for primary tumour resection. MPRAGE MRI or CT data were fused with FDG- and FET-PET data using the neuronavigation planning software (Brainlab VectorVision2, Heimstätten, Germany). For target setting, the region of maximum FDG and FET uptake was marked and navigated biopsies were taken immediately after opening of the dura in order to avoid brain shift after partial tumour resection. The biopsy forceps (Decker Rongeurs, Codman, Raynham, MA), providing 2 × 6 mm specimens, were registered in the optical neuronavigation system as previously described [30].
In both the stereotactic and the neuronavigated procedure, the neuropathologist performed frozen section examinations of the specimens to ensure diagnostic suitability of the material.
Finally, the remaining tumour bulk was investigated by histology to assess whether any regions of increased malignancy were present.
Results
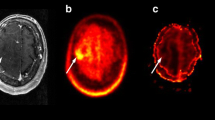
PET-guided stereotactic biopsies were performed in five patients and neuronavigated resections in 10 patients (Figs. 1 and 2). Histology showed HGG (WHO grade III) in 6/15 and LGG (WHO grade II) in 9/15 patients. Investigation of the remaining tumour bulk did not reveal any regions of higher malignancy. FET-PET showed an increased intratumoural tracer uptake in 8/9 LGG and in 5/6 HGG. FDG-PET depicted hypermetabolic foci in 2/9 LGG and in 4/6 HGG. Thus, FET-PET provided a biopsy target in 13/15 patients (86%), whereas FDG-PET allowed a target delineation in only 6/15 patients (40%). The comparison of PET findings in relation to histology is shown in Table 2. In six patients we observed an increased intratumoural uptake of both tracers. In four of them, the area of highest FET accumulation in the tumour corresponded to the focus of increased FDG uptake. In another patient with LGG, a focus of increased FDG uptake was inside a more extensive area of a homogeneous FET uptake. In one patient with HGG, FET-PET demonstrated two tumour lesions (both detectable as “hot spots”), only one of which showed an increased FDG uptake. Both lesions were biopsied and verified as HGG by histology. In two patients (one with LGG, another one with HGG), neither FET-PET nor FDG-PET demonstrated an increased tracer uptake. Tumours with increased FDG uptake, but absent FET uptake were not observed.
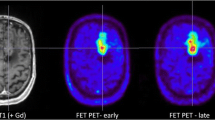
A 29-year-old female patient (no. 8) with a non-contrast-enhancing lesion in the left frontal lobe as shown by MRI (A). FET-PET (C) and fused FET-PET/MRI images (B) demonstrate a focus with increased amino acid uptake in the rostral part of the lesion (B, C). On the FDG-PET image (E) and fused FDG-PET/MRI images (D), a corresponding area of preserved intratumoural glucose metabolism is detectable, although its differentiation from an unaffected cortical area appears somewhat challenging. After PET-guided tumour resection, an anaplastic astrocytoma (WHO grade III) was diagnosed by histology
A 39-year-old female patient (no. 4) presented with a recurrent oligodendroglioma WHO grade II as a non-contrast-enhancing lesion in the left frontal lobe on MRI (A). FET-PET (C) and fused FET-PET/MRI images (B) show a circumscribed area of increased amino acid uptake, whereas FDG-PET (E) detects a decreased glucose metabolism in the lesion (D). FET-PET images were used for biopsy selection during the neuronavigated tumour resection; histology confirmed an oligodendroglioma WHO grade II
Discussion
The feasibility of the FET-PET-guided stereotactic biopsy or resection using FET-PET information was shown previously [18, 23, 31, 32]. In the present study we focused on non-contrast-enhancing brain tumours, since biopsy targeting is particularly difficult in this subgroup [5–7]. Our results showed that FET-PET is clearly superior to FDG-PET for selecting of biopsy targets. Indeed, in seven out of 15 studied patients the biopsy targeting was based on the results of FET-PET only (FDG-PET was negative in all these cases, see Table 2). Importantly, FDG-PET failed to detect a malignant area in one of these seven patients. A low sensitivity of FDG-PET for detection of malignant transformation of gliomas was already demonstrated by our previous data [18]. As shown by Pirotte et al. for MET [19], the areas with highest FET and FDG uptake were concordant in tumours (n = 6), which showed an increased glucose metabolism (Fig. 1). However, in one case (patient no. 9), FDG-PET failed to detect the second tumour in another brain region. Recently, Pauleit and co-workers [26] compared FDG-PET and FET-PET in 52 patients with cerebral tumours. In the subgroup of 43 glioma patients a focal maximum was reported in 76% using FET and only 35% for FDG. These findings are strongly in line with our results, with diagnostic yield for FET and FDG of 86% and 40%, respectively. In the subgroup of 28 non-enhancing brain lesions the authors found nine high-grade gliomas of WHO grade III or IV (32%), which again matches with our observation of 40% high-grade gliomas. However, some important differences between two studies are noteworthy. First, in our setting FDG- and FET-PET images were performed on separate days, which might be advantageous from the theoretical point of view, as such a study design excludes interference between the tracers. The fact that our data confirm the results by Pauleit et al. provides evidence that almost simultaneous images with FDG and FET do not influence the results of each individual tracer. The second and the most important distinction is that in our investigation a strict protocol implementing PET-guided stereotaxy or neuronavigation ensured biopsies from regions with raised tracer uptake, increasing the validity of our data. Finally, we could confirm the previous data in a different institution, with a different study protocol and patient set.
MET-PET has been evaluated for biopsy targeting more thoroughly than FET-PET. Thus, Pirotte et al. showed that integration of MET-PET in the procedure of stereotactic biopsy allowed a PET-guided target selection in all 32 studied patients, whereas FDG-PET was of limited ability to define a target, showing hypermetabolic lesions in only 14 patients of the same group [20]. Importantly, areas with highest MET and FDG uptake were concordant in those gliomas, which showed an increased glucose metabolism. The value of MET-PET for targeting the biopsy is supported by the results of Roessler et al. [33]: an increased MET uptake was detected in 26 of 27 gliomas, and allowed detection of anaplasia in five gliomas showing no Gd enhancement in MRI. The uptake in gliomas and the image contrast of FET appear to have similarities to those of MET [25]. However, some differences between the tracers must be taken into consideration. FET is not incorporated into proteins like MET and its accumulation only reflects amino acid transport [24]. On the other hand, FET accumulates to a significantly greater extent in tumour cells than in macrophages [34] and, therefore, seems to reflect the tumour metabolism more specifically than MET. Thus, the available data on the clinical value of MET-PET for biopsy targeting in gliomas cannot be transferred onto FET studies without certain restrictions. In analogy to MET, FET exhibits a variable uptake pattern in LGG, showing no increased accumulation in approximately one-third of them [9]. Recently, we showed that FET uptake correlated with vascular and tumour cell density in non-Gd-enhancing gliomas [35]. Therefore, the FET-PET-based target setting for biopsy may be impossible in tumours with low cellularity and low neoangiogenesis rate, because of the lack of intratumoural tracer uptake.
Use of membrane transport tracers [11C]-choline or [18F]-choline represents an alternative approach for biopsy targeting in brain tumours. Hara et al. evaluated feasibility of using both tracers for PET-guided stereotactic biopsy sampling and found an increased uptake of both tracers in 11/12 of histologically confirmed gliomas [36]. The comparison of these tracers with FET and MET should be a subject of further investigations. Other fluorinated tracer compounds such as phenylalanine derivates 3-O-methyl-6-[18F]-fluoro-DOPA (OMFD) and [18F]-dihydroxyphenylalanine (DOPA), cis-4-[18F]-fluoro-l-proline (cis-FPro), nucleotide analogue 3′-deoxy-3′-[18F]-fluorothymidine (FLT) or hypoxia tracer [18F]-fluoromisonidazole (FMISO) were proposed for brain tumour imaging [37–41]. Until now, no data on their usefulness for the biopsy planning were published. Out of these tracers, phenylalanine derivates and FMISO seem promising and worth being further evaluated. An important disadvantage of cis-FPro and FLT is the dependence of their uptake on blood–brain barrier (BBB) disruption, which limits its utility for imaging of gliomas. Some authors advocate use of SPECT with an unspecific tumour marker 201Tl for biopsy planning in gliomas [42]. However, the value of this method appears limited because of a low spatial resolution of the SPECT technique and low specificity of 201Tl for tumour detection [43, 44].
Conclusion
FET-PET provides valuable complementary information on metabolism of non-Gd-enhancing brain tumours, allowing biopsy targeting in the majority of patients. Because of a higher sensitivity in tumour detection, FET is more suitable for target selection than FDG.
References
Glantz MJ, Burger PC, Herndon JE, Friedman AH, Cairncross JG, Vick NA, Schold SC Jr (1991) Influence of the type of surgery on the histologic diagnosis in patients with anaplastic gliomas. Neurology 41:1741–1744
Brucher JM (1993) Neuropathological diagnosis with stereotactic biopsies. Possibilities, difficulties and requirements. Acta Neurochir 124:37–39
DeAngelis LM (2001) Brain tumors. N Engl J Med 344:114–123
Watanabe M, Tanaka R, Takeda N (1992) Magnetic resonance imaging and histopathology of cerebral gliomas. Neuroradiology 34:463–469
Byrne TN (1994) Imaging of gliomas. Semin Oncol 21:162–171
Ginsberg LE, Fuller GN, Hashmi M, Leeds NE, Schomer DF (1998) The significance of lack of MR contrast enhancement of supratentorial brain tumors in adults: histopathological evaluation of a series. Surg Neurol 49:436–440
Kondziolka D, Lunsford LD, Martinez AJ (1993) Unreliability of contemporary neurodiagnostic imaging in evaluating suspected adult supratentorial (low-grade) astrocytoma. J Neurosurg 79:533–536
Barker FG 2nd, Chang SM, Huhn SL, Davis RL, Gutin PH, McDermott MW, Wilson CB, Prados MD (1997) Age and the risk of anaplasia in magnetic resonance-nonenhancing supratentorial cerebral tumors. Cancer 80:936–941
Scott JN, Brasher PM, Sevick RJ, Rewcastle NB, Forsyth PA (2002) How often are nonenhancing supratentorial gliomas malignant? A population study. Neurology 59:947–949
Francavilla TL, Miletich RS, Di Chiro G, Patronas NJ, Rizzoli HV, Wright DC (1989) Positron emission tomography in the detection of malignant degeneration of low-grade gliomas. Neurosurgery 24:1–5
Goldman S, Levivier M, Pirotte B, Brucher JM, Wikler D, Damhaut P, Stanus E, Brotchi J, Hildebrand J (1996) Regional glucose metabolism and histopathology of gliomas. A study based on positron emission tomography-guided stereotactic biopsy. Cancer 78:1098–1106
Herholz K, Pietrzyk U, Voges J, Schroder R, Halber M, Treuer H, Sturm V, Heiss WD (1993) Correlation of glucose consumption and tumor cell density in astrocytomas. A stereotactic PET study. J Neurosurg 79:853–858
Wong TZ, van der Westhuizen GJ, Coleman RE (2002) Positron emission tomography imaging of brain tumors. Neuroimaging Clin N Am 12:615–626
Chung JK, Kim YK, Kim SK, Lee YJ, Paek S, Yeo JS, Jeong JM, Lee DS, Jung HW, Lee MC (2002) Usefulness of 11C-methionine PET in the evaluation of brain lesions that are hypo- or isometabolic on 18F-FDG-PET. Eur J Nucl Med Mol Imaging 29:176–182
Delbeke D, Meyerowitz C, Lapidus RL, Maciunas RJ, Jennings MT, Moots PL, Kessler RM (1995) Optimal cutoff levels of F-18 fluorodeoxyglucose uptake in the differentiation of low-grade from high-grade brain tumors with PET. Radiology 195:47–52
Spence AM, Muzi M, Mankoff DA, O'Sullivan SF, Link JM, Lewellen TK, Lewellen B, Pham P, Minoshima S, Swanson K, Krohn KA (2004) 18F-FDG PET of gliomas at delayed intervals: improved distinction between tumor and normal gray matter. J Nucl Med 45:1653–1659
Ishizu K, Nishizawa S, Yonekura Y, Sadato N, Magata Y, Tamaki N, Tsuchida T, Okazawa H, Miyatake S, Ishikawa M et al (1994) Effects of hyperglycemia on FDG uptake in human brain and glioma. J Nucl Med 35:1104–1109
Stockhammer F, Thomale UW, Plotkin M, Hartmann C, von Deimling A (2007) 18F-fluorodeoxyglucose uptake is associated with 1p and 19q loss in WHO grade II gliomas. J Neurosurg 106:633–637
Levivier M, Massager N, Wikler D, Lorenzoni J, Ruiz S, Devriendt D, David P, Desmedt F, Simon S, Van Houtte P, Brotchi J, Goldman S (2004) Use of stereotactic PET images in dosimetry planning of radiosurgery for brain tumors: clinical experience and proposed classification. J Nucl Med 45:1146–1154
Pirotte B, Goldman S, Massager N, David P, Wikler D, Lipszyc M, Salmon I, Brotchi J, Levivier M (2004) Combined use of 18F-fluorodeoxyglucose and 11C-methionine in 45 positron emission tomography-guided stereotactic brain biopsies. J Neurosurg 101:476–483
Goldman S, Levivier M, Pirotte B, Brucher JM, Wikler D, Damhaut P, Dethy S, Brotchi J, Hildebrand J (1997) Regional methionine and glucose uptake in high-grade gliomas: a comparative study on PET-guided stereotactic biopsy. J Nucl Med 38:1459–1462
Pirotte B, Goldman S, Massager N, David P, Wikler D, Vandesteene A, Salmon I, Brotchi J, Levivier M (2004) Comparison of 18F-FDG and 11C-methionine for PET-guided stereotactic brain biopsy of gliomas. J Nucl Med 45:1293–1298
Pauleit D, Floeth F, Hamacher K, Riemenschneider MJ, Reifenberger G, Müller HW, Zilles K, Coenen HH, Langen KJ (2005) O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain 128:678–687
Langen KJ, Hamacher K, Weckesser M, Floeth F, Stoffels G, Bauer D, Coenen HH, Pauleit D (2006) O-(2-[18F]fluoroethyl)-L-tyrosine: uptake mechanisms and clinical applications. Nucl Med Biol 33:287–294
Weber W, Wester HJ, Grosu AL, Herz M, Dzewas B, Feldmann HJ, Molls M, Stocklin G, Schwaiger M (2000) O-(2-[18F] fluoroethyl)-L-tyrosine and L-[methyl-11C] methionine uptake in brain tumours: Initial results of a comparative study. Eur J Nucl Med 27:542–549
Pauleit D, Stoffels G, Bachofner A, Floeth FW, Sabel M, Herzog H, Tellmann L, Jansen P, Reifenberger G, Hamacher K, Coenen HH, Langen KJ (2009) Comparison of (18)F-FET and (18)F-FDG PET in brain tumors. Nucl Med Biol 36:779–787
Bjartmarz H, Rehncrona S (2007) Comparison of accuracy and precision between frame- based and frameless stereotactic navigation for deep brain stimulation electrode implantation. Stereotact Funct Neurosurg 85:235–242
McGirt MJ, Villavicencio AT, Bulsara KR, Friedman AH (2003) MRI-guided stereotactic biopsy in the diagnosis of glioma: comparison of biopsy and surgical resection specimen. Surgical neurology 59:277–281, discussion 281-282
Kim JE, Kim DG, Paek SH, Jung HW (2003) Stereotactic biopsy for intracranial lesions: reliability and its impact on the planning of treatment. Acta Neurochir (Wien) 145:547–554, discussion 554-555
Muacevic A, Uhl E, Steiger HJ, Reulen HJ (2000) Accuracy and clinical applicability of a passive marker based frameless neuronavigation system. J Clin Neurosci 7:414–418
Messing-Junger AM, Floeth FW, Pauleit D, Reifenberger G, Willing R, Gärtner J, Coenen HH, Langen KJ (2002) Multimodal target point assessment for stereotactic biopsy in children with diffuse bithalamic astrocytomas. Childs Nerv Syst 18:445–449
Floeth FW, Pauleit D, Sabel M, Stoffels G, Reifenberger G, Riemenschneider MJ, Jansen P, Coenen HH, Steiger HJ, Langen KJ (2007) Prognostic value of O-(2-18Ffluoroethyl)-L-tyrosine PET and MRI in low-grade glioma. J Nucl Med 48:519–527
Roessler K, Gatterbauer B, Becherer A, Paul M, Kletter K, Prayer D, Hoeftberger R, Hainfellner J, Asenbaum S, Knosp E (2007) Surgical target selection in cerebral glioma surgery: linking methionine (MET) PET image fusion and neuronavigation. Minim Invasive Neurosurg 50:273–280
Stöber B, Tanase U, Herz M, Seidl C, Schwaiger M, Senekowitsch-Schmidtke R (2006) Differentiation of tumour and inflammation: characterisation of [methyl-3H]methionine (MET) and O-(2-[18F] fluoroethyl)-L-tyrosine (FET) uptake in human tumour and inflammatory cells. Eur J Nucl Med Mol Imaging 33:932–939
Stockhammer F, Plotkin M, Amthauer H, van Landeghem FK, Woiciechowsky C (2008) Correlation of F-18-fluoro-ethyl-tyrosin uptake with vascular and cell density in non-contrast-enhancing gliomas. J Neuro Oncol 88:205–210
Hara T, Kondo T, Hara T, Kosaka N (2003) Use of 18F-choline and 11C-choline as contrast agents in positron emission tomography imaging-guided stereotactic biopsy sampling of gliomas. J Neurosurg 99:474–479
Chen W, Silverman DH, Delaloye S, Czernin J, Kamdar N, Pope W, Satyamurthy N, Schiepers C, Cloughesy T (2006) 18F-FDOPA PET imaging of brain tumors: comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J Nucl Med 47:904–911
Beuthien-Baumann B, Bredow J, Burchert W, Füchtner F, Bergmann R, Alheit HD, Reiss G, Hliscs R, Steinmeier R, Franke WG, Johannsen B, Kotzerke J (2003) 3-O-methyl-6-[18F]fluoro-L-DOPA and its evaluation in brain tumour imaging. Eur J Nucl Med Mol Imaging 30:1004–1008
Langen KJ, Jarosch M, Hamacher K, Mühlensiepen H, Weber F, Floeth F, Pauleit D, Herzog H, Coenen HH (2004) Imaging of gliomas with cis-4-[18F]fluoro-L-proline. Nucl Med Biol 31:67–75
Jacobs AH, Thomas A, Kracht LW, Li H, Dittmar C, Garlip G, Galldiks N, Klein JC, Sobesky J, Hilker R, Vollmar S, Herholz K, Wienhard K, Heiss WD (2005) 18F-fluoro-L-thymidine and 11C-methylmethionine as markers of increased transport and proliferation in brain tumors. J Nucl Med 46:1948–1958
Valk PE, Mathis CA, Prados MD, Gilbert JC, Budinger TF (1992) Hypoxia in human gliomas: demonstration by PET with fluorine-18-fluoromisonidazole. J Nucl Med 33:2133–2137
Hemm S, Rigau V, Chevalier J, Picot MC, Bauchet L, El Fertit H, Rodriguez MA, Cif L, Vayssière N, Zanca M, Baldet P, Segnarbieux F, Coubes P (2005) Stereotactic coregistration of 201Tl SPECT and MRI applied to brain tumor biopsies. J Nucl Med 46:1151–1157
del Valle M, Torres MD, Gómez Rio M, Rodríguez Fernández A, Sabatel Hernandez G, Ortega Lozano S, Ramos Font C, Bellon Guardia M, López Ramírez E, Llamas Elvira JM (2004) Value of thallium 201-SPECT in typing brain space-occupying lesions. Rev Esp Med Nucl 23:330–337
Källén K, Heiling M, Andersson AM, Brun A, Holtås S, Ryding E, Rosén I (1997) Evaluation of malignancy in ring enhancing brain lesions on CT by thallium-201 SPECT. J Neurol Neurosurg Psychiatry 63:569–574
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Plotkin, M., Blechschmidt, C., Auf, G. et al. Comparison of F-18 FET-PET with F-18 FDG-PET for biopsy planning of non-contrast-enhancing gliomas. Eur Radiol 20, 2496–2502 (2010). https://doi.org/10.1007/s00330-010-1819-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-010-1819-2