Abstract
Introduction
Classically, the first step in the clinical development of drugs in oncology involves assessments of dose limiting toxicity (DLT) and maximum tolerated dose (MTD). New paradigms are needed for antiangiogenic drugs and vascular disrupting agents (VDAs) as they are active at doses well below the MTD and as single agents their use might not translate into anti-tumour efficacy. MRI is able to assess the antivascular effects of antivascular drugs via changes in functional kinetic parameters; however, the usefulness of MRI in decision making has been questioned by many.
Objectives
Our aim is to review the experience of using dynamic contrast-enhanced MRI (DCE-MRI) in early clinical development of vascular directed anticancer therapies over the last decade. Thirty-nine phase I and II studies including data on more than 700 patients have been published as abstracts and/or papers, documenting DCE-MRI changes after the administration of antiangiogenic drugs and VDAs.
Discussion
Perfusion MRI is helpful in assessing whether mechanistic goals are achieved, in assisting dose selection for phase II studies, in selecting subpopulations enriched for response and in predicting patient benefit. Imaging tools are increasingly available. Future challenges for imaging include correlation with clinical measures of efficacy and determining relationships with blood and serum biomarkers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2003, J.M. Collins from the US Food and Drug Administration (FDA) commented in an editorial that the main purpose of phase I trial designs is to guide more rigorous assessments in phase II, where the main design parameters are dose and schedule [1]. He posed a series of questions designed to assess whether perfusion imaging added value to the transition of antiangiogenic agents from phase I to II. These were:
-
1.
Did imaging help to assess whether mechanistic goals were achieved?
-
2.
Did imaging assist dose selection for phase II?
-
3.
Can imaging select subpopulations enriched for response?
-
4.
Are imaging tools widely available for a broad range of probes?
In this article, we will provide a summary of the experience of using functional MRI in the early clinical development of vascular directed anticancer therapies in the last decade and will address these questions directly for two major classes of drugs [antiangiogenic agents and vascular disrupting agents (VDAs)]. An additional question will be addressed, which was not posed by Collins, because it was not relevant to phase I studies but a question posed by many investigators and patients:
-
5.
Can imaging tools predict patient benefit?
As a general background, the clinical development of drugs in oncology traditionally involves the assessment of dose limiting toxicity (DLT) and determination of maximum tolerated dose (MTD) in phase I trials by dose escalation, based on the paradigm that the highest applicable dose will result in the greatest anti-tumour effect. MTD is then taken forward into single-arm phase II trials with response rate as primary end-point. This paradigm appears outdated for drugs with novel anticancer therapies with cytostatic properties such as antiangiogenic drugs and VDAs for a number of reasons including: (1) in experimental tumours it has been shown that most VDAs are active at doses well below the MTD, (2) some DLTs may not be related to antivascular activity, (3) vascular disrupting activity as single agents might not readily translate into anti-tumour efficacy when used in combination therapy with conventional therapies (e.g. chemotherapy and radiation), (4) efficacy of antiangiogenic and VDA treatments appears to vary between tumour types, stage and between patients and (5) clinical experience shows that VDAs in particular have significant toxicity including tumour pain, fistula formation and neuropathy with a narrow safety window. Thus, optimal treatment with antiangiogenic drugs and VDAs requires information on the biology and functional status of the tumour vasculature before and during therapy. There are as yet no validated serum or circulating cellular surrogate markers of angiogenesis, which would allow easy monitoring of activity and efficacy of antiangiogenic drugs and VDAs in patients [2]. Non-invasive imaging characterization of the angiogenic status of tumours may allow rational selection of antiangiogenic agents and VDA treatments in terms of appropriateness, for dose selection and scheduling.
Goals for imaging in drug development
A number of clinically applicable imaging techniques are able to assess the antivascular effects of antivascular drugs via changes induced in functional kinetic parameters. These techniques include dynamic contrast-enhanced MRI (DCE-MRI), dynamic susceptibility-enhanced MRI (DSC-MRI), diffusion MRI, PET with oxygen-labelled water, perfusion/functional CT and dynamic microbubble-enhanced ultrasound. Each of these techniques yields quantitative or semi-quantitative kinetic parameters, which can be related to one or more of the following vascular characteristics: blood flow, blood volume, extraction fraction and microvessel permeability. Changes in these imaging biomarkers can be used during the drug development process because they can serve as pharmacodynamic (PD) indicators of vascular activity in vivo.
Functional vascular imaging techniques have found roles in early drug development (pre-clinical and phase I), whereas morphological imaging methods have maintained their dominance for later phase clinical studies. Thus, phase I studies now go beyond defining just pharmacokinetics (PK) and MTD, with hypothesis testing on mode of action of drugs becoming normal. The information gained using PD biomarkers are used for internal decision making on compound development, either to drop failing compounds or to accelerate development of promising agents. In order to achieve these aims it is first necessary to understand clearly the clinical/pharmaceutical goals in phase I clinical trials and to define corresponding objectives to be met by PD biomarkers. Thus, objectives in pre-clinical and early clinical studies that deploy PD imaging biomarkers could include:
-
1.
To demonstrate mechanism of action in vivo usually via modulation of kinetic parameters when the drug is given.
-
2.
To show drug dose-vascular response relationships which can in turn enable the definition of a biologically active dose.
-
3.
To identify the therapeutic dose window, which lies between the MTD (clinically defined) and biologically active dose (imaging defined). If in vivo studies are well conducted then it may also be possible to define a dosing schedule to be taken into phase II.
-
4.
To demonstrate drug exposure-tumour efficacy relationships by correlating PK (blood concentration of the drug) with PD measurements.
From an imaging perspective, drugs targeting the tumour vasculature can be broadly divided into antiangiogenic drugs and VDAs. At the time this manuscript was written, 39 phase I and II studies had been published as abstracts and/or papers that evaluated the MR imaging effects of antiangiogenic (Table 1) and vascular disrupting (Table 2) drugs on tumour vascularity, including data on more than 700 patients. Studies have been done in different clinical settings including (1) phase I clinical trials in heavily pre-treated patients, (2) studies where antiangiogenic agents are administered as monotherapy and (3) studies where antiangiogenic agents are administered with conventional therapies (usually cytotoxic chemotherapy). In the latter category, a few studies can be found where the first cycle of therapy is the antiangiogenic agent alone, with subsequent cycles having combination treatment. The majority of clinical studies have used MRI for the assessment of tumour vascularity with the most commonly used technique being DCE-MRI. DCE-MRI is thus the focus of this review.
As will be shown, the effects of antiangiogenic drugs and VDAs on DCE-MRI kinetic vascular parameters have been found to be similar with the dominant effect of successful therapy being reductions in blood flow and permeability. Importantly, it is the timing of the onset and duration of vascular changes that enable antiangiogenic drugs and VDAs to be distinguished on imaging. Both xenograft and human imaging studies of antiangiogenic drugs show that antivascular effects are not immediate, arising at least 1–2 days post-drug administration. In contrast, VDAs cause rapid shutdown of the vasculature within minutes to hours of drug administration and reversibility of effects being visible in the short term (usually seen within 24–48 h). “Normalisation” of the vasculature induced by antiangiogenic drugs as described originally by Jain [3] can be detected on DCE-MRI and is evidenced by regional increases in non-enhancing pixels (that is vascular pruning) with reductions in permeability and leakage space. Improved flow in non-pruned vessels can also be detected often in other regions. So it is the combination of vascular pruning, reductions in vascular permeability and leakage space, and regional increases in flow that suggest that “normalisation” is occurring on DCE MRI [4, 5].
DCE-MRI: the technology
There are numerous MRI ways of assessing the functional properties of tissue vasculature, including techniques that make use of exogenously administered contrast agents. A full discussion of these techniques is beyond the scope of this article and interested readers are directed to the article of Brix et al. in this Supplement and a recent review of these methods [6]. In this chapter, we focus on contrast-enhanced MRI methods that use commercially available low molecular weight contrast agents because this is the most commonly used clinical MRI technique.
Dynamic MRI involves the acquisition of serial images before, during and after the intravenous injection of a contrast agent. MR contrast agents leak at variable rates through the vasculature, with leakage rates being dependent on the charge and size of the contrast medium molecules relative to the size of vascular pores. The temporal resolution requirements of dynamic MRI techniques are related to how quickly the contrast medium leaves the vascular compartment. Thus, temporal resolutions for low molecular weight contrast media are relatively fast (in the order of 5–20 s), whereas for larger contrast agents, temporal resolutions in the order of 1–2 min may be adequate to observe permeability. If tissue perfusion is of interest, then temporal resolution requirements are greater (usually in the order of 1–2 s).
Clinical dynamic MRI is usually performed using low molecular weight gadolinium chelate-based contrast agents (molecular weight <1 kDa). When these contrast agents are used, two distinct phenomena can be observed, depending on the experimental set-up. Dynamic relaxivity-based contrast techniques use a rapid series of T1-weighted images to observe the passage of contrast media, usually resulting in tissue “brightening”; by default, this technique is referred to as DCE-MRI. This technique is sensitive to the presence of contrast medium both within vessels and in the extravascular, extracellular space—the latter predominates due to the low blood volumes in tissues and tumours (approximately 5–10%). Conversely, if very fast (every 1–2 s) susceptibility weighted or T2*-weighted sequences are used to monitor contrast medium passage, then transient “darkening” of tissue is observed during the first and second pass of contrast media through tissues. Because this technique is sensitive to the presence of concentrated contrast medium within the vascular space, it is usually referred to as dynamic susceptibility-weighted MRI (DSC MRI).
For full data quantification (see below), it is usually necessary to obtain, or to estimate, an arterial input function (AIF) (Fig. 1). This can be achieved by measuring signal intensity changes in arteries near to the anatomical location of the organs or tumour being studied and can be performed before or at the same time as the dynamic data acquisitions. If accurately measured, the AIF helps to compensate for changes related to the rate of injection and the cardiac output of patients. To enable quantification of signal intensity changes, it is also necessary to incorporate methods that allow concentration of contrast agent to be obtained at each time point during the measurement period. For DCE-MRI, this is often done by obtaining “T1 maps” prior to contrast medium injection, which effectively allows conversion of the MR signal intensity into contrast agent concentration. For DSC MRI, conversion of signal intensity changes into contrast agent concentration is more problematic and it is for this reason that measurements of blood flow and blood volume are prefaced with the term “relative”: relative blood flow (rBF) and relative blood volume (rBV).
Body compartments accessed by low molecular weight, gadolinium-containing contrast media injected intravenously. AIF arterial input function. These images are consistent with the phenomenon of “pseudo-response” where there is an apparent decrease in enhancement because of vascular normalisation and re-establishment of the blood-brain barrier and yet the tumour is unchanged
Quantification of DCE-MRI
Dynamic contrast images can be analysed by quantitative model dependent or semi-quantitative (non-model dependent) means. The parameters extracted provide information on blood flow, blood volume, microvessel permeability, extraction fraction, and on plasma and interstitial volumes. PK analysis of DCE-MRI is the most widely used method of measuring vessel permeability changes, analyses typically being derived from variations of the Tofts’ two-compartment kinetic model (Fig. 1) which, in turn, has its roots in Kety’s dynamic model [7, 8]. In this model, an injected contrast agent leaks into the extravascular-extracellular space (EES) and assessments of tissue perfusion and permeability can be derived from the shape of the observed wash-in and wash-out curves. The transfer constant Ktrans (often called wash-in rate; unit: min-1) describes the forward leakage rate of the contrast medium. For blood vessels where leakage is rapid (that is when extraction fraction during the first pass of the contrast agent is high, as typically found in tumours), perfusion will determine contrast agent distribution and Ktrans approximates to tissue blood flow per unit volume [9].
There are circumstances where transport out of the vasculature does not significantly deplete intravascular contrast medium concentration (that is tissue with a lower first pass extraction fraction). This is typically found in some brain tumours which have a largely intact blood-brain barrier, but also occurs in extracranial tumours after treatment with chemotherapy and/or late after radiotherapy, and in fibrotic lesions and in some normal tissues, then Ktrans approximates to the product of permeability and the surface area (permeability surface area product, PS) [10, 11]. After a variable time, the contrast agent diffuses back into the vasculature (described by the wash-out rate constant or kep; unit: min-1) from where it is excreted usually by the kidneys. Wash-out of contrast medium is faster when capillary permeability is very high, due to a typically rapid return of contrast medium into the blood. Other quantitative kinetic parameters that can be derived from PK modelling of DCE-MRI data include the fraction plasma volume (vp; unit: %) and the fractional extravascular, extracellular space (ve, or simply leakage space; unit: %).
Quantitative parameters such as Ktrans are complicated to derive, which can deter their use at the workbench. Difficulties arise from more complex data acquisition requirements and from the fact that kinetic models may not exactly fit the DCE-MRI data observed, because all models make assumptions that may not be valid for every tissue or tumour type. On the other hand, semi-quantitative parameters are simple to acquire, but tend to be more dependent on the exact injection and acquisition protocol used in the study. The initial area under the gadolinium concentration curve (IAUGC) is a relatively robust and simple kinetic parameter to derive and is able to characterize all enhancing regions without the problems associated with model fitting failures. IAUGC has been recommended as a practical substitute for Ktrans in clinical studies by several authors [12–16]. If IAUGC is to be used then it needs to be validated for this purpose. The strength of correlations against Ktrans depends on the exact cut-off time used for calculation, with 60 s recommended by international consensus panels [14]. However, readers should remember that IAUGC, as a semi-quantitative parameter, does not have a simple relationship to the physiological parameters of interest (perfusion, permeability and leakage space) [17], being dependent on both transfer constant and leakage space to varying degrees. On the other hand, transfer constant provides a more direct insight into underlying tissue pathophysiological processes.
Another semi-quantitative parameter that appears to be helpful for monitoring the effects of antivascular drugs is the % of non-enhancing (NE) pixels. This parameter is analogous to the CT density parameter incorporated into the Choi criteria [18] for the evaluation of imatinib mesylate on gastrointestinal stromal tumours (GIST). Recent analyses by us have shown that increasing numbers of NE pixels is a specific measure of effectiveness of drugs that target directly the tumour microvasculature but not of drugs that cause vascular shutdown via indirect mechanisms such as chemotherapy-induced tumour cell death [19]. These NE pixels represent non-vascularised tissues and anatomically represent cystic degeneration and/or necrosis (Fig. 2). Our experience shows that combretastatin A4 phosphate (CA4P) and bevacizumab both cause such profound vascular shutdown that results in increased numbers of NE pixels but these effects are not seen with chemotherapy.
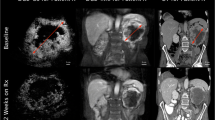
Ktrans map (colour overlay on a morphological T1-weighed MR image) of a 57-year-old patient with colon cancer liver metastasis (white dotted line) before (a) and 4 h after (b) first treatment with CA1P (OXi4503). Part of the liver metastasis shows reduced transfer constant (dark blue region) or complete vascular shutdown (black region) after 4 h. These images are consistent with the phenomenon of “pseudo-response” where there is an apparent decrease in enhancement because of vascular normalisation and re-establishment of the blood-brain barrier and yet the tumour is unchanged
There are uncertainties in the accuracy of kinetic parameter estimates derived from the application of tracer kinetic models in clinical DCE-MRI experiments. These derive from model-based assumptions and from assumptions made for the determination of tissue contrast agent concentrations. For example, in the original implementation of Tofts’ model, population-based AIFs were used (although this is not a strict requirement) [20] and it was assumed that tissue blood volume contributes negligible signal compared with that arising from contrast medium in the interstitial space [21]. It was recognised by Buckley that the application of these model-based assumptions leads to systematic overestimation of the transfer constant in tumours [22]. Modern two-compartment model implementations leave AIF choice to investigators and allow derivation of plasma volume fraction provided that AIF choice and temporal data sampling rates are appropriate. An important point for readers to remember is that the presence of gadolinium-containing contrast medium is detected only indirectly, by its effect on tissue water (that is the contrast medium itself is not detected). In tissues, contrast medium is confined to the extracellular space, whereas the bulk of water is intracellular. As a result, transmembrane water exchange can affect the accuracy of the tissue contrast agent concentration estimates [23, 24] which additionally needs to be taken into account.
Validation of DCE-MRI as a vascular biomarker
DCE-MRI has been widely validated in the last decade in a number of ways including direct correlative studies against immunohistochemical microvessel density measurements and tissue expressions of pro-angiogenic growth factors including vascular endothelial growth factor (VEGF) (broad correlations in some studies and no correlations in others) [25, 26]. Tissue validation studies have also come from correlative studies against widely accepted surrogates of tissue perfusion including 14C-aminoisobutyric acid quantitative autoradiography [27]. More recently, in vivo correlative imaging studies have been performed. Thus, transfer constant as a marker of tumour blood flow has now been validated against blood volume/blood flow derived from DSC MRI studies [13], 15O-water PET [28] and microbubble ultrasound [29]. These cross imaging validation studies have shown that the relationship is not upheld in every tumour type (e.g. in gliomas, because a variably intact blood-brain barrier reduces the first pass extraction of the contrast agent [10]). Similarly, the strength of correlations also decreases when therapies that reduced microvessel permeability are used, again because the first pass extraction fraction of small molecular weight contrast agents is reduced.
Functional imaging in the development of antiangiogenic agents (Table 1)
Phase I studies
Both anti-VEGF antibodies and receptor tyrosine kinase inhibitors (TKIs) demonstrate changes in DCE-MRI parameters in phase I studies. The most convincing DCE-MRI data have come from studies where tyrosine kinase receptor inhibitors that target multiple pathways (multitarget tyrosine kinase inhibitors, MTKIs) were used. Effective MTKIs all appear to inhibit KIT, platelet-derived growth factor (PDGF), and vascular endothelial growth factor receptor 2 (VEGFR-2). Most also inhibit VEGFR-1, some inhibit VEGFR-3, and with other additional receptor tyrosine kinases, including fibroblastic growth factor. It is the targets that are inhibited at variable drug concentrations that introduce distinct but subtle differences between the agents [30–34].
One of the first MTKI to be evaluated was PTK787/ZK222584 (PTK/ZK, vatalanib succinate), an oral angiogenesis inhibitor targeting all known VEGF receptor tyrosine kinases, PDGF receptor tyrosine kinase and the KIT tyrosine kinase. Phase I studies showed a 40–58% reduction of Ki (equivalent to Ktrans) after 2 days of the first treatment. These studies concluded that both DCE-MRI and PK data were helpful for defining the biologically active dose. Interestingly, changes in Ki also appear to predict responses and disease progression in patients with metastatic colorectal cancer to the liver [30–33]. Patients with a best response of stable disease had significantly greater reductions in Ki at both day 2 and at the end of cycle 1 compared with progressors. They confirmed significant negative correlations between changes in tumour Ki and increases in PTK/ZK oral dose and plasma levels, indicating a relationship between drug exposure and changes in tumour pharmacodynamics. Regrettably, these early promising results did not ultimately translate into patient benefit in two phase III clinical trails when PTK/ZK was used in combination with chemotherapy.
DCE-MRI assessment in a study with the TKI AZD2171 (cediranib), targeting VEGFR-1, -2, -3, PDGFR-α, and -β, showed reduced tumour flow and evidence of a drug dose-vascular response relationship [34]. This study formed the basis for the clinical development of this compound as will be commented on further below.
AG013736 (axitinib) is another orally bioavailable MTKI. Mechanistically, AG013736 inhibits the tyrosine kinase activities of all known VEGF receptors, PDGF-ß and KIT at low nanomolar concentrations. Rapid decreases in Ktrans and IAUC90 were observed on day 2 (>40% reductions in Ktrans in 11 of 17 evaluable patients) [35]. Interestingly, statistically significant decreases were noted for both mean Ktrans and IAUC90 for increasing values of log-transformed drug AUC and Cmax. It was evident that higher exposures of AG013736 were associated with a greater decrease in mean Ktrans with similar correlations between mean IAUC90 with respect to plasma AG013736 AUC0-24 concentrations.
However, not all phase I studies have shown reductions in DCE-MRI kinetic parameters or clear relationships between plasma drug exposure and tumour DCE-MRI changes. For example, studies with SU5416 (semaxanib) showed mixed results, with progressive disease despite reduced IAUC [36] and with two other studies not documenting consistent changes in DCE-MRI parameters [37, 38]. Negative results were seen in a small study with SU6668, a PDGFR, VEGFR-2 and FGFR-1 TKI inhibitor [39]. Another orally bioavailable MTKI is AMG-706 (motesanib), which targets VEGFR-1, -2, -3 and PDGFR. In a phase I study incorporating DCE-MRI, Rosen et al. showed decreases in Ktrans or IAUC ranged from −52% to +62%, but there was no significant correlation of either Ktrans or IAUC with AMG-706 AUC at either day 3 or 21 [40]. The anti-VEGF antibody HuMV833 reduced rate constant (kep) in tumours; however, no dose relationship was observed. This was ascribed by the authors to the heterogeneous antibody distribution and clearance between and within patients and between and within individual tumours [41].
Antiangiogenic agents as monotherapy
BAY 43-9006 (sorafenib), a tumour proliferation and angiogenesis inhibitor which works through blockade of the Raf/MEK/ERK pathway at the level of Raf kinase and the receptor tyrosine kinases VEGFR-2 and PDGFR-β, has been evaluated with DCE-MRI in renal cell carcinoma patients.
In a large prospective study (n = 56), Hahn et al. investigated DCE-MRI changes in renal cancer patients who were randomly assigned to placebo or 200 or 400 mg twice per day of sorafenib. DCE-MRI was performed at baseline and after 4 weeks [42]. DCE-MRI parameters included Ktrans and IAUGC90. Dose-response relationships in both Ktrans and IAUGC90 were observed with greater effects at higher doses. However, changes in DCE-MRI parameters after 4 weeks of sorafenib were not predictive of progression-free survival (PFS).
In a trial by Flaherty et al. with 17 renal cell carcinoma patients, 4 (24%) partial responses according to WHO criteria were observed [43]. DCE-MRI data from 15 evaluable patients demonstrated significant declines in Ktrans with changes in Ktrans correlating significantly with TTP and response judged by CT scanning. Interestingly, in both studies, a high baseline Ktrans value indicated a trend toward larger decreases in Ktrans although only significantly in the Hahn et al. study.
As noted above, vascular normalisation due to vessel pruning and remodelling has been demonstrated in a phase II study of AZD2171 (cediranib) in patients with recurrent glioblastoma. Interestingly, increases in vessel size and blood volume have been observed, while the decrease of Ktrans persisted. This “uncoupling” correlated with the elevation of basic fibroblast growth factor (bFGF) and stromal cell-derived factor (SDF)1α levels [5]. Importantly, decreases in Ktrans, microvessel volume and circulating collagen IV either alone or summarised together as a “vascular normalisation index” were predictive of overall survival (OS) when measurements were made as early as after 1 day of therapy [44]. An example of multiparametric MRI for the evaluation of antiangiogenic drug action on brain tumours is shown in Fig. 3.
Multiparametric imaging of anti-VEGF antibody therapy response in glioblastoma multiforme. Rows: serial images obtained before, after 2 and 8 weeks of bevacizumab. a Columns show T2-weighted (T2W), post-contrast-enhanced T1-weighted (T1W+c) images and apparent diffusion coefficient (ADC) maps of the tumour area and contralateral normal brain. Reduction in enhancement without decrease in tumour size is seen after 2 weeks with almost no enhancement visible after 8 weeks. At the 8-week time point the mass is a little larger and the necrotic cavity within the mass is smaller (arrow). b Columns show changes in relative cerebral blood volume (rCBV; arbitrary units) derived from dynamic susceptibility contrast-enhanced MRI and leakage space (ve; scale 0–50%) and transfer constant (Ktrans; scale 0–0.3 min-1) from DCE-MRI studies. Note reductions in the transfer constant and leakage space consistent with vascular normalisation (arrows) after 2 weeks. Note also some areas of decreased rCBV (*) with some areas of increasing rCBV (arrowheads). These images are consistent with the phenomenon of “pseudo-response” where there is an apparent decrease in enhancement because of vascular normalisation and re-establishment of the blood-brain barrier and yet the tumour is unchanged
SU11248 (sunitinib), which targets the VEGF and PDGFR receptor TKI, also demonstrates rapid reductions of tumour vessel leakiness in patients with hepatocellular carcinoma (decreases in Ktrans and Kep of 50% on average in all 25 patients). Ktrans decreases at 14 days correlated with partial remission/stable disease in 17 patients, whereas no decrease was found in the 8 patients with progressive disease [45].
Antiangiogenic agents with conventional therapies
A few studies have evaluated the antiangiogenic effects of a single cycle of bevacizumab, an anti-VEGF monoclonal antibody in patients with newly diagnosed locally advanced and inflammatory breast cancer [16, 46, 47]. All studies show that after a single dose of bevacizumab Ktrans and kep and IAUGC180 were significantly reduced from baseline values. In subsequent cycles, patients received combination bevacizumab therapy and chemotherapy, and DCE-MRI parameters all decreased substantially. Thukral et al. noted that parameter values measured between cycles 1 and 4 showed greater differences than did those measured between cycles 4 and 7, suggesting that the greatest effect on tumour microvascularity occurred early in the course of therapy. Importantly, DCE-MRI parameter changes after the first cycle 1 were not reflective of the eventual success of combination therapy [16, 46].
An interesting new study has attempted to separate the antiangiogenic effects of chemotherapy from those due to angiogenesis inhibitors. Baar et al. performed a randomised phase II trial designed to evaluate the additional biomarker effect on angiogenesis when bevacizumab is added to docetaxel in women with breast cancer [48]. Forty-nine patients were randomised and no differences in overall clinical response, PFS or OS were observed. IAUGC90 showed greater decreases in the bevacizumab+docetaxel arm with overall greater decreases in tumour volume, suggesting a greater antiangiogenic effect for the combination therapy.
Functional imaging in the development of vascular disrupting agents
At the time the manuscript was written, data from 10 studies including a total of 100 patients had been published as papers or abstracts (Table 2). Galbraith et al. were the first to use DCE-MRI with a clinical study of a VDA. They showed significant reductions in median IAUC90 in the majority of 16 patients at various time points after treatment with 5,6-dimethylxanthenone-4-acetic acid (DMXAA), [49]. There was no evidence of a dose-response reduction in tumour IAUC90 after the administration of DMXAA to patients. However, in a further safety phase I study performed in New Zealand, no reductions in DCE MRI parameters were seen at 26 h post-DMXAA but increases in leakage space (ve) were seen [50]. It has been speculated that the acute antivascular effects disappear within 26 h, but these results contradict the observations of Galbraith et al. [49]. Increases in ve may be explained by DMXAA mechanisms of action, which include induction of cytokines [particularly tumour necrosis factor alpha (TNF-α), serotonin and nitric oxide (NO)] as well as its antivascular and antiangiogenic effects. Several studies have shown that cytokines, TNF-α in particular, can increase vascular permeability but also decreases tumour blood flow by inducing vascular collapse and haemorrhage. Since changes in Ktrans and IAUGC are related to both tumour blood flow and vessel permeability, the two physiological parameters cannot be decoupled so the net effect may be inconsistent.
The classic description of VDA activity on DCE-MRI (rapid shutdown of the vasculature and reversibility of effects) was noted in a limited phase I trial of the tubulin binding colchicin analogue VDA ZD6126, where significant decreases in IAUC60 were seen in six of eight patients 6 h post-infusion. This was then maintained at 24 h, with partial recovery at 18–21 days [51, 52]. There was also a significant trend of greater antivascular effect with increasing drug exposure (p <0.01).
DCE-MRI changes following infusion of the small-molecule tubulin binding VDA CA4P have been assessed in six phase I and II oncology clinical studies where considerable variations in dosing ranged from 27 to 114 mg/m2 [53–60]. In all of these studies, decreases in tumour perfusion following CA4P administration have been demonstrated by DCE-MRI and in most studies these achieved statistical significance. In the first phase I trial [54] using DCE-MRI in 18 patients, measuring transfer constant (Ktrans) and IAUGC over the first 24 h after initial treatment with CA4P [57, 59], significant reductions in tumour Ktrans were seen in 6 of 16 patients treated at ≥52 mg/m2, with significant group mean reductions of 37 and 29% at 4 and 24 h, respectively, after treatment. No reductions were seen in muscle Ktrans or in kidney IAUGC. These DCE-MRI data helped to confirm the antivascular activity of CA4P at doses below the maximum tolerated dose (68 mg/m2). No significant changes were seen in patients treated at 20–40 mg/m2, indicating a threshold dose level below which effects on the microvasculature are not seen at the 4-h time point.
Pre-clinical models have demonstrated that the addition of an anti-VEGF antibody to a VDA significantly increases anti-tumour activity, possibly by inhibiting neovascularisation of the surviving rim [61]. The first clinical study combining a VDA with an antiangiogenic drug was to establish the safety of the CA4P/bevacizumab combination and to demonstrate synergy of action in vivo using DCE-MRI. The study showed statistically significant reductions in Ktrans after one dose of CA4P, which as anticipated, reversed on drug wash-out. However, there was a failure of tumour vasculature to recover when CA4P was given with bevacizumab [62].
DCE-MRI: decoration or decision making tool?
Based on the experience gained over the last decade, we can now address more fully the questions posed in 2003 by J.M. Collins from the US FDA [1] as follows:
-
1.
Does imaging help to assess whether mechanistic goals are achieved?
From the data that we have presented, it is clear that it would not be possible to determine directly if tumour perfusion is reduced when perfusion imaging had not been undertaken. For example, studies support the interpretation that CA4P causes local disruption of blood flow within the tumour, rather than being mediated via more global decreases in cardiac output. Evidence for this comes from direct measurements of cardiac output at the time of observed reductions in tumour flow, heterogeneity of blood flow reductions which occurs mostly within tumour centres, increases in the proportion of non-enhancing pixels, and reversibility of action when wash-out of drug is allowed to occur. PET imaging has demonstrated modest decreases in cardiac output with small changes in perfusion of organs such as the kidneys with CA4P administration [63]. However, cardiac output changes tend to be of brief duration compared to reductions in blood flow to tumours which last longer. Imaging studies also support the specificity of vascular disruption for tumour neovasculature compared to normal tissues.
Imaging studies have also shown that there are some differences in the patterns between the antivascular effects of antiangiogenic drugs and VDAs. The magnitude of blood flow reduction appears similar but the speed of onset of action and recovery differ significantly. The often-stated difference that antiangiogenic agents affect the centre and periphery of tumours whereas VDAs affect only tumour centres has not been realised in practice; both classes of agents appear to affect tumour centres predominantly. Furthermore, within substance classes, differences have been noted on imaging. Thus, within the antiangiogenic class of agents, the anti-permeability effects of anti-VEGF therapies can be counteracted in the short term by anti-PDGF activity of some small molecular weight TKR inhibitors [64]. This may explain in part the observation that therapy with BIBF-1120, a potent orally active, triple angiokinase inhibitor of VEGFR-1, -2 and -3, PDGFR-α and -β and FGFR-1 and -3, did not show early reductions of Ktrans or IAUGC but rather that these were seen much later after 28–56 days of therapy [65, 66].
Even within the VDA class of agents, differences between DMXAA and CA4P have been noted. Both pre-clinical and clinical studies have shown inconsistent responses in Ktrans and IAUGC (with both increases and decreases having been documented); this inconsistency has not been shown for CA4P, which in general causes blood flow reductions only. This difference can be explained by the mechanism of action of DMXAA, which, despite culminating in the same overall anti-tumour affect as other VDAs (i.e. induction of tumour necrosis through blood vessel disruption), is actually very different. DMXAA is an unusual VDA because it does not work through tubulin binding, but instead stimulates the induction of cytokines, which have both antivascular and anti-tumour effects. Several studies have shown that cytokines, TNF-α in particular, can increase vascular permeability but also decrease tumour blood flow by inducing vascular collapse and haemorrhage. Since changes in Ktrans and IAUGC are related to changes in both tumour blood flow and vessel permeability, the two physiological parameters cannot be decoupled so the net effect maybe inconsistent. Increases in interstitial fluid volume (leakage space - ve) would be expected regardless of the effect on Ktrans and IAUGC, which has been noted in clinical studies.
-
2.
Does imaging assist in dose selection and scheduling for phase II studies?
Studies have consistently shown that the biologically active dose is below the MTD in most cases. However, the therapeutic window for VDAs is smaller than anticipated from rodent models, and more severe DLTs are seen compared to antiangiogenic agents. Dose-response relationships in these studies have been variable, probably in part due to the small numbers of patients and heterogeneity of patient populations in terms of highly variable histological subtypes and the number and types of prior therapies. In two studies [54, 57], correlations between changes in tumour CA4P Ktrans values and/or blood CA4 exposure were seen; however, in the Dowlati et al. study [55], change in Ktrans values correlated with Cmax rather than AUC. In the Galbraith et al. study, significant changes were only seen in the patients who received ≥52 mg/m2 [54]. In both the Bilenker et al. and the Akerley et al. trials, greater effects were observed at the intermediate rather than the higher dose levels [57, 59]. Despite some differences in details, all of these studies indicated that CA4P significantly reduces tumour perfusion, and that the minimal efficacious dose is in the range of 36–52 mg/m2.
As far as we are aware, only one study has evaluated dose finding and scheduling in the setting of a phase I clinical trial [67]. Jonker et al. reported a phase I study of brivanib alaninate, an oral dual inhibitor of VEGFR and FGFR tyrosine kinases. This study was designed and powered to assist in determining dose and schedule. This study evaluated patients in two parts with standard dose escalation to MTD in patients with advanced or metastatic cancer (part A). Part B was enriched by including only those tumour types known to respond to anti-VEGF therapies (renal cancer, colorectal malignancies and hepatocellular cancers). In part B, several dose schedules were evaluated using DCE-MRI responses in patient cohorts of 12–15 patients per schedule. Using this methodology Jonker et al. were able to use DCE-MRI as the basis for schedule selection for clinical phase II. Such dose-response relationships using DCE-MRI have also been shown for sorafenib in renal cancer [42].
-
3.
Can imaging help identify subpopulations enriched for response?
Tumour types that appear best for responding to antiangiogenic agents include colorectal, hepatocellular cancer, renal cancers and brain tumours. Indeed, for these tumour types antiangiogenic agents have gained clinical approval for use as mono- or combination therapy. From the data accumulated to date and personal experience, it has been noted that the liver as a metastatic site is more likely to respond to antiangiogenic agents than metastatic tumours elsewhere. For a number of antivascular therapies, there have been strong correlations between baseline Ktrans values and decreases following therapy. This finding has been seen for both antiangiogenic agents as well as for VDAs. Indeed, it can now be stated that if DCE-MRI responses are expected to occur, but are not seen, then there is probably no major impact on vascular function. Of course there are exceptions as noted above for drugs having strong anti-PDGF effect and of course false-negative results may occur if scanning schedule in relation to drug dosing is incorrect. Experience suggests that tubulin binding VDAs should be imaged within hours to demonstrate antivascular effects and again after more than a day to show reversibility. Anti-VEGF drug effects typically occur within days to weeks and are more persistent, so that imaging must be performed at appropriate time points.
-
4.
Are imaging tools widely available?
Previously, the lack of availability of tools for DCE-MRI data analysis was an impediment to its implementation into clinical trials. However, appropriate software is increasingly available and can be obtained from most major equipment manufacturers, academic sources and commercial software companies. Multicentre working has been well documented with appropriate quality assurance/quality control protocols in place, and reasonable reproducibility has been shown. Three international consensus panels have laid out the requirements and standards of DCE-MRI imaging in early clinical trials. The first consensus meetings took place in October 1999 and November 2000. Reports and recommendations can be found on the National Cancer Institute (NCI) web site [68]. A second workshop was held in London in March 2002 with recommendations published in 2005 [69]. A third meeting sponsored by NIH/NCI met in 2004 and recommendations can be found on the NCI website [70].
-
5.
Can imaging tools predict patient benefit?
This question was not posed by Collins, because it was not relevant to phase I studies but is frequently posed by investigators and patients. There are four recent studies, which found a correlation between antiangiogenic treatment and patient benefit. In general, OS is considered a stronger end-point than PFS or the comparison between non-progressing patients showing partial remission (PR) or stable disease (SD), and patients with progressing disease (PD). In two studies, correlations have been made between the DCE-MRI parameters and progressing vs non-progressing patients. Morgan et al. found a significantly greater reduction in Ki (equivalent to Ktrans) in non-progressors in patients with advanced colorectal cancer and liver metastasis 2 days and after the first cycle of treatment with vatalanib [31]. In a study of sunitinib monotherapy in advanced hepatocellular carcinoma, Zhu et al. observed significant decreases in Ktrans and kep in patients with PR or SD, compared to patients with PD after 14 days of treatment [45]. The correlations between Ktrans or IAUC90 and PFS have been examined in two studies of sorafenib therapy in patients with renal cell carcinoma. In the study by Flaherty et al., percent declines in Ktrans were significantly associated with PFS [43], whereas in the Hahn et al. study, Ktrans or IAUC90 changes were not [42]. The only study where Ktrans (in combination with microvessel volume and circulating collagen IV concentrations) correlated with OS was a study of patients with recurrent glioblastoma after a single dose of cediranib [44]. The validation of functional imaging parameters as a predictor of OS has proved to be difficult due to the inherent small number of patients treated in a phase I or II trial.
Outlook: multiparametric MRI exploring secondary effects on tumour microenvironment and cellular metabolic disruption
As the pharmaceutical industry moves towards increasingly complex multitargeted therapies, the anticipated effects on tumour tissues de novo have become more difficult to predict. As we have seen, studies to date have tended to look at treatment effects using only a single functional imaging modality such as DCE-MRI. There are relatively few clinical studies that have used multifunctional imaging approaches to explore the secondary effects of antiangiogenics or VDAs on the tumour microenvironment or on cellular metabolic disruption. For example, changes in perfusion and glucose metabolism may not occur in parallel. Decoupling of perfusion and glucose metabolism has been shown by Willett at al. in patients with rectal cancer treated with bevacizumab [71]. Dose-related perfusion-metabolism decoupling has also demonstrated by Herbst et al. [72] who noted that endostatin (an antivascular agent) when given in high doses decreased tumour perfusion but increased glucose metabolism. These examples of uncoupling of blood flow and glucose metabolism following antiangiogenic therapy probably result from drug-induced tumour hypoxia and secondary upregulation of glucose metabolism.
Another good example of multifunctional MR imaging used to assess the sequential tissue effects of antiangiogenic compounds in humans was published by Batchelor et al. [5]. They evaluated patients with recurrent glioblastomas who were treated with cediranib. Multifunctional MRI assessments were undertaken including contrast-enhanced tumour volume, vessel size index, microvessel permeability, extracellular leakage space, water diffusivity and diffusion tensor imaging. Using this panel of imaging tests, they were able to show rapid reductions in Ktrans, extracellular leakage space and water diffusivity following treatment, which was interpreted as evidence of microvessel normalisation. They noted that decreases in vessel size index were short-lived despite persistent reductions of transfer constant. Interestingly, tumour volume, which initially decreased, began to expand despite persistent decreases in microvessel permeability (Fig. 3), suggesting that continued tumour growth was not mediated via angiogenesis using the VEGF pathway [73]. Intriguingly, the anticipated effects of cell death on diffusion-weighted (DW) MRI were not seen. Tumour cell death via apoptosis should cause increases in apparent diffusion coefficient (ADC) values [74], but in fact the reverse was observed (reduced ADC values). The latter finding is likely to be due to water shifts out of tumours caused by reductions of microvessel permeability, a finding supported by reduced leakage space estimates and one that has been observed at other tumour sites with antiangiogenic therapy.
These multifunctional imaging observations suggest that it is only by combining biomarker data from a number of imaging techniques that one may begin to truly understand how antiangiogenic therapies affect tumour cells and tissue microenvironments. Such observations can provide unique insights into the mechanism of drug action in vivo and provide useful PD information. However, if multifunctional imaging is to take up the unique position of enhancing decision making at critical milestones in the early phases of the drug development process, then procedural rigour will be needed to establish each biomarker and/or biomarker combination for such a role.
Conclusion
Perfusion imaging provides unique information on the vascular properties of tissues and tumours and their responses to antiangiogenic and VDAs in pre-clinical and early clinical studies. Their successful use is dependent on the need to clearly define imaging objectives, which can be achieved provided that studies are well planned and conducted with appropriate quality assurance/quality control (QA/QC) steps. If done well, observations from such imaging studies do improve our biological understanding of mechanisms of drug action, interactions, and on their magnitude and duration of effects. Many studies show that in general there is good concordance with pre-clinical data and with other angiogenesis biomarkers. Such studies also show considerable heterogeneity in responsiveness between and within lesions and between and within patients (even primary and secondary lesions differ in their responses). It is clear from imaging observations that not all antiangiogenic and VDAs have the same effects in terms of onset times and duration of effects. Future challenges for imaging include correlation with clinical measures of efficacy and determining the relationship with blood and serum biomarkers.
References
Collins JM. Functional imaging in phase I studies: decorations or decision making? J Clin Oncol 2003;21:2807–9.
Rüegg C, Meuwly JY, Driscoll R, Werffeli P, Zaman K, Stupp R. The quest for surrogate markers of angiogenesis: a paradigm for translational research in tumor angiogenesis and anti-angiogenesis trials. Curr Mol Med 2003;3:673–91.
Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med 2001;7:987–9.
Kamoun WS, Ley CD, Farrar CT, Duyverman AM, Lahdenranta J, Lacorre DA, et al. Edema control by cediranib, a vascular endothelial growth factor receptor-targeted kinase inhibitor, prolongs survival despite persistent brain tumor growth in mice. J Clin Oncol 2009;27:2542–52.
Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 2007;11:83–95.
Oostendorp M, Post MJ, Backes WH. Vessel growth and function: depiction with contrast-enhanced MR imaging. Radiology 2009;251:317–35.
Kety S. Measurement of local blood flow by the exchange of an inert, diffusible substance. Methods Med Res 1960;8:228–36.
Kety S. Blood-tissue exchange methods. Theory of blood-tissue exchange and its application to measurement of blood flow. Methods Med Res 1960;8:223–7.
Tofts P, Brix G, Buckley D, Evelhoch J, Henderson E, Knopp M, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging 1999;10:223–32.
Wilkinson ID, Jellineck DA, Levy D, Giesel FL, Romanowski CA, Miller BA, et al. Dexamethasone and enhancing solitary cerebral mass lesions: alterations in perfusion and blood-tumor barrier kinetics shown by magnetic resonance imaging. Neurosurgery 2006;58:640–6. discussion 640-6.
O’Connor JP, Carano RA, Clamp AR, Ross J, Ho CC, Jackson A, et al. Quantifying antivascular effects of monoclonal antibodies to vascular endothelial growth factor: insights from imaging. Clin Cancer Res 2009;15:6674–82.
Lankester KJ, Maxwell RJ, Pedley RB, Dearling JL, Qureshi UA. E, et al. Combretastatin A-4-phosphate effectively increases tumor retention of the therapeutic antibody, 131I-A5B7, even at doses that are sub-optimal for vascular shut-down. Int J Oncol 2007;30:453–60.
Lankester KJ, Taylor JN, Stirling JJ, Boxall J, d’Arcy JA, Collins DJ, et al. Dynamic MRI for imaging tumor microvasculature: comparison of susceptibility and relaxivity techniques in pelvic tumors. J Magn Reson Imaging 2007;25:796–805.
Leach MO, Brindle KM, Evelhoch JL, Griffiths JR, Horsman MR, Jackson A, et al. The assessment of antiangiogenic and antivascular therapies in early-stage clinical trials using magnetic resonance imaging: issues and recommendations. Br J Cancer 2005;92:1599–610.
Lankester KJ, Taylor NJ, Stirling JJ, Boxall J, D’Arcy JA, Leach MO, et al. Effects of platinum/taxane based chemotherapy on acute perfusion in human pelvic tumours measured by dynamic MRI. Br J Cancer 2005;93:979–85.
Thukral A, Thomasson DM, Chow CK, Eulate R, Wedam SB, Gupta SN, et al. Inflammatory breast cancer: dynamic contrast-enhanced MR in patients receiving bevacizumab–initial experience. Radiology 2007;244:727–35.
Walker-Samuel S, Parker CC, Leach MO, Collins DJ. Reproducibility of reference tissue quantification of dynamic contrast-enhanced data: comparison with a fixed vascular input function. Phys Med Biol 2007;52:75–89.
Benjamin RS, Choi H, Macapinlac HA, Burgess MA, Patel SR, Chen LL, et al. We should desist using RECIST, at least in GIST. J Clin Oncol 2007;25:1760–4.
Taylor NJ, Tunariu N, Stirling JJ, Collins DJ, Nathan P, d’Arcy JA, et al. Non-enhancing pixels: a specific additional DCE-MRI kinetic parameter for assessing antivascular effects of anti-angiogenic and vascular disruptive agents. Proceedings of the joint meeting of International Society of Magnetic Resonance in Medicine, 16th Scientific Meeting and Exhibition, Honolulu; 2009. p. 2262.
Weinmann HJ, Laniado M, Mützel W. Pharmacokinetics of GdDTPA/dimeglumine after intravenous injection into healthy volunteers. Physiol Chem Phys Med NMR 1984;16:167–72.
Tofts PS. Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging 1997;7:91–101.
Buckley DL. Uncertainty in the analysis of tracer kinetics using dynamic contrast-enhanced T1-weighted MRI. Magn Reson Med 2002;47:601–6.
Landis CS, Li X, Telang FW, Coderre JA, Micca PL, Rooney WD, et al. Determination of the MRI contrast agent concentration time course in vivo following bolus injection: effect of equilibrium transcytolemmal water exchange. Magn Reson Med 2000;44:563–74.
Buckley DL. Transcytolemmal water exchange and its affect on the determination of contrast agent concentration in vivo. Magn Reson Med 2002;47:420–4.
Schlemmer HP, Merkle J, Grobholz R, Jaeger T, Michel MS, Werner A, et al. Can pre-operative contrast-enhanced dynamic MR imaging for prostate cancer predict microvessel density in prostatectomy specimens? Eur Radiol 2004;14:309–17.
Padhani AR, Dzik-Jurasz A. Perfusion MR imaging of extracranial tumor angiogenesis. Top Magn Reson Imaging 2004;15:41–57.
Ferrier MC, Sarin H, Fung SH, Schatlo B, Pluta RM, Gupta SN, et al. Validation of dynamic contrast-enhanced magnetic resonance imaging-derived vascular permeability measurements using quantitative autoradiography in the RG2 rat brain tumor model. Neoplasia 2007;9:546–55.
Eby PR, Partridge SC, White SW, Doot RK, Dunnwald LK, Schubert EK, et al. Metabolic and vascular features of dynamic contrast-enhanced breast magnetic resonance imaging and (15)O-water positron emission tomography blood flow in breast cancer. Acad Radiol 2008;15:1246–54.
Niermann KJ, Fleischer AC, Huamani J, Yankeelov TE, Kim DW, Wilson WD, et al. Measuring tumor perfusion in control and treated murine tumors: correlation of microbubble contrast-enhanced sonography to dynamic contrast-enhanced magnetic resonance imaging and fluorodeoxyglucose positron emission tomography. J Ultrasound Med 2007;26:749–56.
Conrad C, Friedman H, Reardon D, Provenzale J, Jackson E, Serajuddin H, et al. A phase I/II trial of single-agent PTK 787/ZK 222584 (PTK/ZK), a novel, oral angiogenesis inhibitor, in patients with recurrent glioblastoma multiforme (GBM). J Clin Oncol (Meeting Abstracts), 2004. Vol. 22.
Morgan B, Thomas AL, Drevs J, Hennig J, Buchert M, Jivan A, et al. Dynamic contrast-enhanced magnetic resonance imaging as a biomarker for the pharmacological response of PTK787/ZK 222584, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinases, in patients with advanced colorectal cancer and liver metastases: results from two phase I studies. J Clin Oncol 2003;21:3955–64.
Mross K, Drevs J, Müller M, Medinger M, Marmé D, Hennig J, et al. Phase I clinical and pharmacokinetic study of PTK/ZK, a multiple VEGF receptor inhibitor, in patients with liver metastases from solid tumours. Eur J Cancer 2005;41:1291–9.
Thomas AL, Morgan B, Horsfield MA, Higginson A, Kay A, Lee L, et al. Phase I study of the safety, tolerability, pharmacokinetics, and pharmacodynamics of PTK787/ZK 222584 administered twice daily in patients with advanced cancer. J Clin Oncol 2005;23:4162–71.
Drevs J, Medinger M, Mross K, Zirrgiebel U, Strecker R, Unger C, et al. Phase I clinical evaluation of AZD2171, a highly potent VEGF receptor tyrosine kinase inhibitor, in patients with advanced tumors. J Clin Oncol, 2005 ASCO Annual Meeting Proceedings 2005;23:3002.
Liu G, Rugo HS, Wilding G, McShane TM, Evelhoch JL, Ng C, et al. Dynamic contrast-enhanced magnetic resonance imaging as a pharmacodynamic measure of response after acute dosing of AG-013736, an oral angiogenesis inhibitor, in patients with advanced solid tumors: results from a phase I study. J Clin Oncol 2005;23:5464–73.
Medved M, Karczmar G, Yang C, Dignam J, Gajewski TF, Kindler H, et al. Semiquantitative analysis of dynamic contrast enhanced MRI in cancer patients: variability and changes in tumor tissue over time. J Magn Reson Imaging 2004;20:122–8.
O’Donnell A, Padhani A, Hayes C, Kakkar AJ, Leach M, Trigo JM, et al. A Phase I study of the angiogenesis inhibitor SU5416 (semaxanib) in solid tumours, incorporating dynamic contrast MR pharmacodynamic end points. Br J Cancer 2005;93:876–83.
Dowlati A, Robertson K, Radivoyevitch T, Waas J, Ziats NP, Hartman P, et al. Novel Phase I dose de-escalation design trial to determine the biological modulatory dose of the antiangiogenic agent SU5416. Clin Cancer Res 2005;11:7938–44.
Xiong HQ, Herbst R, Faria SC, Scholz C, Davis D, Jackson EF, et al. A phase I surrogate endpoint study of SU6668 in patients with solid tumors. Invest New Drugs 2004;22:459–66.
Rosen LS, Kurzrock R, Mulay M, Van Vugt A, Purdom M, Ng C, et al. Safety, pharmacokinetics, and efficacy of AMG 706, an oral multikinase inhibitor, in patients with advanced solid tumors. J Clin Oncol 2007;25:2369–76.
Jayson GC, Zweit J, Jackson A, Mulatero C, Julyan P, Ranson M, et al. Molecular imaging and biological evaluation of HuMV833 anti-VEGF antibody: implications for trial design of antiangiogenic antibodies. J Natl Cancer Inst 2002;94:1484–93.
Hahn OM, Yang C, Medved M, Karczmar G, Kistner E, Karrison T, et al. Dynamic contrast-enhanced magnetic resonance imaging pharmacodynamic biomarker study of sorafenib in metastatic renal carcinoma. J Clin Oncol 2008;26:4572–8.
Flaherty KT, Rosen MA, Heitjan DF, Gallagher ML, Schwartz B, Schnall MD, et al. Pilot study of DCE-MRI to predict progression-free survival with sorafenib therapy in renal cell carcinoma. Cancer Biol Ther 2008;7:496–501.
Sorensen AG, Batchelor TT, Zhang WT, Chen PJ, Yeo P, Wang M, et al. A “vascular normalization index” as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res 2009;69:5296–300.
Zhu AX, Sahani DV, Duda DG, di Tomaso E, Ancukiewicz M, Catalano OA, et al. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study. J Clin Oncol 2009;27:3027–35.
Wedam SB, Low JA, Yang SX, Chow CK, Choyke P, Danforth D, et al. Antiangiogenic and antitumor effects of bevacizumab in patients with inflammatory and locally advanced breast cancer. J Clin Oncol 2006;24:769–77.
Overmoyer B, Silverman P, Leeming R, Shenk R, Lyons J, Ziats N, et al. Phase II trial of neoadjuvant docetaxel with or without bevacizumab in patients with locally advanced breast cancer. J Clin Oncol, 2004 ASCO Annual Meeting Proceedings. 2004;22:Abstract 727.
Baar J, Silverman P, Lyons J, Fu P, Abdul-Karim F, Ziats N, et al. A vasculature-targeting regimen of preoperative docetaxel with or without bevacizumab for locally advanced breast cancer: impact on angiogenic biomarkers. Clin Cancer Res 2009;15:3583–90.
Galbraith SM, Rustin GJ, Lodge MA, Taylor NJ, Stirling JJ, Jameson M, et al. Effects of 5,6-dimethylxanthenone-4-acetic acid on human tumor microcirculation assessed by dynamic contrast-enhanced magnetic resonance imaging. J Clin Oncol 2002;20:3826–40.
McKeage MJ, Fong P, Jeffery M, Baguley BC, Kestell P, Ravic M, et al. 5,6-Dimethylxanthenone-4-acetic acid in the treatment of refractory tumors: a phase I safety study of a vascular disrupting agent. Clin Cancer Res 2006;12:1776–84.
Evelhoch J, LoRusso P, DelProposto Z, Stark K, Latif Z, Morton P, et al. Dynamic contrast-enhanced MRI evaluation of the effects of ZD6126 on tumor vasculature in a phase I clinical trial, Proc. ISMRM, Honolulu, Hawaii, 2002. Vol. 10.
LoRusso PM, Gadgeel SM, Wozniak A, Barge AJ, Jones HK, DelProposto ZS, et al. Phase I clinical evaluation of ZD6126, a novel vascular-targeting agent, in patients with solid tumors. Invest New Drugs 2008;26:159–67.
Rustin GJ, Galbraith SM, Anderson H, Stratford M, Folkes LK, Sena L, et al. Phase I clinical trial of weekly combretastatin A4 phosphate: clinical and pharmacokinetic results. J Clin Oncol 2003;21:2815–22.
Galbraith SM, Maxwell RJ, Lodge MA, Tozer GM, Wilson J, Taylor NJ, et al. Combretastatin A4 phosphate has tumor antivascular activity in rat and man as demonstrated by dynamic magnetic resonance imaging. J Clin Oncol 2003;21:2831–42.
Dowlati A, Robertson K, Cooney M, Petros WP, Stratford M, Jesberger J, et al. A phase I pharmacokinetic and translational study of the novel vascular targeting agent combretastatin A-4 phosphate on a single-dose intravenous schedule in patients with advanced cancer. Cancer Res 2002;62:3408–16.
Stevenson JP, Rosen M, Sun W, Gallagher M, Haller DG, Vaughn D, et al. Phase I trial of the antivascular agent combretastatin A4 phosphate on a 5-day schedule to patients with cancer: magnetic resonance imaging evidence for altered tumor blood flow. J Clin Oncol 2003;21:4428–38.
Bilenker JH, Flaherty KT, Rosen M, Davis L, Gallagher M, Stevenson JP, et al. Phase I trial of combretastatin A-4 phosphate with carboplatin. Clin Cancer Res 2005;11:1527–33.
Nathan P, Judson I, Padhani A, Harris A, Carden CP, Smythe J, et al. A phase I study of combretastatin A4 phosphate (CA4P) and bevacizumab in subjects with advanced solid tumors. J Clin Oncol 2008;26:3550.
Akerley WL, Schabel M, Morrell G, Horvath E, Yu M, Johnsson B, et al. A randomized phase 2 trial of combretastatin A4 phosphate (CA4P) in combination with paclitaxel and carboplatin to evaluate safety and efficacy in subjects with advanced imageable malignancies. J Clin Oncol, ASCO Annual Meeting Proceedings Part I. 2007;25:14060.
Meyer T, Gaya AM, Dancey G, Stratford MR, Othman S, Sharma SK, et al. A phase I trial of radioimmunotherapy with 131I-A5B7 anti-CEA antibody in combination with combretastatin-A4-phosphate in advanced gastrointestinal carcinomas. Clin Cancer Res 2009;15:4484–92.
Shaked Y, Ciarrocchi A, Franco M, Lee CR, Man S, Cheung AM, et al. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science 2006;313:1785–7.
Padhani AR, Koh DM, Taylor NJ, Stirling JJ, Collins DJ, Rustin GJ, et al. DCE-MRI demonstration of antivascular effects of combretastatin A4 phosphate (CA4P) given in combination with bevacizumab to human subjects with advanced solid tumours. Proceedings of the joint meeting of International Society of Magnetic Resonance in Medicine and European Society for Magnetic Resonance in Medicine and Biology 2008:Oral presentation. Program number 773.
Anderson H, Yap JT, Wells P, Miller MP, Propper D, Price P, et al. Measurement of renal tumour and normal tissue perfusion using positron emission tomography in a phase II clinical trial of razoxane. Br J Cancer 2003;89:262–7.
Jayson GC, Parker GJ, Mullamitha S, Valle JW, Saunders M, Broughton L, et al. Blockade of platelet-derived growth factor receptor-beta by CDP860, a humanized, PEGylated di-Fab', leads to fluid accumulation and is associated with increased tumor vascularized volume. J Clin Oncol 2005;23:973–81.
Padhani AR, Taylor NJ, d’Arcy JA, Walker-Samuel S, Collins DJ, Knowles B, et al. Dynamic MRI evaluation of the triple receptor tyrosine kinase inhibitor BIBF 1120 in patients with advanced solid tumours. Proc Int Soc Magn Reson Med 2006;14:765.
Strecker R, Mross K, Stefanic M, de Rossi L, Hennig J. Evaluation of the effects of the triple angiokinase inhibitor BIBF 1120 on tumor vasculature in a phase I clinical trial using dynamic contrast-enhanced MRI (DCE-MRI). Proc Int Soc Magn Reson Med 2006;14:764.
Jonker DJ, Rosen LS, Sawyer M, Wilding G, Noberasco C, Jayson G, et al. A phase I study of BMS-582664 (brivanib alaninate), an oral dual inhibitor of VEGFR and FGFR tyrosine kinases, in patients (pts) with advanced/metastatic solid tumors: safety, pharmacokinetic (PK), and pharmacodynamic (PD) findings. J Clin Oncol, 2007 ASCO Annual Meeting Proceedings Part I. 2007;25:Abstract 3559.
NCI. Recommendations for MR measurement methods at 1.5-Tesla and endpoints for use in Phase 1/2a trials of anti-cancer therapeutics affecting tumor vascular function. http://imaging.cancer.gov/images/Documents/14a39040-dad1-46f8-bf88-bae2e7401ebe/DCE_RecommendationfromNov2004NCIWorkshop(2).pdf, 2004.
Leach MO, Brindle KM, Evelhoch JL, Griffiths JR, Horsman MR, Jackson A, et al. The assessment of antiangiogenic and antivascular therapies in early-stage clinical trials using magnetic resonance imaging: issues and recommendations. Br J Cancer 2005;92:1599–610.
Evelhoch J, Garwood M, Vigneron D, Knopp M, Sullivan D, Menkens A, et al. Expanding the use of magnetic resonance in the assessment of tumor response to therapy: workshop report. Cancer Res 2005;65:7041–4.
Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med 2004;10:145–7.
Herbst RS, Mullani NA, Davis DW, Hess KR, McConkey DJ, Charnsangavej C, et al. Development of biologic markers of response and assessment of antiangiogenic activity in a clinical trial of human recombinant endostatin. J Clin Oncol 2002;20:3804–14.
Loges S, Mazzone M, Hohensinner P, Carmeliet P. Silencing or fueling metastasis with VEGF inhibitors: antiangiogenesis revisited. Cancer Cell 2009;15:167–70.
Patterson DM, Padhani AR, Collins DJ. Technology insight: water diffusion MRI—a potential new biomarker of response to cancer therapy. Nat Clin Pract Oncol 2008;5:220–33.
O’Dwyer PJ, Rosen M, Gallagher M, Schwartz B, Flaherty KT. Pharmacodynamic study of BAY 43-9006 in patients with metastatic renal cell carcinoma. J Clin Oncol (Meeting Abstracts). 2005;23:3005.
Rosen LS, Wilding G, Sweeney C, Casale D, Kollia G, Wu C, et al. Phase I dose escalation study to determine the safety, pharmacokinetics and pharmacodynamics of BMS-582664, a VEGFR/FGFR inhibitor in patients with advanced/metastatic solid tumors. J Clin Oncol (Meeting Abstracts). 2006;24:3051.
Mross KB, Gmehling D, Frost A, Baas F, Strecker R, Hennig J, et al. A clinical phase I, pharmacokinetic (PK), and pharmacodynamic study of twice daily BIBF 1120 in advanced cancer patients. J Clin Oncol (Meeting Abstracts), 2005. Vol. 23.
Eder Jr JP, Supko JG, Clark JW, Puchalski TA, Garcia-Carbonero R, Ryan DP, et al. Phase I clinical trial of recombinant human endostatin administered as a short intravenous infusion repeated daily. J Clin Oncol 2002;20:3772–84.
Thomas JP, Arzoomanian RZ, Alberti D, Marnocha R, Lee F, Friedl A, et al. Phase I pharmacokinetic and pharmacodynamic study of recombinant human endostatin in patients with advanced solid tumors. J Clin Oncol 2003;21:223–31.
Watson Y, Cheung S, Roberts C, Buonaccorsi G, Davies K, Jackson A, et al. Prognostic power of DCE-MRI heterogeneity analysis in patients with advanced solid tumors. Proc Int Soc Magn Reson Med 2006;14:755.
Perotti A, Sessa C, Mancuso A, Noberasco C, Cresta S, Locatelli A, et al. Clinical and pharmacological phase I evaluation of Exherin (ADH-1), a selective anti-N-cadherin peptide in patients with N-cadherin-expressing solid tumours. Ann Oncol 2009;20:741–5.
Patterson DM, Charnley N, Saleem A, Stratford M, Dickinson C, Asselin M, et al. Phase I evaluation of vascular disrupting agent OXi4503. J Clin Oncol. 2008;26:Abstract 3551.
Evelhoch JL, LoRusso PM, He Z, DelProposto Z, Polin L, Corbett TH, et al. Magnetic resonance imaging measurements of the response of murine and human tumors to the vascular-targeting agent ZD6126. Clin Cancer Res 2004;10:3650–7.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zweifel, M., Padhani, A.R. Perfusion MRI in the early clinical development of antivascular drugs: decorations or decision making tools?. Eur J Nucl Med Mol Imaging 37 (Suppl 1), 164–182 (2010). https://doi.org/10.1007/s00259-010-1451-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-010-1451-z