Abstract
Purpose
Convection-enhanced delivery (CED) of paclitaxel is a new locoregional approach for patients with recurrent glioblastoma. The aim of this study was to evaluate O-(2-[18F]fluoroethyl)-l-tyrosine (FET) positron emission tomography (PET) in monitoring the effects of this type of direct drug delivery.
Methods
Eight patients with recurrent glioblastoma underwent CED of paclitaxel, which was infused over stereotactically placed catheters into the tumour. FET PET and MRI were performed before and 4 weeks after therapy and then at 3-month intervals to document follow-up. For quantitative evaluation, SUVmax(tumour)/SUVmean(background) ratios were calculated.
Results
At baseline all tumours showed gadolinium enhancement and high FET uptake (SUVmax/BG 3.2±0.8). Four weeks after CED, a statistically significant decrease in FET uptake was seen (SUVmax/BG −17%; p<0.01). During follow-up, no recurrence was observed within the CED area. Two out of eight patients with extended tumours died 4 and 5 months after treatment, most probably from local complications. Temporarily stable disease with stable FET uptake was observed in six of eight patients; this was followed by progression and increasing FET uptake ratios (+46%) distant from the CED area in five of the six patients 3–13 months after CED. One patient still presents stable FET uptake 10 months after CED. MRI showed unchanged/increasing contrast enhancement and oedema without ability to reliably assess disease progression.
Conclusion
FET PET is a valuable tool in monitoring the effects of CED of paclitaxel. In long-term follow-up, stable or decreasing FET uptake, even in contrast-enhancing lesions, is suggestive of reactive changes, whereas increasing ratios appear always to be indicative of recurrence. Therefore, FET PET is more reliable than MRI in differentiating stable disease from tumour regrowth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Glioblastomas represent one of the most rapidly proliferating malignancies [1, 2]. Standard treatment modalities like surgery and radiation therapy are unable to control tumour progression for a longer duration since in almost all cases a considerable number of occult, microscopic cell clusters located in the normal-appearing brain close to the tumour are left and become the starting point for tumour recurrence [3, 4]. In cases of recurrent glioblastoma, additional systemic chemotherapy has only a limited effect in lengthening survival, probably due to the poor penetration of chemotherapeutic drugs across the blood–brain barrier (BBB) [5]. In an attempt to bypass the BBB, various methods of intratumoural chemotherapy have been applied, including direct injection, chronic low-flow infusion and controlled release implants [6–8]. The efficacy of such intratumoural delivery of chemotherapeutic agents, however, is restricted by the poor diffusion of the drugs, which results in limited tissue penetration. Therapeutic drug levels are present in a very small volume surrounding the chemotherapeutic source. To overcome these limitations, a promising new approach to drug delivery, convection-enhanced delivery (CED), was introduced by Oldfield and colleagues in 1994 [9, 10]. This method is based on high-flow microinfusion, which generates a constant pressure gradient to ensure convective flow into the extracellular space. Compared with simple diffusion, this method enhances the distribution of drugs within the tumour and also to areas of normal-appearing brain already containing microscopic tumour infiltrates.
When employing this locoregional therapeutic approach, monitoring of therapeutic effects and differentiation of tumour regrowth from therapy-induced reactive lesions is a diagnostic challenge for neuro-imaging modalities. Diffusion-weighted magnetic resonance imaging (DWMRI) has been suggested to be suitable to continuously monitor the progression of the convection process [11]. Conventional magnetic resonance imaging (MRI) and computed tomography (CT) assess morphological parameters such as changes in the size of tumour or necrosis and in the extent of oedema or contrast enhancement. However, in the long-term follow-up, alterations in BBB properties are not always related to tumour regrowth; rather, they can also represent treatment-related inflammatory reactions or tumour necrosis, especially after locoregional drug administration [12, 13]. Functional imaging methods enable non-invasive visualisation of viable tumour and have therefore been proposed as an alternative for treatment monitoring and claimed to be more specific than structural imaging methods.
Positron emission tomography (PET) using radiolabelled amino acid analogues, particularly [methyl-11C]-l-methionine (MET) and O-(2-[18F]fluoroethyl)-l-tyrosine (FET), has been employed in metabolic studies of malignant brain tumours [14–20]. Amino acid uptake has been shown to be increased relative to normal brain tissue in most low- and high-grade tumours and is therefore suitable for brain tumour imaging [15, 17, 21–23]. PET using MET or FET has also been shown to reliably differentiate between tumour recurrence and post-therapeutic benign lesions after standard treatment modalities like surgery, external radiation therapy or chemotherapy [15, 20, 21, 24–26].
The aim of this study was to evaluate the diagnostic value of FET PET in monitoring the therapeutic effects on locoregional CED of paclitaxel, an antineoplastic agent with proven antimitotic and antitumour activity against gliomas [27–29].
Materials and methods
Approval for this phase II, open label, prospective clinical study was obtained from the local ethics committee.
Patients
Eight patients (six males, two females, mean age 53±10 years) with histopathologically proven recurrent glioblastomas (WHO grade IV) who had a measurable contrast-enhancing tumour on MRI without involvement of the corpus callosum, the brainstem or the cerebellum were included in the study. The main inclusion criteria were age (18–70 years), clinical status (Karnofsky performance status ≥70), the absence of any other concomitant life-threatening disease and the ability to comprehend and sign an informed consent. Prior radiotherapy or chemotherapy given within the previous 6 weeks, hepatic failure, predefined pre-treatment laboratory findings (neutrophil count <1,500, platelet count <100,000, total haemoglobin ≤8 g/dl and creatinine ≥3 mg/dl), known hypersensitivity to paclitaxel and presence of neuropathies such as multiple sclerosis were exclusion criteria. Patient and tumour characteristics are listed in Table 1.
For clinical follow-up, patients underwent clinical examinations in parallel with MRI and PET examinations in order to assess outcome and to monitor complications, e.g. focal neurological deficits.
Surgical procedure in preparation for CED
For treatment planning, preoperatively obtained MR images were co-registered to images obtained by stereotactic CT performed on the day of surgery. Taking into account the location and distribution of Gd enhancement, the stereotactic coordinates of one or two targets for biopsy were defined and documented. Then patients underwent stereotactic biopsy in the operating room. Provided that the diagnosis of recurrent glioblastoma was confirmed histopathologically from the stereotactic serial biopsies (smear preparations), the tip of a silicone catheter was stereotactically placed in the geometrical centre of the tumour mass to provide the best possible distribution of paclitaxel to all tumour areas. The catheters were tunneled subcutaneously and secured to the scalp. Each catheter was subcutaneously connected to its own port, which was linked to an external drug pump (Medfusion 2010 syringe pump). In all patients, antibiotics were routinely given preoperatively according to the standard of care in neurosurgical procedures. After completion of the surgical procedure, the patients underwent CT scanning again to ensure the appropriate placement of the catheters at the desired target. Afterwards, patients were transferred to the neurosurgical intensive care unit to initiate treatment.
Convection-enhanced delivery
Paclitaxel was administered continuously via the placed catheter(s) into the tumour for 120 h with a constant flow rate of 0.3 ml/h and, at first, a concentration of 0.5 mg paclitaxel/ml. The concentration was reduced to 0.25 mg paclitaxel/ml after the first four patients because of side-effects, including skin necrosis and development of extensive brain oedema. After completion of the treatment, the catheters were removed.
Magnetic resonance imaging
All MRI investigations were performed on a high-resolution 1.5-T system (Siemens Symphony) using a circularly polarised head coil. Conventional MRI examinations consisting of axial T2- and T1-weighted [± gadolinium (Gd)] sequences with a slice thickness of 5 mm were obtained before and 4 weeks after paclitaxel CED and at 3-month intervals thereafter for the purpose of follow-up. For documentation of CED, in addition DWMRI was performed at day 4 of treatment and 1 day after the end of treatment since this technique has previously been shown [11] to provide information on the extent of the convection wave, which cannot be derived from conventional MR images.
Positron emission tomography
FET PET scans were obtained with a Siemens ECAT EXACT HR+ scanner at the same time points as conventional MRI (before and 4 weeks after CED, and then at 3-month intervals). Patients fasted for a minimum of 6 h before injection of FET to ensure standardised metabolic conditions. The scanner acquires 63 contiguous transaxial planes, simultaneously covering a 15.5 cm axial field of view. After a 15-min transmission scan (68Ge sources), 180 MBq [18F]FET was injected intravenously. Five frames of 10 min duration each were acquired from 10 to 60 min post injection (128×128 matrix, 3D acquisition). Images were reconstructed by filtered back-projection using a Hann filter and were corrected for scatter and attenuation. From the raw data, finally three added 10-min frames were used for homogeneous data evaluation covering the period from 20 to 50 min post injection. For further evaluation, data were transferred to a HERMES work station (Hermes Medical Solutions, Sweden). As described earlier, for quantitative evaluation a manually drawn, large region of interest (ROI) including all tumour areas was established [20]. Next, the slice with the highest FET uptake was determined by scrolling slice by slice. For this slice the maximal SUV (SUVmax) was calculated. Background information (SUVmean) was derived from a 70% isocontour ROI mirrored to the opposite, non-tumour-bearing hemisphere. Quantitative evaluation was based on the ratio of SUVmax within the tumour to SUVmean of the background ROI (SUVmax/BG).
For further regional assessment, all corresponding MRI and PET scans were co-registered using the Multi Modality Software package. On these images, a circular ROI (diameter arbitrarily set to 2 cm) was positioned on four consecutive slices surrounding the tip of the catheter, thus covering the CED area, which in all patients showed a fluid-like signal on MRI on day 4 of CED. In those patients who developed recurrence distant from the CED area, another volume of interest (VOI), consisting of irregular ROIs on four consecutive slices, was drawn on the latest PET scan, which covered the area of increased FET uptake. Then both the VOI covering the CED area and the VOI covering the recurrent tumour were applied to all available scans for each patient and the mean SUVmax/BG ratios were calculated to document follow-up.
Statistical analysis
For statistical analysis of changes in FET uptake during follow-up investigations, paired t-test analysis was used. Data were considered statistically significant when p<0.05.
Results
At baseline, prior to CED of paclitaxel, all patients showed pathological contrast enhancement on MRI as well as high FET uptake by recurrent glioblastoma, with a mean SUVmax/BG ratio of 3.2±0.8. The geometrical centre of the tumour assessed by MRI corresponded to the area of highest FET uptake in only three of the eight patients. In one patient with two implanted catheters, only one was located in the high uptake area while the other was located within tumour necrosis. In four of the eight patients, maximum FET uptake was located eccentrically and therefore was not directly affected by the catheter tip (mean SUVmax/BG surrounding the tip before therapy: 2.5±0.6).
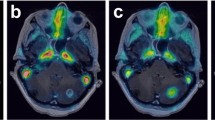
DWMRI, obtained at day 4 and 1 day after the end of CED, showed a hyperintense signal which was believed to illustrate the convection wave. Convection was mostly observed in the white matter, with less signal within the grey matter. Most interestingly, the distribution of paclitaxel as visualised by DWMRI co-localised with the treatment-induced changes in FET uptake on follow-up PET scans, as illustrated in Fig. 1. On visual reading, areas showing no convection on DWMRI presented higher local FET uptake during follow-up, whereas areas with successful CED of paclitaxel presented lower FET uptake.
DWMR images (left side) of a glioblastoma patient (case no. 6) obtained 1 day after CED of paclitaxel show a higher signal spreading from the resection cavity located in the right temporoparietal area to the white matter compared with the cortex. Fusion with FET PET images reveals higher FET uptake in this cortical area, which shows less convection (red arrow). Co-registered MRI and FET PET images (right side) show the extent of perifocal oedema (T2-w), of contrast enhancement surrounding the tumour cavity (T1-w with Gd) and of viable tumour tissue ventrolateral to the cavity (FET PET)
Therapeutic details, results of FET PET and clinical outcome for all patients are summarised in Table 2.
Four weeks after CED of paclitaxel, FET PET results demonstrated at least a short-term therapeutic effect as documented by a statistically significant decrease in overall FET utilisation (mean SUVmax/BG 2.6±0.8; −17%; p<0.01) and especially in FET uptake within the area surrounding the catheter tip (mean SUVmax/BG 2.0±0.4; −20%; p<0.01) in all patients.
During further follow-up, no tumour regrowth was seen within the immediate vicinity of the catheter tip, as documented by a stable FET uptake ratio within the respective VOI between 4 weeks after therapy and the last follow-up investigation.
Two out of eight patients presented with clinical deterioration early after CED therapy. Both patients showed increasing necrosis after therapy, documented by an increasing defect size on MRI and PET accompanied by markedly increasing perifocal oedema. Also, overall FET uptake ratios had decreased slightly in both patients 4 weeks after therapy (SUVmax/BG −11%). These patients died 4 and 5 months after therapy, most probably from local complications.
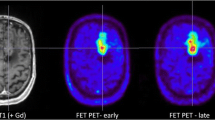
Six out of eight patients clinically presented with temporarily stable disease followed in five cases by development of recurrence 3, 7, 9, 12 and 13 months after therapy, in all instances distant from the CED area. During the clinically stable period, MRI demonstrated stable or even increasing extent of contrast enhancement and oedema surrounding the tumour. FET PET, however, showed stable or even slightly decreasing values during the temporarily stable follow-up period. At the time of progression, FET uptake ratios increased in the area of recurrent tumour tissue distant from the CED area (SUVmax/BG +46%). In this group, patients died 9, 10, 13, 15 and 16 months after therapy. A representative example of a patient with stable PET findings within the CED area and progression distant from the CED area is shown in Fig. 2. One patient unfortunately decided to discontinue the follow-up investigations 12 months after therapy; therefore the final location of recurrence was unknown. During the stable period, however, MRI demonstrated an increasing extent of Gd enhancement as well as markedly increasing oedema 6 months after therapy, which was interpreted as clear tumour progression. In contrast, PET showed unchanged or even decreasing FET uptake ratios, indicating stable disease. After initiation of high-dose steroid therapy, oedema decreased again and the FET PET diagnosis was confirmed by stable follow-up (Fig. 3).
MRI and PET images of a temporarily stable patient (case no. 7). a High FET uptake and Gd enhancement of the recurrent tumour surrounding the resection cavity located in the left temporal lobe before CED (B). Follow-up studies one (1), three (3), six (6) and nine (9) months after CED show nearly unchanged FET uptake and MRI findings at the level of the catheter tip (CED area). b Distant from the CED area (lower left temporal lobe), tumour progression is seen on FET PET images nine months after therapy (9), where previously no tumour was visible. The SUVmax/BG ratios within the CED area (a) and within the area of tumour regrowth (b) are listed on the right
MRI and PET images of a clinically stable patient (case no. 5) show high FET uptake and Gd enhancement of recurrent glioblastoma located in the right frontotemporal area before CED (B). MRI shows an increasing extent of Gd enhancement as well as markedly increasing perifocal oedema at the 1- and 3-month follow-ups (1 and 3), with midline shift at 6 months (6) after treatment, clearly indicating tumour progression. PET shows unchanged FET uptake, indicating stable disease, which was confirmed by stable follow-up and decrease in MRI changes following steroid therapy. The respective SUVmax/BG ratios of overall FET uptake are listed on the right
One of the six patients has presented with clinically stable disease with a post-therapy observation period now extending to 10 months. In the absence of signs of progression, this patient has shown a decreasing FET uptake ratio (SUVmax/BG −30%) compared with baseline and stable uptake compared with the 4-week control.
There was no surgery-related morbidity or mortality. Side-effects were seen coincidentally with the intracerebral infusion. Temporary worsening of (pre-existing) neurological symptoms was seen in five of the eight patients, with permanent worsening in one. Two patients experienced poor wound healing due to a leakage of paclitaxel through one of the burr holes. Two patients experienced neurological deterioration due to markedly increased perifocal oedema, which could be adequately treated with steroids. There were no signs of any systemic toxicity.
Discussion
To our knowledge, this is the first study to have used metabolic imaging to monitor the effects of CED of a chemotherapeutic agent (in this case, paclitaxel) in patients with recurrent glioblastoma. Paclitaxel is a novel antitumour alkaloid derived from Taxus species which has been tested for its anti-migrational, anti-invasive and anti-proliferative effect on human glioma cells [28] and which has shown significant clinical potency against human brain tumours [27–29]. Since paclitaxel has been shown not to cross the BBB, this chemotherapeutic agent is suited only to therapeutic regimens that make use of local delivery methods. Compared with other methods of intratumoural chemotherapy of brain tumours [6, 8], the bulk flow properties of CED permit safe, reliable and homogeneous distribution of an infusate over predefined volumes in the CNS [9], which is not limited by molecular weight, concentration or diffusivity [10]. The direct convective infusion of paclitaxel therefore permits a tissue penetration depth that allows treatment of even distant spread of disease [11, 30]. In the rat glioma model, CED of various chemotherapeutic agents has shown a positive effect on tumour size and survival times [31, 32]. To our knowledge, only one group has thus far reported on CED of paclitaxel for the treatment of recurrent glioblastoma in humans. In a phase I/II clinical study of 15 patients, a beneficial effect on survival time was observed after this treatment [30]. The same group had previously reported on the use of DWMRI to monitor the immediate response to CED in a small sample of three patients [11]. However, no report to date has correlated clinical or MRI findings after CED of chemotherapeutic agents in brain tumours with the results of functional imaging methods like PET. Therefore, in this study we applied FET PET to monitor the effects of CED of paclitaxel and to relate them to clinical findings and MRI results.
In all our patients, the convection process was visualised by DWMRI images during and immediately after treatment. The feasibility of this approach has been previously shown in the same setting [11, 30]. In our patients, convection was predominantly observed in the white matter, most probably following the path of least resistance along fibre tracts inside white matter. Less convection was seen within the grey matter. These findings are in line with the results of Voges et al. [33], who infused Gd-DTPA intracerebrally as a surrogate for CED. They also observed Gd distribution mostly in the white matter, and no distribution within the cortical regions. Most interestingly, the distribution of paclitaxel as visualised by the DWMRI images co-localised with the treatment-induced changes in FET uptake on follow-up PET scans. Cortical areas showing no convection on DWMRI presented higher local FET uptake in the follow-up compared with areas showing a high signal on DWMRI, indicating more successful distribution of paclitaxel. This first impression, based on visual assessment, needs to be further substantiated in a larger group of patients by directly relating the area of CED with post-therapeutic FET uptake. For this purpose, a strategy has to be developed to objectively assess the extent of DWMRI signal.
Our preliminary data indicate that FET PET is suitable to monitor the response of recurrent glioblastomas to intratumoural CED of paclitaxel. The statistically significant decrease in overall FET uptake 4 weeks after therapy, and particularly the long-term persisting decrease in FET uptake within the CED area in all patients, was interpreted as a therapeutic effect indicating a decrease in tumour viability. However, whether these observations might in part have resulted from a more severe disruption of the BBB following CED (which is indicated by increasing contrast enhancement on MRI) has yet to be answered. Hypothetically, a more severe disruption of the BBB could allow easier back-diffusion especially of unbound FET out of the tumour cells, which might lead to a decrease in FET uptake ratios in the corresponding area during the late acquisition period used (20–50 min p.i.). Thus, an aggravation of the disruption of the BBB may theoretically have contributed to the reduction in FET uptake observed particularly early after CED of paclitaxel. However, since increasing FET uptake ratios during follow-up were seen in areas with tumour regrowth, which also presented with increasing contrast enhancement on MRI, the degree of BBB disruption is unlikely to be the only explanation for decreasing FET uptake ratios. In all cases, increasing FET uptake ratios were a valuable indicator of tumour regrowth. Unfortunately, however, neither the initial fall in FET uptake, which was observed in all patients independent of later outcome, nor the intensity of FET uptake before and after CED allowed prediction of the clinical outcome. It is of note that none of the patients showed tumour regrowth within the immediate vicinity of the catheter tip during follow-up. Unfortunately, under the current protocol the catheter tip was placed in the geometrical centre of the tumour mass as assessed by MRI. This corresponded completely to the area of highest FET uptake in only three of the patients, while it did so partially in one further patient. Whether therapy would have been even more beneficial had the catheter tip been placed in the area of highest FET uptake in all the patients is uncertain.
Clinical presentation and outcome in the patients studied so far has been heterogeneous. Two of the eight patients showed rapid clinical worsening early after therapy, resulting in death at 4 or 5 months after therapy. Both patients had presented with an extensive recurrent tumour mass, large necrotic areas and perifocal oedema before CED. Furthermore, both were treated with the higher concentration of paclitaxel. Since FET uptake showed no significant increase during clinical worsening in these two patients, it might be speculated that rapid enlargement of necrosis and increasing oedema, rather than tumour regrowth, were the cause of death. Moreover, CED seems to be less effective in large tumours with large necrotic portions than in small tumours without necrotic areas. This has previously been suggested by Lidar et al., who reported that large necrotic areas within a tumour led to an arrest of convection and absence of subsequent tumour response [30].
A temporarily stable course of disease was seen in six of the eight patients. During this period, PET showed an initial decrease in FET uptake 4 weeks after therapy, with stable or even further slight reductions thereafter; by contrast, contrast enhancement and oedema in MR images, which actually increased in some of the cases, clearly suggested tumour recurrence. PET, therefore, seems to be superior to conventional MRI in the assessment of reactive lesions induced by CED of paclitaxel. Development of recurrent tumour distant from the CED area in five of the six patients with temporarily stable disease was accompanied by increasing FET uptake. In one of these patients, tumour progression started as early as 3 months after therapy. In this case the tumour was located near the left ventricle and post-therapeutic DWMRI showed that the convection wave stopped at the border of the ventricle. This perhaps explains the minor response to therapy in this patient. Lidar et al. also observed that the convection wave appeared to stop when reaching a cystic cavity such as the ventricular system or a tumour cyst [30]. As mentioned above, one of eight patients is clinically stable without progression after an observation period of 10 months.
Thus, FET PET seems to fulfil the clinical requirement for a method that is sensitive enough to assess therapeutic effects early on and to distinguish between harmless reactive lesions induced by this type of treatment and tumour regrowth. In the latter context, stable or even decreasing FET uptake ratios during long-term follow-up, even in patients with increasing Gd enhancement or perifocal oedema on conventional MRI, pointed to a stable course of disease whereas increasing FET uptake ratios always indicated tumour regrowth. These findings are in line with other preliminary results from studies on FET PET for monitoring the effects of intralesional radioimmunotherapy [34] and also on MET PET for monitoring the effects of systemic chemotherapy [24] or brachytherapy with 125I seeds [15], which similarly indicated that PET using amino acid tracers is sensitive enough to indicate therapeutic efficacy during and after different treatment modalities.
Conclusion
FET PET has been shown to be a valuable tool in monitoring the effects of CED of paclitaxel, which, based on our first experiences, seems to be an effective locoregional therapeutic approach in patients with recurrent glioblastoma. This inference is supported by the observation of a statistically significant decrease in FET uptake early after this new type of drug delivery and the absence of any increase of FET uptake within the immediate vicinity of the catheter tip. For long-term follow-up, FET PET seems to be a reliable tool to differentiate reactive, therapy-induced changes in stable disease from those induced by tumour regrowth. In contrast, conventional MRI in this context is often hampered by reactive alterations of the BBB and increasing extent of oedema following this local approach.
In the future, catheter placement should take into account the area of highest FET uptake rather than the geometrical centre of the tumour mass. This approach may further improve treatment since most therapeutic effects were seen in the vicinity of the catheter tip. For patients showing more than one area with high focal FET uptake, two or more catheters could be placed as necessary. After CED of paclitaxel, sensitive assessment of tumour regrowth with FET PET may play an important role in early planning of retreatment.
References
Black PM. Brain tumors: part 1. N Engl J Med 1991;324:1471–6.
Black PM. Brain tumor: part 2. N Engl J Med 1991;324:1555–64.
Harbaugh KS, Black PM. Strategies in the surgical management of malignant gliomas. Semin Surg Oncol 1998;14:26–33.
Devaux BC, O’Fallon JR, Kelly PJ. Resection, biopsy, and survival in malignant glial neoplasms. A retrospective study of clinical parameters, therapy, and outcome. J Neurosurg 1993;78:767–75.
Brandes AA, Fiorentino MV. The role of chemotherapy in recurrent malignant gliomas: an overview. Cancer Investig 1996;14:551–9.
Read TA, Thorsen F, Bjerkvig R. Localised delivery of therapeutic agents to CNS malignancies: old and new approaches. Curr Pharm Biotechnol 2002;3:257–73.
Patchell RA, Regine WF, Ashton P, Tibbs PA, Wilson D, Shappley D, et al. A phase I trial of continuously infused intratumoral bleomycin for the treatment of recurrent glioblastoma multiforme. J Neurooncol 2002;60:37–42.
Westphal M, Lamszus K, Hilt D. Intracavitary chemotherapy for glioblastoma: present status and future directions. Acta Neurochir, Suppl 2003;88:61–7.
Lieberman DM, Laske DW, Morrison PF, Bankiewicz KS, Oldfield EH. Convection-enhanced distribution of large molecules in gray matter during interstitial drug infusion. J Neurosurg 1995;82:1021–9.
Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A 1994;91:2076–80.
Mardor Y, Roth Y, Lidar Z, Jonas T, Pfeffer R, Maier SE, et al. Monitoring response to convection-enhanced taxol delivery in brain tumor patients using diffusion-weighted magnetic resonance imaging. Cancer Res 2001;61:4971–3.
Kumar AJ, Leeds NE, Fuller GN, Van Tassel P, Maor MH, Sawaya RE, et al. Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology 2000;217:377–84.
Earnest F, Kelly PJ, Scheithauer BW, Kall BA, Cascino TL, Ehman RL, et al. Cerebral astrocytomas: histopathologic correlation of MR and CT contrast enhancement with stereotactic biopsy. Radiology 1988;166:823–7.
Weber WA, Wester HJ, Grosu AL, Herz M, Dzewas B, Feldmann HJ, et al. O-(2-[18F]fluoroethyl)-l-tyrosine and l-[methyl-11C]methionine uptake in brain tumours: initial results of a comparative study. Eur J Nucl Med 2000;27:542–9.
Voges J, Herholz K, Holzer T, Wurker M, Bauer B, Pietrzyk U, et al. 11C-methionine and 18F-2-fluorodeoxyglucose positron emission tomography: a tool for diagnosis of cerebral glioma and monitoring after brachytherapy with 125I seeds. Stereotact Funct Neurosurg 1997;69:129–35.
Ogawa T, Inugami A, Hatazawa J, Kanno I, Murakami M, Yasui N, et al. Clinical positron emission tomography for brain tumors: comparison of fludeoxyglucose F 18 and l-methyl-11C-methionine. Am J Neuroradiol 1996;17:345–53.
Kaschten B, Stevenaert A, Sadzot B, Deprez M, Degueldre C, Del Fiore G, et al. Preoperative evaluation of 54 gliomas by PET with fluorine-18-fluorodeoxyglucose and/or carbon-11-methionine. J Nucl Med 1998;39:778–85.
Goldman S, Levivier M, Pirotte B, Brucher JM, Wikler D, Damhaut P, et al. Regional methionine and glucose uptake in high-grade gliomas: a comparative study on PET-guided stereotactic biopsy. J Nucl Med 1997;38:1459–62.
Derlon JM, Bourdet C, Bustany P, Chatel M, Theron J, Darcel F, et al. [11C]l-methionine uptake in gliomas. Neurosurgery 1989;25:720–8.
Popperl G, Gotz C, Rachinger W, Gildehaus FJ, Tonn JC, Tatsch K. Value of O-(2-[18F]fluoroethyl)-l-tyrosine PET for the diagnosis of recurrent glioma. Eur J Nucl Med Mol Imaging 2004;31:1464–70.
Wurker M, Herholz K, Voges J, Pietrzyk U, Treuer H, Bauer B, et al. Glucose consumption and methionine uptake in low-grade gliomas after iodine-125 brachytherapy. Eur J Nucl Med 1996;23:583–6.
Ogawa T, Shishido F, Kanno I, Inugami A, Fujita H, Murakami M, et al. Cerebral glioma: evaluation with methionine PET. Radiology 1993;186:45–53.
Herholz K, Holzer T, Bauer B, Schroder R, Voges J, Ernestus RI, et al. 11C-methionine PET for differential diagnosis of low-grade gliomas. Neurology 1998;50:1316–22.
Herholz K, Kracht LW, Heiss WD. Monitoring the effect of chemotherapy in a mixed glioma by C-11-methionine PET. J Neuroimaging 2003;13:269–71.
Tsuyuguchi N, Sunada I, Iwai Y, Yamanaka K, Tanaka K, Takami T, et al. Methionine positron emission tomography of recurrent metastatic brain tumor and radiation necrosis after stereotactic radiosurgery: is a differential diagnosis possible? J Neurosurg 2003;98:1056–64.
Lilja A, Lundqvist H, Olsson Y, Spannare B, Gullberg P, Langstrom B. Positron emission tomography and computed tomography in differential diagnosis between recurrent or residual glioma and treatment-induced brain lesions. Acta Radiol 1989;30:121–8.
Walter KA, Cahan MA, Gur A, Tyler B, Hilton J, Colvin OM, et al. Interstitial taxol delivered from a biodegradable polymer implant against experimental malignant glioma. Cancer Res 1994;54:2207–12.
Terzis AJ, Thorsen F, Heese O, Visted T, Bjerkvig R, Dahl O, et al. Proliferation, migration and invasion of human glioma cells exposed to paclitaxel (taxol) in vitro. Br J Cancer 1997;75:1744–52.
Cahan MA, Walter KA, Colvin OM, Brem H. Cytotoxicity of taxol in vitro against human and rat malignant brain tumors. Cancer Chemother Pharmacol 1994;33:441–4.
Lidar Z, Mardor Y, Jonas T, Pfeffer R, Faibel M, Nass D, et al. Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: a phase I/II clinical study. J Neurosurg 2004;100:472–9.
Bruce JN, Falavigna A, Johnson JP, Hall JS, Birch BD, Yoon JT, et al. Intracerebral clysis in a rat glioma model. Neurosurgery 2000;46:683–91.
Kaiser MG, Parsa AT, Fine RL, Hall JS, Chakrabarti I, Bruce JN. Tissue distribution and antitumor activity of topotecan delivered by intracerebral clysis in a rat glioma model. Neurosurgery 2000;47:1391–8.
Voges J, Reszka R, Gossmann A, Dittmar C, Richter R, Garlip G, et al. Imaging-guided convection-enhanced delivery and gene therapy of glioblastoma. Ann Neurol 2003;54:479–87.
Popperl G, Gotz C, Rachinger W, Gildehaus FJ, Holtmannspoetter M, Tonn JC, et al. [18F]FET PET for monitoring the effects of intralesional radioimmunotherapy in patients with malignant glioma. Eur J Nucl Med Mol Imaging 2003;30:S189–90.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pöpperl, G., Goldbrunner, R., Gildehaus, F.J. et al. O-(2-[18F]fluoroethyl)-l-tyrosine PET for monitoring the effects of convection-enhanced delivery of paclitaxel in patients with recurrent glioblastoma. Eur J Nucl Med Mol Imaging 32, 1018–1025 (2005). https://doi.org/10.1007/s00259-005-1819-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-005-1819-7