Abstract
Objectives
To identify dynamic contrast-enhanced (DCE) imaging parameters from MRI, CT and US that are prognostic and predictive in patients with metastatic renal cell cancer (mRCC) receiving sunitinib.
Methods
Thirty-four patients were monitored by DCE imaging on day 0 and 14 of the first course of sunitinib treatment. Additional scans were performed with DCE-US only (day 7 or 28 and 2 weeks after the treatment break). Perfusion parameters that demonstrated a significant correlation (Spearman p < 0.05) with progression-free survival (PFS) and overall survival (OS) were investigated using Cox proportional hazard models/ratios (HR) and Kaplan-Meier survival analysis.
Results
A higher baseline and day 14 value for Ktrans (DCE-MRI) and a lower pre-treatment vascular heterogeneity (DCE-US) were significantly associated with a longer PFS (HR, 0.62, 0.37 and 5.5, respectively). A larger per cent decrease in blood volume on day 14 (DCE-US) predicted a longer OS (HR, 1.45). We did not find significant correlations between any of the DCE-CT parameters and PFS/OS, unless a cut-off analysis was used.
Conclusions
DCE-MRI, -CT and ultrasound produce complementary parameters that reflect the prognosis of patients receiving sunitinib for mRCC. Blood volume measured by DCE-US was the only parameter whose change during early anti-angiogenic therapy predicted for OS and PFS.
Key Points
• DCE-CT, -MRI and ultrasound are complementary modalities for monitoring anti-angiogenic therapy.
• The change in blood volume measured by DCE-US was predictive of OS/PFS.
• Baseline vascular heterogeneity by DCE-US has the strongest prognostic value for PFS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since the early 2000s, the standard response evaluation criteria for solid tumours (RECIST) have been based on changes of the anatomical tumour dimensions measured by routine CT or MRI techniques [1]. The limitations of these response evaluation criteria are widely acknowledged, particularly when they are applied to therapies whose anti-cancer activity is not immediately reflected by tumour shrinkage [2,3,4]. For example, the viability of the tumour microvasculature of metastatic renal cell cancer (mRCC) often begins to change within the first week of targeted therapy, long before any noticeable reduction in bulk tumour volume [5, 6]. This observation has motivated efforts to find and validate early indicators of treatment effect that predict long-term clinical benefit for individual patients [7].
To date, over 100 independent early-phase clinical studies have monitored early tumour response to targeted therapies using dynamic contrast-enhanced (DCE)-CT, MRI and ultrasound (US) techniques, mostly in isolation [8,9,10,11]. These methods take advantage of the pharmacokinetics of intravenously injected contrast agents, which are intricately related to vascular function and cellular metabolism [12,13,14,15,16,17,18]. Although numerous publications discuss the value of these imaging modalities in editorials, review papers [7, 19, 20] and within the pre-clinical literature [21, 22], none of them are accepted as standard of care for kidney cancer patients.
To our knowledge there are no clinical trials that have compared the value of all three modalities (DCE-MRI, DCE-CT and DCE-US) within the same patient cohort. Therefore, the current study seeks to identify and compare prognostic and predictive parameters of DCE-MRI, DCE-CT and DCE-US in the same group of patients receiving sunitinib for metastatic renal cell cancer.
Materials and methods
Clinical study population and imaging schedule
The institutional ethics review board approved this prospective phase II study and all patients gave written informed consent. Eligible patients were to start first-line sunitinib therapy and had histologically confirmed clear cell mRCC at first presentation with either an unresected primary with metastatic disease or abdominal metastases following a prior nephrectomy. Cancer sites had to be amenable to imaging by all three imaging modalities (MR, CT and US) and ECOG performance status 0 or 1. One prior cytokine therapy was allowed.
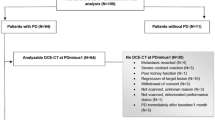
Thirty-four consecutive patients were enrolled and followed between 4 June 2007 and 21 November 2014 (Table 1). Patients were monitored by DCE imaging before and during the first course of sunitinib given on a schedule of 50 mg daily for 4 weeks followed by a 2 week drug holiday. All 34 patients were imaged at baseline and day 14 with DCE-MRI, -CT and -US. Given the relative simplicity of DCE-US imaging, additional scanning was performed by DCE-US only on day 7 (n = 22) or day 28 (n = 9) and after a 2-week treatment break (n = 27). This was to provide insight into perfusion changes that may occur within 1 week of therapy and after the 2-week treatment break before re-initiating therapy. The day 28 DCE-US scan was dropped early into the study due to the extra time burden placed on patients that was leading to poor compliance. After the first course of therapy, standard-of-care CT imaging was performed every 2-3 months until progression. After progression, patients were followed for overall survival. Clinical prognosis was determined using the Heng group criteria for mRCC [23].
Imaging methods - overview
DCE-US was used to identify the target lesion, which was either a primary renal mass if present or an accessible metastasis post-resection. Scanning was performed by one of two radiologists with 26 and 10 years of sonography experience. The slice orientation of the baseline US scan was subsequently reproduced during CT and MRI scanning and image analysis as well as possible. Dynamic data were extracted slice by slice from a region of interest (ROI) that encompassed the entire lesion and fitted to the modality-specific kinetic models described below. Median values for each parameter were calculated from the different image slices for each patient at each treatment time point. Image quantification and parameter extraction were blinded to patient outcome variables.
DCE-MRI
DCE-MRI data were acquired at 1.5 T (GE Signa, GE Healthcare, Milwaukee, WI) with a spoiled gradient recalled (SPGR)-based sequence (12 slices, 3 × 3 × 8 mm3, flip angle 15°, TE time 0.968 ms, repetition time of 3.2 ms) with temporal resolution of 3.7 s for ~ 5 min. Omniscan (GE Healthcare, Milwaukee, WI; 0.2 mmol/kg) was injected 20 s into the scan. Data from each region of interest were fitted to a Kety-Tofts model [18] in MATLAB (R2011a, The MathWorks Inc., Natick, MA) to determine the volume transfer constant from blood plasma to the extravascular extracellular space (K trans ), the volume of the extravascular extracellular space as a fraction of total tissue volume (v e ) and the rate constant, which is their ratio (k ep = K trans /v e ). Pre-contrast T1 values for normalisation were determined using fast SPGR scans at two flip angles (15° and 30°, repetition time of 50 ms).
ROIs were drawn to the tumour boundary as determined by the contrast enhancement within the 15th dynamic image (~ 35 s after injection). The arterial input function was obtained from the signal in the descending aorta with compensation for the delay in the bolus arrival time between the aorta and tumour ROIs.
CT and DCE-CT
Clinical CT scans were performed at baseline (pre-treatment) and follow-up between 26-109 days (mean, 57 ± 23 days) for assessment of tumour progression measured by RECIST [1]. DCE-CT data were acquired using the GE LightSpeed VCT (GE Healthcare, Milwaukee, WI), 120kV and 100mAs. Visipaque (GE Healthcare, Milwaukee, WI) contrast was injected 5 s after the scan started at a dose of 1 ml/kg and rate of 5ml/s followed by 30 ml of saline at the same rate. The slice thickness was 5 mm (total range, 4 cm) with in-plane resolution from 0.5-0.7 mm2. The temporal resolution of the dynamic scan was 1 Hz for the first 55 s and 0.25 Hz for the remaining 52 s.
The slice orientation and location of the dynamic study were aligned with the ultrasound scan as best as possible by a radiologist using an initial non-contrast CT scan as a guide. An arterial input function was determined from a region of interest (ROI) drawn in the largest available artery at the level of the tumour. ROIs were drawn around the lesion of interest, including visually perfused and non-perfused areas (whole tumour ROI). The dynamic data were analysed on an imaging workstation (Advantage Windows 4.2, GE Healthcare, Milwaukee, WI) using CT perfusion software (Perfusion 3.0, GE Healthcare, Milwaukee, WI) that uses a deconvolution model [15].
Median blood flow, mean blood volume, mean transit time and permeability surface area product values for the tumours were calculated.
DCE-US
DCE-US measurements were performed using an iU22 scanner (C5-1 probe, Philips Ultrasound, Bothell, WA, USA) operating in split contrast-specific and B-mode during a constant infusion of 0.9 ml of Definity microbubbles (Lantheus Medical Imaging, Boston MA) diluted in 54 ml of saline, infused with a syringe pump at 250 ml/h (Medfusion 3500; Smiths Medical, Dublin, OH) [24]. Disruption-replenishment measurements were performed over 25-30 s in seven planes spanning the tumour volume [24]. In summary, the ultrasound system parameters including image depth, beam focus, mechanical index, gain and signal compression were optimised for each patient during their baseline scan and duplicated for subsequent visits. Linearised time-intensity data were generated within an ROI defined by the tumour boundary, using QLAB (Philips Ultrasound, Bothell, WA, USA) and imported into MATLAB for curve fitting. The time-intensity data were fitted with the lognormal perfusion model [12, 25] allowing the extraction of microvascular parameters reflective of small vessel blood volume (BV) [26], mean flow speed (MFS), perfusion and vascular heterogeneity [27].
Statistical analysis
Initial exploratory analysis used Spearman’s two-tailed correlation coefficient to test the relationship between the median of the vascular parameters of each patient and time point against PFS and OS. Parameters that demonstrated a correlation with PFS and OS with a level of significance of p < 0.05 were selected for further analysis. Survival analysis was performed two-fold: The first was based on imaging cut-off values that separated the patients into groups with a long and short PFS and OS. Cut-off values for each imaging parameter were determined by grouping the patients above and below their median survival time, followed an ROC analysis to determine the parameter value that maximised the separation of the groups. A Kaplan-Meier survival analysis was used to the compare groups. It is important to recognise the limitations of survival analyses that are based on cut-off values in a small group of patients because they may overestimate the impact of imaging parameters on survival. In response to this limitation, a secondary Cox proportional hazard analysis was used to examine the relationship between the imaging parameters and OS and PFS, treated as continuous variables. The results were reported using hazard ratios and their associated 95% confidence intervals. All analyses were carried out using SAS version 9.3 (SAS Institute, Cary, NC, USA).
PFS was calculated from the starting date of sunitinib therapy to the date of tumour progression or death from any cause. OS was calculated from the starting date of any systemic therapy to the date of death from any cause. The sample size (>30) for this imaging study was linked to the design of the phase II clinical trial from which the patient population was drawn [28].
Results
General changes in DCE parameters with sunitinib therapy
The majority of the DCE parameters of all the modalities decreased relative to their baseline level during the first course of treatment for all patients (Figs. 1 and 2). The exception to this trend was vascular heterogeneity measured by DCE-US that was found to increase or remain constant throughout the cycle (Fig. 2C). The mean flow speed by DCE-US also did not change. Other notable observations included a reduction in tumour blood volume (measured by DCE-US) as early as day 7 of treatment in 11/22 patients (Fig. 2A). DCE-US demonstrated that blood volume was maximally reduced on day 14 in most patients. For the patients that were imaged on day 28, blood volume was noted to in fact increase in 56% of the patients even though they continued to be treated. After therapy was discontinued on day 28, 14/27 patients had a rebound in blood volume during the 2-week treatment break (day 42) while 13 remained stable; however these changes were not significantly correlated with PFS or OS [29].
DCE parameters predictive of PFS and OS
The survival analysis based on cut-off values (Table 2 and Fig. 3) demonstrated that a higher baseline K trans (DCE-MRI) and lower baseline vascular heterogeneity (DCE-US) were associated with a longer PFS (HR, 0.42 and 0.32 respectively). A greater per cent decrease in blood volume (DCE-US) from baseline to day 14 was associated with a longer PFS [hazard ratio (HR), 2.6]; the cut-off value was -56%. Additionally, a larger per cent decrease in blood volume as measured by DCE-US on day 14, and a higher K trans value on day 14 (DCE-MRI) corresponded to a longer OS (HR, 3.0 and 0.42 respectively). Furthermore, higher baseline values for blood volume (DCE-CT) and K trans (DCE-MRI) and a lower baseline heterogeneity parameter (DCE-US) were associated with a longer OS (HR, 0.29, 0.42 and 2.3 respectively).
Kaplan-Meier survival plots show a significant difference between the two groups separated using imaging parameter cut-off values (reported in Table 2)
Cox proportional hazard analysis found that a higher baseline and day 14 value for K trans (DCE-MRI) and a lower baseline vascular heterogeneity parameter (DCE-US) were significantly associated with a longer PFS (HR, 0.62, 0.36 and 5.5, respectively) (Table 3). Additionally, a larger per cent decrease in blood volume as measured by DCE-US was associated with a longer OS (HR, 1.45). Of note, we did not find significance between any of the DCE-CT parameters and PFS or OS.
Discussion
Our DCE-MR findings are in line with the studies performed by Hahn et al [30] and Flaherty et al [31] who reported that high pre-treatment K trans predicts a favourable treatment response to sorafenib. Of note, relative week-to-week changes in MRI parameters during therapy were generally not indicative of a survival benefit, although one report on ten patients found a correlation between the mean decrease in K trans (r, -0.617, p = 0.043) and k ep (r, -0.598, p = 0.052) with PFS [32]. This general observation can be explained by considering the assumptions of the pharmacokinetic model from which the parameters are derived. The K trans /k ep parameters are generally interpreted as a mixture of flow and vascular permeability whose relative weighting is likely to shift during the course of anti-angiogenic therapy [33]. Because RCCs are typically highly vascularised, baseline K trans measurements most likely reflect the permeability of the tumour microvasculature. The known microvascular disruption that is caused by TKIs is a strong confounder of this assumption [5] and offers an explanation for the lack of support for pre-post treatment tests in the MR/mRCC literature.
DCE-CT is also capable of quantifying tumour vascularity and its response to vascular-targeted therapies. Han et al [34] studied RCC patients receiving sunitinib or sorafenib and found a correlation between tumour enhancement at baseline and response and PFS. In contrast, our study, like that reported by Fournier et al [35], found that baseline perfusion parameters were higher in patients with longer survival times but were not significantly predictive of outcome except when a cut-off analysis was employed. Alternative CT based methods continue to be developed to evaluate tumour response to anti-angiogenic therapy and predict clinical outcome. These include techniques that supplement standard measurements of tumour size with radiologic density [36,37,38], morphology and structure [39]. CT texture heterogeneity, entropy and uniformity are additional parameters that show promise for evaluating the anti-angiogenic response of metastatic renal cell carcinoma [40].
Our DCE-US findings are in agreement with those of Lassau et al [8] who identified that a 40% or greater decrease in blood volume, measured from the area under the DCE-US bolus curve, predicted a longer PFS in 539 patients on different types of anti-angiogenic therapy (157 RCC patients) imaged before and during therapy. In a second report, Lassau et al [41] studied 38 RCC patients on sunitinib with DCE-US at baseline and on day 15 and identified two DCE-US parameters (time to peak intensity and slope of the wash-in) that were significantly associated with PFS. Furthermore, they found that the time to peak intensity was significantly associated with OS. Although both our and Lassau’s work report the use of DCE-US, it is important to recognise the differences in how the modality is implemented. Unlike other groups that administer the contrast agent as a bolus, our study protocol relied on a continuous infusion of contrast and the method of disruption-replenishment [24]. The disruption-replenishment principle [42] removes the uncertainty of the arterial input function by introducing a negative bolus directly in the tissue under study. This simplifies the interpretation of the DCE-US time-intensity curve and introduces a parameter unique to DCE-US that reflects the level of vascular flow heterogeneity [27]. It is commonly accepted from biological studies that anti-angiogenic therapy first disrupts the smaller vessels of the microvasculature [5, 26]. This selective pruning asymmetrically reduces the slower flow contributions in the pharmacokinetics model, effectively increasing the width of the probability density function of the flow distribution along with the magnitude of the heterogeneity parameter. The converse may be true when therapy is ineffective, in which case a decrease in the heterogeneity parameter would indicate that the microvasculature is continuing to develop. The rebound that was observed following treatment cessation, although not correlated with outcome [29], informed the design of a prospective phase II study where the dose and schedule of sunitinib were individualised based on toxicity. Many patients were able to remain on a higher dose for a shorter duration (7-14 days) and with a shortened treatment break of 7 days (thus minimising the opportunity for ‘rebound’) [43].
As reported, each modality produced parameters that relate the pharmacokinetics of their respective exogenous contrast agents to properties of the tissue microvasculature. All three modalities produced parameters that were predictive of PFS and/or OS with similar power, sensitivity and specificity. DCE-CT parameters were only predictive of outcome at baseline, while DCE-MR and DCE-US parameters were predictive of outcome at baseline and with respect to on-treatment time points. It is difficult to distinguish the difference between a predictive and prognostic marker in a baseline-only setting because one cannot say with certainty whether the observed outcome was related to the natural course of the disease or influenced by the prescribed therapy. In our study, DCE-US was the only modality that produced parameters where a per cent change during therapy was associated with treatment benefit. Analyses based on changes during therapy are potentially more powerful in a treatment-monitoring setting, as there is a lower intrinsic variability in the relationship between the observed change in the tumour and drug’s activity, with each tumour acting as its own ‘control’.
Differences in the predictive level of the three modalities arise from the kinetics of their respective contrast agents through the tissue and tumour microcirculation. Crucial to extracting meaningful quantitative information from the dynamics of a contrast injection is an understanding of how the presence of the agent impacts the modality’s measured signal and its relation to the local agent’s tissue concentration. Of the three modalities studied here, the iodinated contrast agents used in DCE-CT are the simplest to quantify as the change in the signal attenuation following contrast injection is directly related to its local concentration [15]. MR contrast enhancement in the presence of gadolinium-based agents is due to indirect and multifactorial effects that disrupt the local magnetic fields and natural tissue relaxation rates. Both of these agents are small molecules that diffuse through the vascular endothelium at a rate influenced by the enhanced permeability of the tumour vasculature. Ultrasound contrast agents are unique among the three as they remain intravascular throughout their transit and travel with comparable rheology to that of red blood cells. The DCE-US signal intensity also bears a linear relationship with contrast concentration; however, a direct comparison of absolute signal levels between sites is confounded by ultrasound attenuation by the skin and other intervening tissues [44]. As such, each imaging modality has its own strengths and challenges.
DCE-CT scans are fast and readily available in many centres, but rely on non-negligible doses of ionising radiation. MRI is a powerful modality that can leverage additional contrast mechanisms (diffusion, chemical exchange, saturation transfer, etc.). However, challenges for widespread adoption of MRI for therapy monitoring include a substantial technical infrastructure as a barrier to access. Ultrasound and ultrasound contrast agents are known to be safe, especially in patients with compromised renal function, and are widely accessible. Imaging protocols have been developed that minimise the impact of tissue attenuation and other confounding factors and operate with a respectable reproducibility of 9-14%. However, ultrasound imaging is operator-dependent and limited to abdominal organs and other soft tissue targets that are clear from bone, air and gas. After the acquisition of the scans, the time required to perform the perfusion calculations is primarily limited by offline region of interest placement that delineates the tumour border within each image plane. The efficiency of DCE perfusion calculations could be optimised with dedicated software applications that streamline this quantification process. In their current form, the imaging techniques described in this article are not yet applicable to every day practice. Clinical translation may come with increased exposure and consensus among practitioners on which technique, modality and parameters most reliably impact clinical decision-making. However, DCE- perfusion techniques are not without meaningful utility. For example, they could be used to determine the best treatment schedule for novel anti-angiogenic drugs before large trials are commenced. Specifically, the DCE-US data from the current study were the basis for the dosing schedules used in a phase II study of individualised sunitinib where some patients receive a compressed regimen of therapy with a shorter time both on and off drug [43].
Future studies could explore how the predictive power of imaging could be improved by multi-modality combinations of pre-treatment parameters with early changes following treatment using generalised linear models. Such a strategy would leverage the advantages of each modality (e.g. diffusible versus intravascular contrast agents) by incorporating measures of the pre-existing tumour state with the tumour’s early sensitivity to the prescribed therapy.
Our study has several weaknesses. For ease of imaging with all modalities, 24 patients with intact primary tumours were included in the study. This group of patients is generally sicker and has a poorer outcome compared to the general RCC population. Our study would have been improved with increased patient recruitment and by examining additional lesions in each patient since response measured at one localised site may misrepresent the global response.
In summary, dynamic contrast-enhanced MRI, CT and ultrasound produce complementary parameters that reflect the prognosis of patients receiving sunitinib therapy for metastatic renal cell carcinoma. Blood volume measured by DCE-US was the only parameter whose change during early anti-angiogenic therapy predicted overall survival and progression-free survival. Of all the parameters studies, baseline measurements of vascular heterogeneity by DCE-US have the strongest prognostic value for PFS.
References
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Weber WA (2009) Assessing tumor response to therapy. J Nucl Med 50:1S–10S
Desar IME, Herpen CMLV, Laarhoven HWMV, Barentsz JO, Oyen WJG, Graaf WTAVD (2009) Beyond RECIST: Molecular and functional imaging techniques for evaluation of response to targeted therapy. Cancer Treat Rev 35:309–321
Sirous R, Henegan JC, Zhang X, Howard CM, Souza F, Smith AD (2016) Metastatic renal cell carcinoma imaging evaluation in the era of anti-angiogenic therapies. Abdom Radiol 41:1086–1099
Mancuso M, Davis R, Norberg S et al (2006) Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Investig 116:2610–2621
Desar IME, Van Herpen CML, Van Laarhoven HWM, Barentsz JO, Oyen WJG, Van Der Graaf WTA (2009) Beyond RECIST: Molecular and functional imaging techniques for evaluation of response to targeted therapy. Cancer Treat Rev 35:309–321
O'Connor J, Aboagye E, Adams J, Aerts H, Barrington S, Beer A (2017) Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol 14:169–186
Lassau N, Vilgrain V, Taieb S et al (2012) Evaluation with DCE-US of antiangiogenic treatments in 539 patients allowing the selection of one surrogate marker correlated to overall survival. J Clin Oncol abstract 4618
Leen E, Averkiou M, Arditi M et al (2012) Dynamic contrast enhanced ultrasound assessment of the vascular effects of novel therapeutics in early stage trials. Eur Radiol 22:1442–1450
O'Connor JPB, Jackson A, Parker GJM, Roberts C, Jayson GC (2012) Dynamic contrast-enhanced MRI in clinical trials of antivascular therapies. Nat Rev Clin Oncol 9:167–177
Prezzi D, Khan A, Goh V (2015) Perfusion CT imaging of treatment response in oncology. Eur J Radiol 84:2380–2385
Hudson JM, Karshafian R, Burns PN (2009) Quantification of flow using ultrasound and microbubbles: A disruption replenishment model based on physical principles. Ultrasound Med Biol 35:2007–2020
Katabathina VS, Lassau N, Pedrosa I, Ng CS, Prasad SR (2012) Evaluation of treatment response in patients with metastatic renal cell carcinoma: Role of state-of-the-art cross-sectional imaging. Curr Urol Rep 13:70–81
Lamuraglia M, Bridal SL, Santin M et al (2010) Clinical relevance of contrast-enhanced ultrasound in monitoring anti-angiogenic therapy of cancer: current status and perspectives. Crit Rev Oncol Hematol 73:202–212
Lee TY (2002) Functional CT: Physiological models. Trends Biotechnol 20:S3–S10
Nathan P, Vinayan A (2013) Imaging techniques as predictive and prognostic biomarkers in renal cell carcinoma. Ther Adv Med Oncol 5:119–131
O'Connor JPB, Jackson A, Parker GJM, Jayson GC (2007) DCE-MRI biomarkers in the clinical evaluation of antiangiogenic and vascular disrupting agents. Br J Cancer 96:189–195
Tofts PS, Brix G, Buckley DL et al (1999) Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging 10:223–232
Ammari S, Thiam R, Cuenod CA et al (2014) Radiological evaluation of response to treatment: Application to metastatic renal cancers receiving anti-angiogenic treatment. Diagn Interv Imaging 95:527–539
Lassau N, Chami L, Benatsou B, Peronneau P, Roche A (2007) Dynamic contrast-enhanced ultrasonography (DCE-US) with quantification of tumor perfusion: a new diagnostic tool to evaluate the early effects of antiangiogenic treatment. Eur Radiol Suppl 17:89–98
Yankeelov TE, Niermann KJ, Huamani J et al (2006) Correlation between estimates of tumor perfusion from microbubble contrast-enhanced sonography and dynamic contrast-enhanced magnetic resonance imaging. J Ultrasound Med 25:487–497
Kim E, Kim J, Maelandsmo GM, Johansen B, Moestue SA (2016) Anti-angiogenic therapy affects the relationship between tumor vascular structure and function: A correlation study between micro-computed tomography angiography and dynamic contrast enhanced MRI. Magn Reson Med 25:25
Heng DYC, Xie W, Regan MM et al (2009) Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor–targeted agents: results from a large, multicenter study. J Clin Oncol 27:5794–5799
Williams R, Hudson JM, Lloyd BA et al (2011) Dynamic microbubble contrast-enhanced us to measure tumor response to targeted therapy: A proposed clinical protocol with results from renal cell carcinoma patients receiving antiangiogenic therapy. Radiology 260:581–590
Hudson JM (2013) Lognormal Perfusion Model. http://www.mathworks.com/matlabcentral/fileexchange/40786-lognormal-perfusionmodel
Hudson JM, Williams R, Lloyd B et al (2011) Improved flow measurement using microbubble contrast agents and disruption-replenishment: Clinical application to tumour monitoring. Ultrasound Med Biol 37:1210–1221
Hudson JM, Williams R, Karshafian R et al (2013) Quantifying vascular heterogeneity using microbubble disruption-replenishment kinetics inpatients with renal cell cancer. Investig Radiol 49(2):116–123
Ebos JML, Mastri M, Hudson JM et al (2015) Effect of the timing of sunitinib administration on the predictive value of biomarkers in renal cell cancer (mRCC). J Clin Oncol 33:11096–11096
Bjarnason GA, Khalil B, Hudson JM et al (2014) Outcomes in patients with metastatic renal cell cancer treated with individualized sunitinib therapy: Correlation with dynamic microbubble ultrasound data and review of the literature. Urol Oncol 32:480–487
Hahn OM, Yang C, Medved M et al (2008) Dynamic contrast-enhanced magnetic resonance imaging pharmacodynamic biomarker study of sorafenib in metastatic renal carcinoma. J Clin Oncol 26:4572–4578
Flaherty K, Rosen M, Heitjan D et al (2008) Pilot study of DCE-MRI to predict progression-free survival with sorafenib therapy in renal cell carcinoma. Cancer Biol Ther 7:496–501
Desar IME, ter Voert EGW, Hambrock T et al (2012) Functional MRI techniques demonstrate early vascular changes in renal cell cancer patients treated with sunitinib: a pilot study. Cancer Imaging 11:259–265
Sourbron SP, Buckley DL (2011) On the scope and interpretation of the Tofts models for DCE-MRI. Magn Reson Med 66:735–745
Han KS, Jung DC, Choi HJ et al (2010) Pretreatment assessment of tumor enhancement on contrast-enhanced computed tomography as a potential predictor of treatment outcome in metastatic renal cell carcinoma patients receiving antiangiogenic therapy. Cancer 116:2332–2342
Fournier L, Oudard S, Thiam R et al (2010) Metastatic renal carcinoma: Evaluation of antiangiogenic therapy with dynamic contrast-enhanced CT1. Radiology 256:511–518
van der Veldt AA, Meijerink MR, van den Eertwegh AJ, Haanen JB, Boven E (2010) Choi response criteria for early prediction of clinical outcome in patients with metastatic renal cell cancer treated with sunitinib. Br J Cancer 102:803–809
Nathan P, Vinayan A, Stott D, Juttla J, Goh V (2010) CT response assessment combining reduction in both size and arterial phase density correlates with time to progression in metastatic renal cancer patients treated with targeted therapies. Cancer Biol Ther 9:15–19
Tirkes T, Hollar MA, Tann M, Kohli MD, Akisik F, Sandrasegaran K (2013) Response criteria in oncologic imaging: review of traditional and new criteria. Radiographics 33:1323–1341
Smith AD, Shah SN, Rini BI, Lieber ML, Remer EM (2010) Morphology, Attenuation, Size, and Structure (MASS) criteria: assessing response and predicting clinical outcome in metastatic renal cell carcinoma on antiangiogenic targeted therapy. AJR Am J Roentgenol 194:1470–1478
Goh V, Ganeshan B, Nathan P, Juttla JK, Vinayan A, Miles KA (2011) Assessment of response to tyrosine kinase inhibitors in metastatic renal cell cancer: CT texture as a predictive biomarker. Radiology 261:165–171
Lassau N, Koscielny S, Albiges L et al (2010) Metastatic renal cell carcinoma treated with sunitinib: Early evaluation of treatment response using dynamic contrast-enhanced ultrasonography. Clin Cancer Res 16:1216–1225
Wei K, Jayaweera A, Firoozan S, Linka A, Skyba D, Kaul S (1998) Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation 97:473–483
Bjarnason GA, Knox JJ, Kollmannsberger CK et al (2017) Phase II study of individualized sunitinib (SUN) as first-line therapy for metastatic renal cell cancer. J Clin Oncol 35:4514–4514
Stride E, Tang M-X, Eckersley RJ (2009) Physical phenomena affecting quantitative imaging of ultrasound contrast agents. Applied Acoustics 70:1352–1362
Funding
This study has received funding from the Terry Fox Programme of the National Cancer Institute of Canada, the Canadian Institutes of Health Research and an investigator-initiated grant to Georg A. Bjarnason from Pfizer Canada.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Georg Bjarnason.
Conflict of interest
The authors of this manuscript declare relationships with the following companies:
Georg A. Bjarnason: Pfizer Canada: grant support for this study, CME presentations and travel support to oncology meetings. The remaining authors have no further conflicts to disclose.
Statistics and biometry
Alex Kiss (author) has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
A subset of the patient cohort included in this article has been reported in the following journal publications. The referenced articles were reports on technical protocol development (3), a new DCE-US parameter development (2) or describing using DCE-US vascular volume changes during and after sunitinib therapy to justify changes in sunitinib scheduling (1):
1. Bjarnason G A, Khalil B, Hudson JM, Williams R, Milot LM, Atri M, et al. Outcomes in patients with metastatic renal cell cancer treated with individualized sunitinib therapy: Correlation with dynamic microbubble ultrasound data and review of the literature. Urol Oncol. Elsevier; 2013;32(4):1–8.
2. Hudson JM, Williams R, Karshafian R, Milot L, Atri M, Burns PN, et al. Quantifying vascular heterogeneity using microbubble disruption-replenishment kinetics in patients with renal cell cancer. Invest Radiol [Internet]. 2014;49(2):116–23.
3. Williams R, Hudson JJM, Lloyd BBA, Sureshkumar AR, Lueck G, Bjarnason GA, et al. Dynamic microbubble contrast-enhanced US to measure tumor response to targeted therapy: a proposed clinical protocol with results from renal cell carcinoma patients receiving antiangiogenic therapy. Radiology [Internet]. 2011 [cited 2012 Oct 12];260(2):581–90.
Methodology
• prospective
• diagnostic or prognostic study
• performed at one institution
Rights and permissions
About this article
Cite this article
Hudson, J.M., Bailey, C., Atri, M. et al. The prognostic and predictive value of vascular response parameters measured by dynamic contrast-enhanced-CT, -MRI and -US in patients with metastatic renal cell carcinoma receiving sunitinib. Eur Radiol 28, 2281–2290 (2018). https://doi.org/10.1007/s00330-017-5220-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-5220-2