Abstract
The purpose of this article is to describe the use of ultrasound for the diagnosis and treatment of painful joint arthroplasty. Ultrasound plays a crucial role in the diagnosis of the painful joint arthroplasty, especially given its unique dynamic capabilities, convenience, and high resolution. Ultrasound guidance is also instrumental for procedures in both diagnosing and in select cases, treating the painful joint arthroplasty. Topics to be discussed in this article include trends in arthroplasty placement, benefits of the use of ultrasound overall, and ultrasound evaluation of periprosthetic joint infections. We will also review the sonographic findings with dissociated/displaced components and adverse reaction to metallic debris including metallosis, trunnionosis, and metal-on-metal pseudotumors. Additionally, we will discuss ultrasound evaluation of tendon pathologies with arthroplasties, including dynamic maneuvers to evaluate for tendon impingement/snapping. Finally, we will cover ultrasound-guided joint arthroplasty injection indications and precautions.
Key points
• Ultrasound is preferred over MRI in patients with joint arthroplasty and plays a crucial role in diagnosis, especially given its unique dynamic capabilities, convenience and high resolution.
• It is especially beneficial for US-guided aspiration in periprosthetic joint infections; effectively used to evaluate periprosthetic fluid collections, facilitating differentiation between abscesses and aseptic collections, and tracking sinus tracts.
• Recently, the diagnosis of periprosthetic joint infections has shifted focus to biomarkers in the periprosthetic fluid, specifically α‐defensin, which has a high sensitivity and specificity for diagnosing infection.
• Cutibacterium acnes is a major pathogen responsible for shoulder arthroplasty infections, often presenting with normal laboratory values and since slow growing, must be kept for a minimum of 14 days.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Total joint arthroplasty (TJA) is among the most common orthopedic surgeries performed for the highly effective treatment of osteoarthritis-related pain [1,2,3,4]. This pertains to mainly an elderly population in whom several common medical conditions may coexist, potentially increasing the risk of complications [4,5,6,7,8,9]. Currently, approximately 700,000 total knee arthroplasties (TKA), 470,000 total hip arthroplasties (THA), and 90,000 arthroplasties, other than hip or knee (the majority being shoulder), are performed in the USA annually. Approximately, 10% of these are revision arthroplasties [1, 10, 11]. Over the next several decades, the number of TJA surgeries as well the incidence of revision arthroplasties will continue to increase [4, 10, 11]. The number of these arthroplasties performed annually continues to steadily rise due to the increasing life expectancy and the associated high prevalence of osteoarthritis. It is estimated that by 2030, up to nearly 3.5 million TKA and 600,000 THA will be performed in the USA annually [4, 6, 12,13,14,15]. Advancements in THA and TKA techniques and implant designs and technologies, as well as the resurgence in unicompartmental knee arthroplasty, continue to improve outcomes while decreasing complications [4, 16,17,18]. Furthermore, multiple studies have demonstrated that robotic-assisted joint replacement allows for improved precision of implant positioning for THA, TKA, and unicompartmental knee arthroplasty when compared to conventional jig-based techniques, potentially decreasing complications [4, 5, 19,20,21,22]. Despite continued improvements, studies demonstrate that many joint replacement patients are not satisfied with their outcomes [4, 5, 16,17,18,19,20,21,22].
Subsequently, daily inpatient and outpatient consultations regarding painful arthroplasties are directed to musculoskeletal radiology for diagnostic guidance and therapeutic interventions. The workup of a painful arthroplasty is performed as a collaborative effort between primary care physicians, orthopedic surgeons, and radiologists. Radiographs are the first-line imaging modality when there is concern for complications following arthroplasty and can be used to assess the integrity and positioning of the arthroplasty components, as well as to evaluate the adjacent osseous structures [7].
The use of musculoskeletal ultrasound (US) has significantly increased over the past few decades given its ability to perform dynamic imaging while interacting directly with the patient, the ability to compare with the contralateral side, and the ease of accessibility and portability, as well as its lower cost when compared to magnetic resonance imaging (MRI) [7, 23,24,25,26,27]. Furthermore, contrary to radiographs and computed tomography, US has no ionizing radiation [4]. There has also been significantly increased utilization of US in the evaluation of TKA-related pain and for US-guided joint aspirations [25, 26]. US is especially beneficial for US-guided aspiration of infectious materials in periprosthetic joint infection (PJI) [23, 28,29,30,31,32]. US is preferred over MRI in patients with TJA in whom MRI is difficult to perform because of magnetic susceptibility artifacts, requiring the use of special metal artifact reduction sequences to reduce the extent and intensity of susceptibility artifacts [33,34,35,36].
Periprosthetic joint infection
Background
PJI is one of the most challenging complications of TJA. It is sometimes difficult to confidently diagnose but critical to identify expediently as it must be treated vigorously to avoid catastrophic complications [37]. Newly validated criteria hinge on clinical findings, laboratory results, imaging findings, and synovial fluid analysis (Table 1). Furthermore, a dry aspiration often necessitates surgical intervention to fully elucidate [37]. Although the incidence of PJI is relatively low, it predictably follows TJA in some patients and can cause profound medical, financial, and socioeconomic burdens on patients with a reduction in the quality of life. PJI is the most common cause for revision TJA. The incidence of PJI following primary TKA and THA is approximately 1–4% and 1% within 2 years, respectively. Furthermore, in regard to revision TKA and THA, these percentages are actually doubled [38].
Radiographs are the first-line imaging modality in the evaluation of a PJI and can demonstrate a suspected joint effusion or synovial hypertrophy and be used to evaluate the integrity of the components and the adjacent bone [7]. Computed tomography can also be used in select cases as it is more sensitive at detecting subtle osteolysis and characterizing the full extent of bone loss for surgical planning [7].
Sonographic imaging
US is especially beneficial for US-guided aspiration of infectious materials in PJI and can be effectively used to evaluate periprosthetic fluid collections, facilitating differentiation between abscesses and aseptic collections, and to track the presence of sinus tracts within soft tissues [23, 28,29,30,31,32]. US can be used to evaluate the joint capsule for hypoechoic distention indicating either a joint effusion, synovial hypertrophy, or a combination of both. The use of dynamic compressibility during US of a distended joint capsule allows an advantage to both radiographs and MRI in differentiating between synovitis and joint effusion. Synovitis will not compress while simple joint fluid will completely compress and a complex joint effusion in combination with synovial hypertrophy will partially compress or occasionally demonstrate mobile debris (Fig. 1). In order to evaluate the knee joint, for example, the US transducer is placed in the long axis, at the suprapatellar location, paralleling the quadriceps tendon (Fig. 1) and scanning both laterally and medially. The transducer is then turned horizontally to evaluate the suprapatellar recess in the short axis as well. Real-time Doppler can also be easily applied, without the need for intravenous contrast, to observe if there is associated hyperemia, indicating acute synovitis and active inflammation [7].
Images of a 74-year-old woman with an existing right total knee arthroplasty. a Cross-table lateral radiograph demonstrates a right total knee arthroplasty (the arrow points to the femoral component) with distention of the suprapatellar recess (star) compatible with a joint effusion and/or synovial hypertrophy. Q denotes the shadow of the quadriceps tendon. b Long-axis sonographic image of the anterior aspect of the same knee shows compressible hypoechoic distention of the suprapatellar recess (star) compatible with a joint effusion. Q denotes the quadriceps tendon and the arrow points to the shadowing femoral component of the total knee arthroplasty. A subsequent ultrasound-guided aspiration resulted in 24 mL of purulent fluid
US guidance can then be used for the aspiration (Figs. 2 and 3). Using constant sonographic guidance, the needle can be directed towards areas of simpler fluid to avoid clogging the needle. For example, in the hip joint, the US transducer is placed in the long axis, paralleling the femoral neck, and the needle is inserted using an in-plane approach, from distal to proximal (Fig. 2). If there is no fluid return, US can allow visualization of the debris blocking the needle tip. Simply reinserting the stylet can then clear that debris. Additionally, real-time US imaging allows the avoidance of passing through wounds, ulcers, cellulitis, and subcutaneous fluid collections such as abscesses when approaching the deeper joint space. We recommend that while using local anesthetic prior to a joint aspiration, constant US guidance should be utilized to avoid the intraarticular administration of the anesthetic, which could have bacteriostatic properties and alter the aspirate results [7, 23, 28,29,30,31,32].
A 62-year-old man with a right total hip arthroplasty presenting with pain. a Anteroposterior radiograph reveals a right total hip arthroplasty without radiographic evidence of hardware complication. The open arrow points to the femoral head component and the star denotes the location of the joint capsule near the level of the trunnion. b Long-axis ultrasound image of the anterior hip displays the right total hip arthroplasty (the open arrow points to the shadowing femoral head component) with no significant distention of the hip joint capsule (star). c Long-axis image shows the needle (solid arrows) entering the joint capsule (star) during an attempted right hip ultrasound-guided aspiration using an in-plane technique with a distal to proximal approach. The open arrow points to the femoral head component
An 81-year-old woman presenting with pain with an existing left reverse total shoulder arthroplasty. a Anteroposterior (Grashey) radiograph of the left shoulder demonstrates a left reverse total shoulder arthroplasty without evidence of hardware complication. The solid arrow points to the glenosphere. b Long-axis sonographic image of the posterior aspect of the same left shoulder during an ultrasound-guided aspiration shows shadowing artifact (solid arrow) consistent with the glenosphere of the reverse total shoulder arthroplasty. The needle (open arrows) is seen in-plane, entering the joint capsule (star) with a medial to lateral approach. A total of 12 mL thick red fluid was aspirated
α‐defensin
More recently, the diagnosis of PJI has shifted focus to biomarkers in the periprosthetic fluid, specifically α‐defensin (Synovasure, Zimmer Biomet, Warsaw, IN) [37, 38]. α‐defensin is an antimicrobial peptide released by neutrophils in response to pathogens. It acts as a natural peptide antibiotic by inducing depolarization of the cell membrane, which leads to the rapid death of the microorganism. It has been described as an ideal biomarker for PJI due to its high sensitivity and specificity. Quantitative measurements of α-defensin levels in joint fluid have been shown to have a sensitivity ranging from 85 to 100% and similar specificity and accuracy for diagnosing PJI [38]. Furthermore, it has been shown to be effective even in the presence of antibiotics and low virulence organisms and with patient inflammatory comorbidities. The evaluation is typically overnight shipped to an outside facility for processing, although if available, a newer α‐defensin lateral flow test can be processed in-house and the same day, with only slightly decreased sensitivity, specificity, and accuracy [38]. An α‐defensin test should be included in the clinical diagnostic criteria for PJI when performing an US-guided joint aspiration [37, 38].
Cutibacterium acnes
PJI of the shoulder is rare, but remains a serious complication and one of the most frequent causes of a painful shoulder arthroplasty requiring revision shoulder arthroplasty [39,40,41]. PJI of the shoulder has been reported to occur in 1.1–4% of those with a total shoulder arthroplasty and in 3.8–18% of those following a reverse total shoulder arthroplasty [39, 41]. Cutibacterium acnes (formerly known as Propionibacterium acnes) is a non-spore-forming, anaerobic, Gram-positive bacillus commonly found in hair follicles and sebaceous glands deep in the dermis. It can be isolated in the flora of the face, chest, axilla, and lateral shoulder and has emerged as a major pathogen responsible for postoperative shoulder infections following arthroplasty procedures. C. acnes adheres to cells, biofilms, and surfaces by means of antigenic proteins, which can then initiate an inflammatory response within the joint. C. acnes can occur up to 2 years after the initial surgical placement. Among those undergoing shoulder arthroplasty, younger men are particularly at risk, especially in those with shoulder arthroplasties performed following trauma. Posttraumatic shoulder arthroplasty has a 3 times higher risk than elective surgery [41]. In those undergoing shoulder arthroplasty, PJI can reach 10% in the male subgroup, a 2.5 times higher risk than females [39, 41].
Patients with a C. acnes shoulder infection often present with normal laboratory values, including normal white blood cells, erythrocyte sedimentation rate, and C-reactive protein. Furthermore, these bacteria on cultures are slow-growing. These challenging characteristics result in a difficult and sometimes delayed diagnosis that can result in significant disease and increased morbidity and lead to undetected prosthesis failure. US-guided synovial fluid aspiration samples sent for C. acnes culture must be specified in the orders to be kept for a minimum of 14 days to optimize the sensitivity and specificity to detect C. acnes [41] (Fig. 4).
Images of a 74-year-old woman following stage 1 revision of a left total shoulder arthroplasty due to a periprosthetic joint infection. a Anteroposterior (Grashey) radiograph of the left shoulder demonstrates stage 1 revision changes, status posthardware removal of the left total shoulder arthroplasty with placement of an antibiotic cement spacer (arrow). G denotes the glenoid. b Long-axis sonographic image of the posterior aspect of the same left shoulder prior to an ultrasound-guided aspiration shows shadowing artifact consistent with the antibiotic cement spacer (arrow) with the adjacent glenoid (G). The image reveals complex, incompletely compressible hypoechoic distention of the glenohumeral joint capsule (star) consistent with a combination of a complex joint effusion and synovial hypertrophy. A subsequent ultrasound-guided aspiration resulted in 19 mL thin reddish fluid, which was sent for the requested analysis including a Cutibacterium acnes culture and α‐defensin which were both negative
Saline lavage
Frequently encountered dilemmas with US-guided TJA aspirations arise when there is no synovial fluid visualized or a very small effusion is suspected, and an aspiration is then attempted but there is no fluid return (so-called dry tap).
There is considerable debate in the literature in regard to whether or not to perform a sterile saline lavage of the joint using US guidance with the injection of sterile, non-bacteriostatic saline and sending that fluid for analysis. Ting and Della Valle [42] state that in the event there is no fluid aspirated, they do not recommend performing a joint lavage with sterile saline. Porrino and colleagues [43] are also opposed, stating that there are no high-quality studies supporting the diagnostic value of that method, which can dilute microorganism concentration and be unrepresentative of the joint fluid and could pose a risk of actually causing an infection. Finally, Abdel Karim et al. [44] recommend against its use as well, except in certain circumstances when it is performed by a dedicated radiologist using sterile technique.
On the contrary, multiple studies have shown that lavage is useful in providing a positive diagnostic yield and in assisting in preoperative decision-making [45,46,47,48,49]. These studies have shown the potential of obtaining positive cultures following joint lavage; however, it is recommended that the sample be clearly labelled as a saline lavage as it will alter the cell count. Lee et al. [49] demonstrated that in “dry joints” that were lavaged with sterile non-bacteriostatic saline, then aspirated and cultured, there was an 83% sensitivity, 93% specificity, and 83% accuracy yield when compared with tissue cultures obtained at the time of the revision surgery. As a large institution, performing a high number of these TJA saline lavage procedures, we can also attest to its benefit in the management of PJI and recommend its use. However, if there is ever doubt, direct communication with the ordering orthopedic surgeon is strongly advised.
Dissociated/displaced components
Background
Aseptic loosening and wear are second to only infection as the most common causes of arthroplasty failure. Radiographs are also the first-line imaging modality in the evaluation of dissociated/displaced components and can demonstrate signs of wear and component malalignment, as well as be used to evaluate the integrity of the components.
Sonographic imaging
Sonographic findings of dissociated/displaced components, including polyethylene component dissociation and displacement, are infrequently described in the literature with often only reference to the use of US to complement other modalities in the assessment of periarticular fluid collections, such as joint effusions and soft tissue changes [7]. The direct evaluation of hardware using US is limited due to shadowing artifacts and positioning. However, as the use of MSK US significantly increases, especially for the evaluation of soft tissues and the joint, knowledge of the appearance of dissociated/displaced components is crucial. Furthermore, US is preferred over MRI in patients with TJA in whom MRI is difficult to perform because of magnetic susceptibility artifacts and requires the use of metal artifact reduction sequences [33,34,35,36].
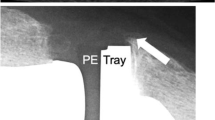
The smooth surfaces of the arthroplasty (Fig. 3) and the osseous contour can be assessed on US while evaluating for linear echogenic structures in unexpected locations, such as displaced into the intraarticular fat pads or extraarticular soft tissues, which indicates displaced components and warrants further evaluation and correlation with radiographs. For example, the polyethylene component in a TKA can dissociate from its locking mechanism and be seen displaced into the infrapatellar (Hoffa) fat pad [7] (Fig. 5a). Furthermore, a unique advantage of US is its capability to detect fragments of polyethylene, indicating component fracture. Also, displacement of the patellar resurfacing component of the TKA is a known and described complication [7, 50, 51] that can be identified with US when evaluating the periarticular soft tissues.
A 62-year-old man presented for evaluation of a painful right total knee arthroplasty, 6 years following surgery. a Panoramic long-axis sonographic image of the anterior knee, obtained with the knee in extension, at the level of the quadriceps tendon (Q), patella (Pat), patellar tendon (P), and tibial tubercle (T). The image reveals a large complex partially compressible hypoechoic suprapatellar joint effusion (star) with curvilinear echogenic areas of shadowing (solid arrows) along the anterior aspect compatible with metallic debris and metallosis. A rectangular-shaped geographic echogenicity, centrally anechoic, (open arrow) is noted, inferior to the patella compatible with anterior displacement of the polyethylene insert/liner into the infrapatellar (Hoffa) fat pad. b Photograph obtained of the aspirate obtained following the ultrasound-guided aspiration of the right knee. Note the dense black color of the resultant aspirate, typical of metallosis and metal-induced synovitis. c During the stage-one revision a synovectomy was performed showing diffuse black-stained synovium, also typical of metallosis and metal-induced synovitis
Adverse reaction to metallic debris
Background
Adverse reaction to metallic debris is an umbrella title encompassing metallosis, trunnionosis, pseudotumors, and aseptic lymphocytic vasculitis associated lesions (histological entity) [52]. Breakdown and loosening of components are inevitable. In the absence of infection, the most common complication in TJA is from the degradation of arthroplasty components, both metal and polyethylene, and their resultant effects on the surrounding tissues [7, 53]. Early degeneration of the polyethylene component can lead to the deposition of small particles of polyethylene into the joint space and surrounding tissues, which is referred to as “plasticosis.” Particularly with older generation polyethylene (before the year 2000), the plastic debris can be very reactive and create osteolysis. Further wear or displacement of the polyethylene component can result in metal-on-metal (MoM) contact and subsequent deposition of metal products, which are predominantly oxides, a condition referred to as “metallosis” [7, 53,54,55,56] (Figs. 5 and 6).
A 65-year-old woman complaining of pain and swelling, 4 years following placement of a left total knee arthroplasty. a Anteroposterior and b lateral radiographs of the left knee demonstrating a left total knee arthroplasty with lobulated dense joint capsular distention (bubble sign) (open arrows) consistent with metal-induced synovitis and metallosis. Note the anterior displacement of the disc-shaped lucent appearing polyethylene liner (solid arrows) resulting in complete loss of the joint space, metal-on-metal contact (star), and significant valgus alignment. c Photographs obtained during the staged revision reveal the densely black-stained synovium (solid arrows) and an anteriorly displaced polyethylene tray component (open arrows). d Photographs of the operative gross specimens show the removed displaced polyethylene liner demonstrating marked asymmetric wear at the posterior aspect (black arrow) and significant corresponding asymmetric metallic erosion of the subjacent posterior aspect of the tibial metallic baseplate component (black arrow). (Operative images courtesy of Dr. Eddie El-Yussif, Henry Ford Macomb Hospital, Clinton Township, MI)
Furthermore, adverse reaction to metallic debris is an emerging problem with MoM hip replacements [52]. Greater than 1.5 million MoM hip arthroplasties have been implanted worldwide [36, 57]. In the early 2000s, MoM arthroplasties made up 35% of all THA performed annually in the USA [36]. Singisetti and colleagues [52] have demonstrated that US is an inexpensive, noninvasive, and dynamic modality that can be used for the reliable diagnosis of adverse reactions to metallic debris including metallosis and MoM pseudotumors.
Metallosis/trunnionosis
Metallosis can cause local cytotoxic effects as well as an innate, adaptive, and cytokine-mediated inflammatory response. These effects can result in a large and sometimes painful joint effusion as well as resorption of adjacent bone with secondary loosening of prosthetic joint components [7, 53,54,55,56]. Though uncommon, metallosis is relatively more prevalent in high-wear joint replacements, such as THA and TKA [7, 58, 59] (Figs. 5 and 6). As the MoM bearing choice has fallen out of favor in regards to THA, traditional metal-on-polyethylene bearing became the preferred surgical construct, at which time trunnionosis was becoming more recognized as another source of adverse reaction to metallic debris/metallosis. Trunnionosis is a generalized term used to encompass wear at the THA modular cobalt-chrome femoral head-titanium femoral stem interface (trunnion) via mechanically assisted crevice corrosion [60] (Fig. 7).
A 58-year-old man with a traditional metal-on-polyethylene right total hip arthroplasty, presenting with pain. a Anteroposterior radiograph of the right hip demonstrating the arthroplasty with lobulated dense joint capsular distention (bubble sign) (open arrows), most pronounced surrounding the femoral head-neck interface inferiorly consistent with trunnionosis. The solid arrow points to the femoral head component. b Long-axis ultrasound image of the anterior aspect of the left hip reveals shadowing artifact (solid arrow) compatible with the femoral head component with adjacent complex echogenicity (open arrows) corresponding with trunnionosis and metal-induced synovitis
Radiographs are again the first-line imaging modality and can demonstrate the characteristic findings of metallosis and its secondary effects. Metallosis has both nonspecific and specific radiographic findings that have been described in the literature. The more specific findings are the appearance of metallic density within the joint effusion or an adjacent extrasynovial collection and include the “bubble sign,” the “metal-line sign,” and the “cloud sign.” The bubble sign refers to metal deposition outlining the entire joint space with metallic density, giving a curvilinear bubble-like appearance. Similarly, the metal-line sign refers to a thin linear and less complete outlining of the joint capsule with metallic density. The cloud sign refers to amorphous fluffy or cloudy metallic densities in the joint space. Periprosthetic osteolysis is a nonspecific but more sensitive finding that can also be seen with metallosis. Computed tomography can also detect the metallic densities and is more sensitive at detecting subtle osteolysis and characterizing the full extent of osseous loss for surgical planning [7].
The sonographic findings of metal-induced synovitis are rarely described in the literature with often only reference to the use of US to complement other modalities in the assessment of periarticular fluid collections such as joint effusions and soft tissue changes [7, 52, 61]. Metallosis is identified sonographically by the presence of synovitis and a complex joint effusion with a heterogeneous echotexture due to the combination of necrotic tissues, inflammatory cells, and metallic as well as plastic debris. Metal deposition around the fluid collection demonstrates echogenic shadowing, which is the US correlate to the radiographic metal-line sign and bubble sign [7, 34] (Figs. 5–7).
Metal-on-metal pseudotumors
A MoM pseudotumor is a mass-like lesion of inflammation that can form surrounding MoM hip arthroplasties and is one presentation of an adverse reaction to metallic debris. MoM pseudotumors present as large, rapidly growing, and painful focal solid or complex cystic masses around hip arthroplasties. They mimic the local effects of an infection or a tumor in the absence of both, causing extensive bone loss and tissue necrosis (Fig. 8). They may also cause a restricted range of motion [36, 52, 57, 61, 62].
A 71-year-old woman who presented for evaluation of a painful revision right total hip arthroplasty, 2 years following revision surgery. a Sagittal computed tomography image of the right hip, in bone windows, demonstrates a complex mass (open arrows) at the anterior aspect of the femoral head component of the arthroplasty, within the iliopsoas bursa consistent with a pseudotumor. Punctate densities (solid arrow) are seen in the mass compatible with metallic densities. There is secondary extensive periprosthetic bone loss, especially involving the posterior acetabulum (solid star) consistent with loosening. b Panoramic long-axis and c short-axis ultrasound images of the anterior aspect of the right hip demonstrate smooth shadowing artifact consistent with the femoral head component of the arthroplasty (open arrowheads). There is redemonstration of the complex echogenic mass (open arrows) anterior to the femoral head component and iliopsoas tendon (open stars), within the iliopsoas bursa, consistent with the pseudotumor with punctate echogenic foci corresponding to the metallic densities
Lainiala et al. [62], in their study, demonstrated that US had a high sensitivity and specificity for the detection of MoM pseudotumors when compared to intraoperative findings at revision surgery. Current guidelines recommend longitudinal monitoring of at-risk MoM arthroplasty patients with either US or metal artifact reduction sequences MRI [36, 57]. During follow-up, the focus is on the relative interval changes in symptoms, radiographs, laboratory tests, and imaging findings. Although MRI has the ability to detect adverse local soft tissue reactions, the potential disadvantages of MRI include its high cost, lack of portability, and the obscuration of periprosthetic tissues by metal artifacts [36]. US images are not comprised of these metal artifacts and have the benefit of superior soft tissue resolution of both intracapsular and extracapsular pseudotumors [27, 62]. A study by Kwon and colleagues [36] demonstrated that US detected the interval change in the adverse local soft tissue reactions with higher accuracy, higher agreement, and smaller variability.
In US, MoM pseudotumors will present as complex heterogeneously hypoechoic masses and can be complex cystic, solid, or mixed with variable wall thicknesses (Fig. 8). Lesions can be extracapsular or less often, intracapsular (Fig. 9). The extracapsular pseudotumors typically demonstrate a connection to the joint capsule and are most commonly located at the posterolateral aspect of the hip joint capsule. The identification of enlarging pseudotumors by US is critical as a predictor of soft tissue injury and pending tissue necrosis, potentially allowing revision prior to periprosthetic loosening [36, 52, 57, 61, 62].
A 63-year-old man who presents for follow-up in regards to his metal-on-metal left total hip arthroplasty. He has no pain but his serum titanium levels are significantly elevated. a Anteroposterior radiograph of the left hip shows a metal-on-metal left total hip arthroplasty without evidence of hardware complication or mass. b Panoramic long-axis (LAX) and c short-axis (SAX) sonographic images of the anterior aspect of the left hip demonstrate smooth artifact consistent with the acetabular component of the arthroplasty (open arrowheads). There is a complex heterogeneous intracapsular mass (open arrows) anteriorly consistent with a metal-on-metal pseudotumor
Tendon pathology in joint arthroplasty
US has proven itself as an instrumental imaging tool for the diagnosis of tendon pathology [7, 23,24,25,26,27]. This is especially true in patients with arthroplasty hardware given the artifact hardware causes on MRI [27, 33,34,35,36]. Furthermore, the unmatched real-time dynamic imaging capabilities of US make it the gold standard for the diagnosis of hardware-related tendon impingement/snapping [7, 23,24,25,26,27, 63, 64].
Subscapularis tendon tears
Subscapularis tendon tears are common in the symptomatic postarthroplasty shoulder. During total shoulder arthroplasty, a subscapularis tenotomy is typically performed, surgically dividing the tendon in order to access the joint. The tendon is then repaired following arthroplasty placement, however, making it susceptible to postoperative tearing and complications. Subscapularis tears are associated with anterior shoulder instability and can in turn lead to postoperative function loss, loss of active motion, and loosening of the glenoid component of the arthroplasty. Early diagnosis is important, not only to avoid these complications but to also prevent muscle belly atrophy and fatty infiltration [65].
US is an accurate method to evaluate the rotator cuff following shoulder arthroplasty and is a better alternative to MRI because of the lack of susceptibility artifact [27, 33,34,35,36, 65, 66]. The subscapularis tendon is evaluated sonographically in both the long axis and short axis by placing the transducer on the anterior aspect of the shoulder which is positioned in external rotation. A full-thickness tear will present as an area of hypoechoic or anechoic echotexture with volume loss extending from the articular surface to the bursal surface and often with underlying bony irregularity and fluid in the adjacent subacromial-subdeltoid bursa (Fig. 10). When a defect is suspected, dynamic compression with the US transducer of any adjacent fluid can be performed to confirm the finding [24, 26, 27]. However, chronic full-thickness tears are more commonly associated with tendon retraction and less commonly present with joint or bursal fluid [24].
An 83-year-old man who complains of anterior shoulder pain during a postoperative visit following placement of a total shoulder arthroplasty. The patient was referred for a shoulder ultrasound to evaluate for a possible tear of the subscapularis tendon repair following an abnormal radiograph. a Axillary radiograph of the right shoulder demonstrates a total shoulder arthroplasty. There is significant anterior subluxation of the humeral head component (solid arrow) in relation to the glenoid component (G). Note that the humeral head component approximates the coracoid (C). The arrowhead denotes the area of the expected insertion of the subscapularis tendon at the lesser tuberosity. b Long-axis sonographic image of the anterior aspect of the right shoulder, at the level of the lesser tuberosity (arrowhead). The image redemonstrates anterior subluxation of the humeral head component (H) in relation to the glenoid component (G). There is a full-thickness retracted tear of the subscapularis tendon with the stump not seen as it is retracted medial to the coracoid (C). Complex hypoechoic fluid fills the expected location of the tendon insertion (open arrows)
Gluteal tendon tears
Gluteal or abductor mechanism tears are a well-known cause of pain and altered gait following THA. This may be caused by the inadvertent intraoperative damage to the superior gluteal nerve, postoperative mechanical failure of a repaired abductor tenotomy at the greater trochanter, postoperative rupture, or altered biomechanics [67, 68]. The incidence of postsurgical gluteal tendon failure has been reported in as high as 22% of patients following THA [67]. Knowledge of and special attention to the THA surgical approach is extremely beneficial as patients having undergone THA utilizing the direct lateral, anterolateral, or transgluteal surgical approaches that involve the release of the abductor tendon insertion from the greater trochanter or gluteal muscle splitting/release are particularly more at risk [67, 68]. For example, Bremer et al. [68] demonstrated that damage of the abductor tendons and fatty atrophy of the gluteus medius and gluteus minimus muscles were significantly less evident and less frequent when the direct anterior approach was performed compared to a transgluteal approach.
US is also instrumental in the evaluation of the abductor/gluteal tendon tears following THA. The gluteus medius and minimus tendons should be evaluated at the greater trochanter in both the long axis and short axis. Similar to any tendon, including the rotator cuff, the sonographic finding of a hypoechoic or anechoic often fluid-filled focal area within the gluteus medius or gluteus minimus tendon with an absence of a uniform fibrillar pattern and tendon detachment from the greater trochanter is consistent with a tear (Fig. 11). Dynamic compression of any adjacent or bursal fluid should also be performed and can occasionally fill an unexpected defect, confirming a tear [24, 26, 27, 33,34,35,36, 65, 66].
An 88-year-old woman, 4 years status postplacement of a left total hip arthroplasty, presenting with weakness with abduction and a suspected gluteal/abductor tear. a Long-axis and b short-axis ultrasound images were obtained at the level of the lateral hip demonstrating a full-thickness retracted tear of the gluteus minimus tendon (solid arrow) from the greater trochanter (GT) with approximately 4.5 cm of tendon retraction (calibers denote the retraction measurement). The open arrow points to the absent gluteus minimus tendon insertion from the greater trochanter
Quadriceps and patellar tendon tears
Extensor mechanism pathology in the knee following TKA and unicompartmental knee arthroplasty should be suspected in the presence of anterior knee soft tissue swelling, pain, and limitation in an active extension of the knee [69, 70]. Among the postoperative complications following TKA and unicompartmental knee arthroplasty, the frequency of extensor mechanism pathology, such as quadriceps and patellar tendon tears or ruptures, is 1–10%. During knee replacement surgery, the quadriceps is often split and the patella and extensor mechanism are subluxed or dislocated laterally, oftentimes for 45–60 min [69]. Postoperative tendon rupture is often related to trauma and is promoted by decreased postoperative tendon vascularization [70].
US of the extensor mechanism is performed by imaging the anterior knee with the patient in the supine position with the knee slightly flexed 20°–30°, which functions to reduce any tendon laxity and minimize anisotropy artifact. The quadriceps and patellar tendons should be imaged in both the long axis and short axis, evaluating for the normal continuous and fibrillar appearance [25]. Creteur and colleagues [69] note that thickening of the quadriceps tendon by more than 50%, thickening of the patellar tendon by more than 90%, shortening of the patellar tendon by 8%, and loss of the normal fibrillar structure and focal hypoechoic areas within the tendons are commonly observed findings in early postoperative US imaging following TKA and should not be considered pathologic findings. However, an anechoic fluid-filled focal area, often with tendon retraction, involving the quadriceps or patellar tendon is consistent with a full-thickness tear (Fig. 12). These full-thickness tears will often lead to the subsequent distal displacement of the patella (patella baja) in the case of a quadriceps tendon rupture and proximal displacement of the patella (patella alta) in the case of a patellar tendon rupture [25].
A 49-year-old woman with a left total knee arthroplasty presents with infrapatellar pain and weakness. a Cross-table lateral radiograph of the left knee demonstrates a left total knee arthroplasty. The solid arrow points to the anterior aspect of the tibial component baseplate. T denotes the proximal anterior tibia and the open arrow points to the expected location of the proximal patellar tendon insertion at the inferior pole of the patella. b Long-axis sonographic image of the anterior aspect of the same knee reveals a full-thickness tear of the proximal aspect of the patellar tendon (open arrows) from the inferior pole of the patella (Pat). The open star corresponds to the anterior aspect of the tibial component baseplate. T denotes the proximal anterior tibia and P overlies the patellar tendon
Iliopsoas tendinopathy, bursitis, and impingement/snapping
Iliopsoas tendinopathy is a known extrinsic cause of hip pain after THA and is usually caused by impingement and friction on the iliopsoas tendon by the anterior aspect of the acetabular component or sometimes by the femoral head component itself in THA. This can occur with an excessive overhang of the acetabular component resulting from less than ideal anteversion, or from larger femoral head components, particularly with dual mobility bearings [27]. The diagnosis is suspected based on history and physical examination. When the lower extremity is in a neutral position, the iliopsoas tendon lays over the acetabular component. In these patients, during hip flexion, abduction, and external rotation, the tendon moves away from the bone/acetabular component. Subsequently, when the patient returns the hip/leg to its neutral position, the tendon then snaps against the acetabular component, making an audible and painful snap. This can result in iliopsoas tendinopathy and iliopsoas bursitis secondary to the repetitive friction on the tendon and may also lead to the enlargement and inflammation of the adjacent bursa [27, 63].
The unique benefit of real-time dynamic imaging with the patient renders US the gold standard for the diagnosis of iliopsoas impingement/snapping. Direct sonographic visualization of the exact tendon translation mechanism during hip movement resulting in the abnormal tendon friction is used to make the diagnosis [27, 63, 64]. The patient is placed in a supine position with the hip in flexion, abduction, and external rotation and then instructed to actively move the hip back to neutral by extending, adducting, and internally rotating the hip while imaging the patient in real-time with the transducer on the anterior hip in the short axis [63]. In our experience, oftentimes instructing the patient to move the leg into whatever position that typically causes the snapping while imaging sonographically is also beneficial in the diagnosis.
Furthermore, US is an excellent imaging modality to evaluate for the secondary iliopsoas tendinopathy and iliopsoas bursitis. In US, iliopsoas tendinosis presents as a thickened tendon with loss of the normal fibrillar structure and is often associated with hypoechogenicity. Attritional wear/partial-thickness tearing of the tendon can also occur and is diagnosed as tendon thinning and heterogeneity. A full-thickness tendon tear, near or at the level of the THA, can also be diagnosed when there is an absence of the tendon. Iliopsoas bursitis will present sonographically as fluid accumulation within the bursa, often complex and with subsequent pain with transducer pressure onto the bursa. Also, by applying real-time Doppler to the bursa, associated hyperemia may be present in the acute state [27, 63].
Joint arthroplasty injections
Traditionally, the placement of corticosteroids within a TJA is avoided in order to reduce the risk of PJI and potentially periprosthetic loosening [71]. Mills et al. [71] in their study state that the use of intraarticular corticosteroids with an existing TKA should be avoided given the dire consequences of the injection. However, in the case of TKA for example, recovery can be challenging. It usually takes at least 3 months to recover and in some may take up to a year [72]. Moreover, in approximately 20% of patients, satisfactory outcomes are not achieved and these patients go on to have persistent pain [71, 72]. In some instances, following thorough clinical and imaging investigations, the source of pain can be diagnosed as infection, aseptic loosening, or periprosthetic fracture. Conversely, in other patients with persistent pain, despite normal clinical and imaging findings, the cause is unknown or multifactorial; possible etiologies include component malposition, instability, arthrofibrosis, and soft tissue inflammation [71].
A more recent study by Klement and colleagues [72] suggests that intraarticular corticosteroids can be used in certain patients with TKA as a viable option for function improvement and symptom relief, but only after fully screening for PJI. Regardless, it is essential that close and direct communication occurs between the radiologist performing the US-guided procedure and the orthopedic surgeon who placed the arthroplasty before any procedure is performed.
US-guided injections in the extraarticular soft tissues in TJA patients, e.g., peritendinous and bursal injections, on the other hand are not uncommonly performed. However, when performing an US-guided injection in the soft tissues adjacent to an arthroplasty, caution must be taken by using constant real-time sonographic imaging of the needle tip in order to avoid passing the needle into the adjacent joint capsule. Similarly, US-guided procedures are often performed for diagnostic purposes by utilizing only anesthetic injected into the extraarticular soft tissues in order to exclude a source of pain other than that related to the intraarticular hardware [27].
One commonly performed US-guided procedure in arthroplasty patients is an US-guided aspiration and injection of the iliopsoas bursa for the treatment of iliopsoas bursitis, which can be caused by iliopsoas snapping as discussed above [27, 63]. Although these injections will often relieve the pain resulting from iliopsoas snapping, the snapping sensation itself often will not resolve [63]. Furthermore, it is critical to note that in 10–15% of the population, the iliopsoas bursa normally communicates with the hip joint, and therefore, a portion of the injectate can migrate to the arthroplasty, which could present a risk [27].
Conclusion
US plays a crucial role in the diagnosis of painful joint arthroplasty, especially given its unique dynamic capabilities, convenience, and high resolution. US guidance is also instrumental for procedures in both diagnosing and in select cases, treating the painful joint arthroplasty.
Abbreviations
- THA:
-
Total hip arthroplasty
- TJA:
-
Total joint arthroplasty
- TKA:
-
Total knee arthroplasty
- MoM:
-
Metal-on-metal
- MRI:
-
Magnetic resonance imaging
- PJI:
-
Periprosthetic joint infection
- US:
-
Ultrasound
References
Fingar KR, Stocks C, Weiss AJ, Steiner CA. Most frequent operating room procedures performed in U.S. hospitals, 2003–2012: Statistical Brief #186. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): Agency for Healthcare Research and Quality; 2006.
Gademan MG, Hofstede SN, VlietVlieland TP, Nelissen RG, Marang-van de Mheen PJ. Indication criteria for total hip or knee arthroplasty in osteoarthritis: a state-of-the-science overview. BMC Musculoskelet Disord. 2016;17:463.
Quintana JM, Arostegui I, Escobar A, Azkarate J, Goenaga JI, Lafuente I. Prevalence of knee and hip osteoarthritis and the appropriateness of joint replacement in an older population. Arch Intern Med. 2008;168:1576–84.
Tran G, Khalil LS, Wrubel A, Klochko CL, Davis JJ, Soliman SB. Incidental findings detected on preoperative CT imaging obtained for robotic-assisted joint replacements: clinical importance and the effect on the scheduled arthroplasty. Skeletal Radiol. 2021;50:1151–61.
Kayani B, Konan S, Pietrzak JRT, Haddad FS. Iatrogenic bone and soft tissue trauma in robotic-arm assisted total knee arthroplasty compared with conventional jig-based total knee arthroplasty: a prospective cohort study and validation of a new classification system. J Arthroplasty. 2018;33:2496–501.
Khalil LS, Darrith B, Franovic S, Davis JJ, Weir RM, Banka TR. Patient-Reported Outcomes Measurement Information System (PROMIS) Global Health Short Forms demonstrate responsiveness in patients undergoing knee arthroplasty. J Arthroplasty. 2020;35:1540–4.
Mallon S, Bussis K, Beswick Z, North WT, Soliman SB. Ultrasonographic and radiographic findings of polyethylene component displacement with severe metallosis and metal-induced synovitis following total knee arthroplasty. Knee. 2019;26:941–50.
North WT, Mehran N, Davis JJ, Silverton CD, Weir RM, Laker MW. Topical vs intravenous tranexamic acid in primary total hip arthroplasty: a double-blind, randomized controlled trial. J Arthroplasty. 2016;31:1022–6.
Srivastava K, Bozic KJ, Silverton C, Nelson AJ, Makhni EC, Davis JJ. Reconsidering strategies for managing chronic periprosthetic joint infection in total knee arthroplasty: using decision analytics to find the optimal strategy between one-stage and two-stage total knee revision. J Bone Joint Surg Am. 2019;101:14–24.
Klug A, Gramlich Y, Rudert M, et al. The projected volume of primary and revision total knee arthroplasty will place an immense burden on future heath care systems over the next 30 years. Knee Surg Sports Traumatol Arthrosc. 2021;29:3287–98.
Schwartz AM, Farley KX, Guild GN, Bradbury TL Jr. Projections and epidemiology of revision hip and knee arthroplasty in the United States to 2030. J Arthroplasty. 2020;35:S79-85.
Arnholdt J, Kamawal Y, Holzapfel BM, Ripp A, Rudert M, Steinert AF. Evaluation of implant fit and frontal plane alignment after bi-compartmental knee arthroplasty using patient-specific instruments and implants. Arch Med Sci. 2018;14:1424–31.
Aujla RS, Esler CN. Total knee arthroplasty for osteoarthritis in patients less than fifty-five years of age: a systematic review. J Arthroplasty. 2017;32:2598-603.e1.
Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–5.
Netravali NA, Shen F, Park Y, Bargar WL. A perspective on robotic assistance for knee arthroplasty. Adv Orthop. 2013;2013:970703.
Lim SJ, Jang SP, Kim DW, Moon YW, Park YS. Primary ceramic-on-ceramic total hip arthroplasty using a 32-mm ceramic head with a titanium-alloy sleeve. Clin Orthop Relat Res. 2015;473:3781–7.
Sobieraj M, Marwin S. Ultra-high-molecular-weight polyethylene (UHMWPE) in total joint arthroplasty. Bull Hosp Jt Dis. 2013;76:38–46.
Delaunay CP, Putman S, Puliero B, Begin M, Migaud H, Bonnomet F. Cementless total hip arthroplasty with metasul bearings provides good results in active young patients: a concise followup. Clin Orthop Relat Res. 2016;474:2126–33.
Bell SW, Anthony I, Jones B, MacLean A, Rowe P, Blyth M. Improved accuracy of component positioning with robotic-assisted unicompartmental knee arthroplasty: data from a prospective, randomized controlled study. J Bone Joint Surg Am. 2016;98:627–35.
Conditt MA, Roche MW. Minimally invasive robotic-arm-guided unicompartmental knee arthroplasty. J Bone Joint Surg Am. 2009;91:63–8.
Nodzo SR, Chang CC, Carroll KM, et al. Intraoperative placement of total hip arthroplasty components with robotic-arm assisted technology correlates with postoperative implant position: a CT-based study. Bone Joint J. 2018;100-B:1303–9.
Sousa PL, Sculco PK, Mayman DJ, Jerabek SA, Ast MP, Chalmers BP. Robots in the operating room during hip and knee arthroplasty. Curr Rev Musculoskelet Med. 2020;13:309–17.
Klauser AS, Tagliafico A, Allen GM, et al. Clinical indications for musculoskeletal ultrasound: a Delphi-based consensus paper of the European Society of Musculoskeletal Radiology. Eur Radiol. 2012;22:1140–8.
Lee MH, Sheehan SE, Orwin JF, Lee KS. Comprehensive shoulder US examination: a standardized approach with multimodality correlation for common shoulder disease. Radiographics. 2016;36:1606–27.
Alves TI, Girish G, Kalume Brigido M, Jacobson JA. US of the knee: scanning techniques, pitfalls, and pathologic conditions. Radiographics. 2016;36:1759–75.
Soliman SB, Spicer PJ, van Holsbeeck MT. Sonographic and radiographic findings of posterior tibial tendon dysfunction: a practical step forward. Skeletal Radiol. 2019;48:11–27.
Long SS, Surrey D, Nazarian LN. Common sonographic findings in the painful hip after hip arthroplasty. J Ultrasound Med. 2012;31:301–12.
Romanò CL, Petrosillo N, Argento G, et al. The role of imaging techniques to define a peri-prosthetic hip and knee joint infection: multidisciplinary consensus statements. J Clin Med. 2020;9:2548.
van Holsbeeck MT, Eyler WR, Sherman LS, et al. Detection of infection in loosened hip prostheses: efficacy of sonography. AJR Am J Roentgenol. 1994;163:381–4.
Signore A, Sconfienza LM, Borens O, et al. Consensus document for the diagnosis of prosthetic joint infections: a joint paper by the EANM, EBJIS, and ESR (with ESCMID endorsement). Eur J Nucl Med Mol Imaging. 2019;46:971–88.
Battaglia M, Vannini F, Guaraldi F, Rossi G, Biondi F, Sudanese A. Validity of preoperative ultrasound-guided aspiration in the revision of hip prosthesis. Ultrasound Med Biol. 2011;37:1977–83.
Eisler T, Svensson O, Engström CF, et al. Ultrasound for diagnosis of infection in revision total hip arthroplasty. J Arthroplasty. 2001;16:1010–7.
Talbot BS, Weinberg EP. MR imaging with metal-suppression sequences for evaluation of total joint arthroplasty. Radiographics. 2016;36:209–25.
Heffernan EJ, Alkubaidan FO, Nielsen TO, Munk PL. The imaging appearances of metallosis. Skeletal Radiol. 2008;37:59–62.
Miller TT. Sonography of joint replacements. Semin Musculoskelet Radiol. 2006;10:79–85.
Kwon YM, Dimitriou D, Liow MH, Tsai TY, Li G. Is ultrasound as useful as metal artifact reduction sequence magnetic resonance imaging in longitudinal surveillance of metal-on-metal hip arthroplasty patients? J Arthroplasty. 2016;31:1821–7.
Parvizi J, Tan TL, Goswami K, et al. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty. 2018;33:1309-14.e2.
Zeng YQ, Deng S, Zhu XY, et al. Diagnostic accuracy of the synovial fluid α-defensin lateral flow test in periprosthetic joint infection: a meta-analysis. Orthop Surg. 2021;13:708–18.
Fink B, Sevelda F. Periprosthetic joint infection of shoulder arthroplasties: diagnostic and treatment options. BioMed Res Int. 2017;2017:4582756.
Saper D, Capiro N, Ma R, Li X. Management of Propionibacterium acnes infection after shoulder surgery. Curr Rev Musculoskelet Med. 2015;8:67–74.
Kadler BK, Mehta SS, Funk L. Propionibacterium acnes infection after shoulder surgery. Int J Shoulder Surg. 2015;9:139–44.
Ting NT, Della Valle CJ. Diagnosis of periprosthetic joint infection-an algorithm-based approach. J Arthroplast. 2017;32:2047–50.
Porrino J, Wang A, Moats A, Mulcahy H, Kani K. Prosthetic joint infections: diagnosis, management, and complications of the two-stage replacement arthroplasty. Skeletal Radiol. 2020;49:847–59.
Abdel Karim M, Andrawis J, Bengoa F, et al. Hip and knee section, diagnosis, algorithm: proceedings of international consensus on orthopedic infections. J Arthroplast. 2019;34:S339–50.
Chan BY, Crawford AM, Kobes PH, et al. Septic arthritis: an evidence-based review of diagnosis and image-guided aspiration. AJR Am J Roentgenol. 2020;215:568–81.
Kung JW, Yablon C, Huang ES, Hennessey H, Wu JS. Clinical and radiologic predictive factors of septic hip arthritis. AJR Am J Roentgenol. 2012;199:868–72.
Partridge DG, Winnard C, Townsend R, Cooper R, Stockley I. Joint aspiration, including culture of reaspirated saline after a ‘dry tap’, is sensitive and specific for the diagnosis of hip and knee prosthetic joint infection. Bone Joint J. 2018;100-B:749–54.
Ali F, Wilkinson JM, Cooper JR, et al. Accuracy of joint aspiration for the preoperative diagnosis of infection in total hip arthroplasty. J Arthroplasty. 2006;21:221–6.
Lee HD, Prashant K, Shon WY. Management of periprosthetic hip joint infection. Hip Pelvis. 2015;27:63–71.
Hanna BC, Thompson NW, Wilson DS, Mollan RA. Extra-articular migration of the patellar component following total knee arthroplasty. Ulster Med J. 2002;71:57–9.
Helito CP, Gobbi RG, Tirico LE, Pecora JR, Camanho GL. Loosening of the patellar component and extra-articular and transcutaneous migration after TKA. Orthopedics. 2014;37:e211–3.
Singisetti K, Raju P, Langton D, Nargol A. Ultrasound is reliable in diagnosis of adverse reactions to metallic debris following metal on metal hip replacement [abstract]. Orthopaedic Proceedings. 2012;94-B:550.
Sansone V, Pagani D, Melato M. The effects on bone cells of metal ions released from orthopaedic implants. A review Clin Cases Miner Bone Metab. 2013;10:34–40.
Romesburg JW, Wasserman PL, Schoppe CH. Metallosis and metal-induced synovitis following total knee arthroplasty: review of radiographic and CT findings. J Radiol Case Rep. 2010;4:7–17.
Rajgopal A, Panda I, Tyagi VC. Early failure with massive metallosis and posteromedial wear following atraumatic anterior cruciate ligament rupture after medial unicompartmental knee arthroplasty. Arthroplast Today. 2017;4:15–9.
Craig R, Vlychou M, McCarthy CL, Gibbons CLMH, Athanasou NA. Metal wear-induced pseudotumour following an endoprosthetic knee replacement for Ewing sarcoma. Skeletal Radiol. 2017;46:967–74.
Matharu GS, Mansour R, Dada O, Ostlere S, Pandit HG, Murray DW. Which imaging modality is most effective for identifying pseudotumours in metal-on-metal hip resurfacings requiring revision: ultrasound or MARS-MRI or both. Bone Joint J. 2016;98-B:40–8.
Weissman BN, Scott RD, Brick GW, Corson JM. Radiographic detection of metal-induced synovitis as a complication of arthroplasty of the knee. J Bone Joint Surg Am. 1991;73:1002–7.
Case CP, Langkamer VG, James C, et al. Widespread dissemination of metal debris from implants. J Bone Joint Surg Br. 1994;76:701–12.
Mistry JB, Chughtai M, Elmallah RK, et al. Trunnionosis in total hip arthroplasty: a review. J Orthop Traumatol. 2016;17:1–6.
Awan O, Chen L, Resnik CS. Imaging evaluation of complications of hip arthroplasty: review of current concepts and imaging findings. Can Assoc Radiol J. 2013;64:306–13.
Lainiala O, Elo P, Reito A, Pajamäki J, Puolakka T, Eskelinen A. Good sensitivity and specificity of ultrasound for detecting pseudotumors in 83 failed metal-on-metal hip replacements. Acta Orthop. 2015;86:339–44.
Piechota M, Maczuch J, Skupiński J, Kukawska-Sysio K, Wawrzynek W. Internal snapping hip syndrome in dynamic ultrasonography. J Ultrason. 2016;16:296–303.
Cardinal E, Buckwalter KA, Capello WN, Duval N. US of the snapping iliopsoas tendon. Radiology. 1996;198:521–2.
Ives EP, Nazarian LN, Parker L, Garrigues GE, Williams GR. Subscapularis tendon tears: a common sonographic finding in symptomatic postarthroplasty shoulders. J Clin Ultrasound. 2013;41:129–33.
Sofka CM, Adler RS. Original report. Sonographic evaluation of shoulder arthroplasty. AJR Am J Roentgenol. 2003;180:1117–20.
Odak S, Ivory J. Management of abductor mechanism deficiency following total hip replacement. Bone Joint J. 2013;95-B:343–7.
Bremer AK, Kalberer F, Pfirrmann CW, Dora C. Soft-tissue changes in hip abductor muscles and tendons after total hip replacement: comparison between the direct anterior and the transgluteal approaches. J Bone Joint Surg Br. 2011;93:886–9.
Creteur V, De Angelis R, Absil J, Kyriakidis T, Madani A. Sonographic and radiographic evaluation of the extensor tendons in early postoperative period after total knee arthroplasty. Skeletal Radiol. 2021;50:485–94.
Cyteval C. Imaging of knee implants and related complications. Diagn Interv Imaging. 2016;97:809–21.
Mills ES, Elman MB, Foran JRH. The risk of acute infection following intra-articular corticosteroid injection into a pre-existing total knee arthroplasty. J Arthroplasty. 2018;33:216–9.
Klement MR, Luzzi AJ, Siddiqi A, Valichka K, Sharkey PF. Intra-articular corticosteroid injection following total knee arthroplasty: is it effective? J Arthroplasty. 2019;34:303–8.
Acknowledgements
We thank Stephanie Stebens, MLIS, AHIP, for her guidance and assistance in the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Soliman, S.B., Davis, J.J., Muh, S.J. et al. Ultrasound evaluations and guided procedures of the painful joint arthroplasty. Skeletal Radiol 51, 2105–2120 (2022). https://doi.org/10.1007/s00256-022-04080-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-022-04080-y