Abstract
The diagnosis of prosthetic joint infection (PJI) remains challenging, despite multiple available laboratory tests for both serum and synovial fluid analysis. The clinical symptoms of PJI are not always characteristic, particularly in the chronic phase, and there is often significant overlap in symptoms with non-infectious forms of arthroplasty failure. Further exacerbating this challenge is lack of a universally accepted definition for PJI, with publications from multiple professional societies citing different diagnostic criteria. While not included in many of the major societies’ guidelines for diagnosis of PJI, diagnostic imaging can play an important role in the workup of suspected PJI. In this article, we will review an approach to diagnostic imaging modalities (radiography, ultrasound, CT, MRI) in the workup of suspected PJI, with special attention to the limitations and benefits of each modality. We will also discuss the role that image-guided interventions play in the workup of these patients, through ultrasound and fluoroscopically guided joint aspirations. While there is no standard imaging algorithm that can universally applied to all patients with suspected PJI, we will discuss a general approach to diagnostic imaging and image-guided intervention in this clinical scenario.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over 1 million prosthetic joint surgeries are performed annually in the USA, most commonly hip and knee joint replacements [1]. As the population ages, along with increasing rates of osteoarthritis, the frequency of these procedures is expected to increase, with an estimated 1.4 million annual hip arthroplasties and 1.4 million annual knee arthroplasties expected by 2040 [2]. Although often successful procedures, joint replacement can be complicated by prosthetic joint infection (PJI) in either the acute or chronic phase, with reported prevalence of 1.63% and 1.55% at 2 years for hip and knee replacements, respectively [3]. Given the implications of a PJI, a timely and accurate diagnosis is paramount to appropriate patient management and prevention of long-term morbidity.
The diagnosis of PJI remains challenging despite the availability of a variety of clinical signs, serum and synovial fluid markers, and microbiological and histological findings. While PJI can be readily diagnosed in the presence of a draining sinus or exposed implant [4], differentiating between septic and aseptic implant failure becomes much more challenging in the presence of nonspecific clinical symptoms and laboratory tests. Further adding to this complexity, there is lack of a universally accepted definition of PJI, which is reflected by the existence of at least six different definitions by reputable independent societies: the Musculoskeletal Infection Society (MSIS), the Infectious Disease Society of America (IDSA), two International Consensus Meetings, the European Bone and Joint Infection Society (EBJIS), and the World Association against Infection in Orthopedics and Trauma (WAIOT) [4,5,6,7,8,9,10]. For example, the MSIS evidence–based diagnostic tool defines PJI using major and minor diagnostic criteria [4]. The presence of one major criterion (two positive cultures of the same organism, or a sinus tract extending from the skin surface to the prosthetic joint) or multiple minor criteria are diagnostic of PJI under this scoring-based definition (Table 1). With the exception of the WAIOT definition, which includes radio-labeled leukocyte scintigraphy as a criterion, all other proposed diagnostic algorithms for PJI rely solely on laboratory tests and clinical features, without a clearly defined role for diagnostic imaging. None of the societies provides a scientific explanation for this exclusion, despite the reported value and frequent clinical utilization of diagnostic imaging in the workup of PJI [5]. One potential explanation for the lack of guidelines for utilization of imaging could be relative paucity of consolidated scientific evidence regarding the additional value of imaging in diagnosis of PJI. A few recent consensus statements have proposed diagnostic algorithms which utilize diagnostic imaging; however, evidence for many recommendations remains at the level of expert consensus, given the lack of robust studies directly comparing different imaging modalities in the workup of PJI [11, 12].
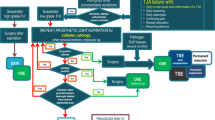
In this article, we will review an approach to diagnostic imaging in the setting of suspected PJI. We will review the benefits and limitations of the various imaging modalities employed to evaluate prosthetic joints and special technical considerations needed to maximize their diagnostic benefit. Additionally, we will discuss the role and available techniques for image-guided prosthetic joint aspiration. The utilization of nuclear medicine studies in the evaluation of PJI is beyond the scope of this article and will be addressed in a separate article in this issue.
Radiography
In the setting of clinically suspected PJI, radiography is the recommended first-line imaging study [13]. Radiographs provide a broad overview of the prosthetic joint and can exclude other potential causes of a painful prosthesis. Radiographs can delineate position of the arthroplasty components, periprosthetic fractures, or periprosthetic osteolysis. However, in the early stages of infection, radiographic appearance of the arthroplasty can be normal, as destruction of 30–60% of the bony trabeculae is required to produce radiographically evident osteolysis [14, 15]. Detection of periprosthetic osteolysis can also be location dependent, and radiographs can underestimate the presence and extent of periprosthetic osteolysis (Fig. 1) [16, 17]. As infection progresses, irregular radiolucency and osseous resorption at the prosthesis-bone or cement-bone interfaces can be seen, as well as lamellated periosteal reaction [17, 18]. If periprosthetic osseous destruction continues to progress, arthroplasty components can become loose and displace from their original position, and the adjacent bone or cement can fracture [19]. However, this appearance is not specific for infection, as aseptic osteolysis due to polyethylene particle disease or other forms of adverse local tissue reaction can appear similar. Comparison with prior imaging, if available, can be helpful in differentiating between septic and aseptic loosening, as more rapid progression of osteolysis is indicative of infection [14].
A 67-year-old man with a left hip arthroplasty, with acute left hip pain. Initial radiograph of the pelvis (A) demonstrates periprosthetic osteolysis within the greater trochanter of the left femur (star). Subsequent CT redemonstrates the greater trochanter osteolysis (B), as well as additional osteolysis within the lesser trochanter (arrow) which was not apparent on radiograph (C). Additionally, an associated pathologic intertrochanteric fracture is demonstrated (D, arrow)
Evaluation of the periprosthetic soft tissues is limited on radiographs, and helpful indicators such as communicating soft tissue collections are often radiographically occult. When in large enough quantities, however, soft tissue gas is radiographically apparent. Soft tissue gas adjacent to an arthroplasty is an expected finding in the immediate post-operative period, but its presence beyond 14 days can suggest the presence of a PJI [20].
In our clinical practice, initial radiographs are often normal or demonstrate nonspecific findings, but are an important initial step in the imaging workup of PJI. In the setting of low clinical suspicion for PJI and normal laboratory workup, an initially normal radiograph may not warrant further imaging. In the presence of high clinical suspicion or radiographic findings suggestive of infection, image-guided aspiration should be pursued to confirm the diagnosis [21]. In cases of radiographically evident osteolysis and loosening with low clinical suspicion for PJI, we either pursue more advanced imaging or an image-guided joint aspiration to clarify the diagnosis.
Ultrasound
Compared with radiography, ultrasound plays a more limited role in the workup of PJI. The ultrasound beam cannot penetrate beyond the metallic arthroplasty components, limiting its assessment to the soft tissues superficial to a prosthetic joint. Although ultrasound is frequently indicated in evaluation for joint effusions and synovitis in native joints, there is less consensus on the utility of ultrasound to evaluate prosthetic joints [22]. Ultrasound can be utilized to assess for hypoechoic distention of a prosthetic joint, which may reflect an underlying effusion, synovial thickening, or a combination of the two (Fig. 2) [23, 24]. In more superficial prosthetic joints (such as the knee), compression can help distinguish between non-compressible synovial thickening and compressible joint effusion; however, this can be more difficult to assess in deeper joints such as the hip. In an attempt to utilize ultrasound to diagnose infected prostheses, van Holsbeeck and colleagues found a significant difference in anterior recess distention between infected and non-infected hip arthroplasties and concluded that anterior recess distention of less than 3.2 mm could be used to exclude the presence of a joint effusion and infection [19]. Weybright and colleagues later contradicted these findings and found no significant difference in anterior recess distention in patients with and without hip joint effusions, when using arthrocentesis as a gold standard [25].
A 69-year-old woman with suspected infection and hip joint effusion. Grayscale ultrasound image of the anterior hip joint (A) demonstrates hypoechoic distention of the anterior recess of the joint (arrowheads). Color Doppler image (B) demonstrates no substantial vascularity within the area of hypoechoic distention. Subsequent aspiration (C) yielded no fluid. Lack of vascularity was unable to distinguish between fluid distention of the joint and synovial thickening
The presence of extra-articular fluid collections or a sinus tract extending to the skin surface can also be detected on ultrasound, and while strongly suggestive of infection, these findings are not always present in the setting of PJI [26]. To the best of the authors’ knowledge, the value of ultrasound in assessing periprosthetic muscle edema and vascularity in the context of PJI has never been studied. As such, in evaluation of a suspected PJI, the American College of Radiology concluded that diagnostic ultrasound “may be appropriate” in the hip, but was “usually not appropriate” in the knee [13, 21]. Given the accessibility of CT and MRI in many clinical settings and superior soft tissue characterization afforded by these modalities compared with ultrasound, the utility of ultrasound for purely diagnostic purposes is limited. Ultrasound plays a more substantial role in the setting of ultrasound-guided prosthetic joint aspirations, which will be discussed in a subsequent section.
Computed tomography
Imaging with computed tomography (CT) can be beneficial in the workup of PJI, as it can delineate areas of periprosthetic osteolysis, and its superior soft tissue contrast compared with radiography can aid in the detection of periarticular soft tissue abnormalities. Similar to radiography, CT evaluation of an infected arthroplasty may initially be normal. The normal CT appearance of an arthroplasty will demonstrate solid osseous fixation of the prosthetic components, with less than 2-mm thin linear lucency visible at the prosthesis-bone or cement-bone interfaces. When osseous abnormalities are evident on CT, they can include focal or non-focal osteolysis (osseous resorption measuring more than 2 mm in thickness) at the prosthesis-bone or cement-bone interfaces, periosteal reaction, and loosening of the arthroplasty components [18, 27]. Compared with radiography, CT is more sensitive in the detection of osteolysis, allowing for superior characterization of the extent of osteolysis, as well as improved detection of small osteolytic lesions (Fig. 1) [16, 28].
Soft tissue CT findings in the setting of PJI include fluid/soft tissue distention of the prosthetic joint, fluid accumulation within the adjacent bursae, and periarticular soft tissue collections [17]. In an effort to distinguish CT findings specific for septic (rather than aseptic) osteolysis, Cyteval et al. found that both periostitis and intramuscular/peri-muscular fluid collections were highly specific for infection, but lacked sensitivity [17]. Isern et al. found that periprosthetic soft tissue extension beyond the joint capsule, osteolysis, and regional lymphadenopathy was predictive of PJI [29]. If clinically appropriate, the administration of intravenous contrast can also be used to better delineate the presence and extent of periarticular soft tissue collections, as well as sinus tract formation [30].
Despite the superior characterization of an arthroplasty with CT compared to radiography, CT is infrequently used in the initial workup for suspected PJI at the authors’ institution. MRI is often considered a more appropriate follow-up examination after initial radiograph, due to high diagnostic performance in the setting of PJI and lack of ionizing radiation, which is reflected in recent consensus statements [11,12,13, 21]. CT does provide value in the setting of a planned arthroplasty revision (whether septic or aseptic), as characterization of the osteolysis and residual bone stock can aid the performing surgeon in preoperative planning.
Technical considerations
When evaluating a suspected PJI with CT, metal-associated artifacts can limit the diagnostic value of CT, the severity of which can vary depending on the type, size, and shape of the implant. Beam hardening artifact and photon starvation substantially contribute to CT metal-associated artifacts generated by an arthroplasty, as well as photon scatter, edge effects, and patient motion to a lesser degree [31,32,33,34,35]. Metal-associated artifacts can not only hinder evaluation of the osseous structures immediately adjacent to the arthroplasty, but can also limit evaluation of the soft tissues somewhat distant from an arthroplasty (i.e., visceral pelvic anatomy in the setting of bilateral hip arthroplasty) [35].
Multiple parameters can be adjusted at the image acquisition and reconstruction phases to overcome metal-associated artifacts. Increasing the tube current (mAs) and tube voltage (kVp) will increase the number of photons that strike the detector, decreasing the effect of photon starvation and beam hardening artifacts, respectively. However, altering these parameters in isolation is often insufficient to produce a diagnostic quality image. Dual-energy CT, in which CT images are simultaneously acquired at both high and lower energy spectra, can be utilized to reconstruct a synthetic monoenergetic image with optimized kVp, minimizing the effect of beam hardening [31, 32, 35, 36]. In addition to modifications made at the acquisition phase, metal artifact reduction (MAR) techniques employ projection completion techniques to remove data corrupted by metal artifact at the reconstruction phase [31, 32, 36]. By identifying projections corrupted by metal, these corrupt projections can be subtracted from the original sinogram and filled in with interpolated data from adjacent detector elements. Each vendor has a proprietary metal artifact reduction sequence (MARS), some of which utilize MAR techniques and some of which combine dual-energy CT with MAR to further mitigate metal-associated artifact [34]. Deep learning–based reconstruction methods may also play a role in metal artifact suppression in the future [37].
However, there are pitfalls to the implementation of all metal-artifact reduction techniques. Increasing the mAs and kVp will increase the radiation dose to the patient [31]. Dual-energy CT can reduce beam hardening artifacts without increasing patient dose, but a high kVp technique will limit soft tissue contrast [32, 34, 35]. The use of MARS algorithms can also result in creation of new artifacts, as well as underestimation of the size of an arthroplasty (Fig. 3) [33, 34, 36]. Given these limitations of MARS, some authors have suggested that images reconstructed with MARS should be reviewed concurrently with non-MARS-reconstructed images to reduce diagnostic error [34].
A 58-year-old man with a right total hip arthroplasty who presented with abdominal pain. Patient underwent a CT of the abdomen and pelvis, and images were reconstructed with and without a metal artifact reduction sequence (iMAR—Siemens Healthcare). In the image without MAR (A), streak artifacts arise from the hip arthroplasty and partially obscure the adjacent bone and soft tissue (arrowheads). These streak artifacts are reduced in the image with MAR (B); however, an additional artifact is generated, with non-visualization of the central aspect of the femoral head component of the arthroplasty (star)
Magnetic resonance imaging
Magnetic resonance imaging (MRI) allows for comprehensive evaluation of a prosthetic joint, with excellent contrast resolution to delineate the prosthesis-bone interface, as well as the adjacent soft tissues. In the setting of PJI, MRI can clearly demonstrate osteolysis, bone marrow edema pattern, and periosteal reaction [38]. While all MR sequences and imaging planes should be reviewed when evaluating an arthroplasty, some planes are more useful in delineating periprosthetic complications. For example, for hip arthroplasty, axial MR images are the most useful for evaluating the prosthesis-bone or cement-bone interfaces around the femoral and acetabular components. In our experience, bone resorption and osteolysis around the femoral component is best seen using axial proton density-weighted sequences, as circumferential thick (> 2 mm) layer of increased signal intensity at the prosthesis-bone interface, which can be surrounded by a layer of low signal intensity, likely reflecting granulation and fibrous tissue, and surrounding reactive osseous sclerosis (Fig. 4) [39]. Images should also be scrutinized for the integrity of the pseudocapsule, presence of synovitis, and distention of the periarticular bursae including popliteal, iliopsoas, subiliac, and trochanteric bursae (Fig. 5).
A 79-year-old woman with knee pain 4 months after knee arthroplasty, concerning for PJI. Axial short tau inversion recovery (STIR) image (A) of the right knee demonstrates multilayered thickened and hyperintense (lamellated) synovitis (arrow) in the suprapatellar recess of the knee joint. Axial proton-density (PD) and STIR images (B and C) demonstrate focal osteolysis (arrows) adjacent to the tibial stem, with adjacent bone marrow edema pattern (star) apparent on the STIR image (C)
A 62-year-old man with hip pain after hip arthroplasty, with left hip PJI. Axial PD MR image of the hip (A) demonstrates a hip joint effusion with synovitis, and disruption of the pseudocapsule with fluid decompressing posteriorly (star). Extracapsular fluid communicates with an adjacent sinus tract to the skin surface (arrowheads). Coronal STIR MR images (B and C) demonstrate distention of the hip joint capsule superiorly (arrow), with mixed signal intensity joint fluid and synovitis (stars), with joint fluid communicating with an adjacent sinus tract (arrowheads)
One of the most sensitive and specific MRI findings of infection is the presence of lamellated synovium, or thickened and hyperintense, multilayered synovium, first described by Plodkowski et al. in the context of knee arthroplasty (Fig. 4) [40]. This finding has been more recently shown by Gao et al. to have high sensitivity and specificity in the setting of hip arthroplasty as well [41]. Similarly, edematous synovitis has reported > 90% sensitivity and specificity for PJI in the setting of shoulder arthroplasty [42]. Additional MRI findings suggestive of PJI include the presence of a joint effusion, periosteal reaction, periprosthetic muscle/soft tissue edema or extracapsular collections, and regional lymphadenopathy (Fig. 6) [27, 38, 42,43,44,45]. The presence of bone marrow signal abnormalities involving both the femoral and acetabular component of a hip arthroplasty has been found more commonly in the setting of infection rather than aseptic loosening in some studies [38], while other studies did not show significant difference [44].
A 53-year-old woman with hip pain after hip arthroplasty, with right hip PJI. Right hip radiograph (A) demonstrates an intact arthroplasty in anatomic position, without radiographic evidence of infection. Sequential coronal STIR images of the right hip from anterior to posterior (B–D) demonstrate a hip joint effusion and synovitis which decompresses into the adjacent iliopsoas bursa (B, arrow), as well as the adjacent adductor musculature (D, dashed arrow). Acetabular bone marrow edema was present, without osteolysis (C, star). Enlarged external iliac chain lymph nodes are also present (D, arrowheads)
Although there is significant imaging overlap between infected and non-infected arthroplasties, there are a few imaging features that can help exclude the presence of PJI. In a cohort of patients with total shoulder arthroplasty, Fritz et al. found a 100% negative predictive value for infection in patients without a joint effusion, or without lymphadenopathy [42]. In their respective study cohorts, lack of lamellated synovitis had high negative predictive value for PJI in the hip and knee, and lack of edematous synovitis had a high negative predictive value for PJI in the shoulder [40,41,42].
The use of gadolinium-based intravenous contrast can provide additional diagnostic benefit in the setting of suspected infection. Contrast can help differentiate between thickened, enhancing synovium from simple joint effusion/intra-articular debris. Contrast-enhanced images can also better delineate sinus tracts and soft tissue collections, which may otherwise be difficult to perceive within a region of soft tissue edema [14]. Given the presence of a sinus tract extending from the skin surface to the prosthetic joint is a diagnostic criterion for PJI according to multiple societies [4, 6,7,8,9,10], diagnostic accuracy in detection of sinus tracts is crucial.
Technical considerations
Similar to CT, MR imaging for the detection of PJI requires certain technical considerations to minimize the impact of metal-associated artifacts, which will vary with implant material and orientation of the implant within the main magnetic field [36]. MR imaging of prosthetic joints is often performed on 1.5T scanners, rather than 3T, as the magnetic susceptibility artifact generated by an arthroplasty is proportional to the main magnetic field strength [46]. However, a recent study by Khodarami et al. demonstrated interchangeability of 1.5T and 3T MRI for diagnosis of periprosthetic abnormalities; more effective metal artifact reduction and superior bone-implant interface characterization was seen at 1.5T, but 3T images demonstrated lower noise, sharper edges, and better visibility of the periprosthetic tissues [47].
Fast spin echo sequences are the mainstay of arthroplasty MR imaging, as the refocusing pulse will minimize spin dephasing near the arthroplasty [36, 48]. A wider receiver bandwidth, smaller slice thickness, and orientation of the static magnetic field along the long axis of the arthroplasty will further minimize metallic susceptibility artifact [36, 46]. View angle tilting (VAT) can also be utilized to reduce in-plane artifacts by tilting the readout direction to account for in-plane misregistration around metal [36]. If fat-suppressed sequences are desired, sequences such as short tau inversion recovery (STIR) should be used for more homogeneous fat suppression, as frequency-selective fat suppression techniques are susceptible to failure around the arthroplasty (Fig. 7) [49].
A 62-year-old man with hip pain after hip arthroplasty. Coronal proton density weighted image with frequency-selective fat suppression (A) demonstrates large areas of failed fat suppression around the hip arthroplasty (stars), most prominent around the femoral head and acetabular cup. The true extent of bone marrow edema pattern adjacent to the arthroplasty could not be determined. A coronal STIR image (B) from the same study demonstrates more homogeneous fat suppression around the arthroplasty
Commercially available metal-artifact reduction techniques include section encoding for metal artifact correction (SEMAC), multi-acquisition variable-resonance image combination (MAVRIC), and the hybrid MAVRIC selective (MAVRIC-SL). SEMAC relies on an additional phase-encoding gradient to excite multiple spatial partitions, which are combined to form a composite image, as well as view angle tilting to reduce in-plane distortion [36]. MAVRIC relies on the acquisition of multiple frequency bins, which are combined to form a composite image [46]. The hybrid MAVRIC-SL excites multiple frequency bins similar to MAVRIC, but utilizes a Z-gradient to resolve through-plane aliasing similar to SEMAC [50]. An example of the hip arthroplasty MR imaging protocol from the authors’ institution, which utilizes SEMAC for metal-artifact reduction, can be found in Table 2.
There are disadvantages associated with each of the previously described modifications. A wider receiver bandwidth, for example, will result in decreased signal-to-noise ratio (SNR), which can be overcome by increasing the number of excitations, at the expense of total scan time [46, 51]. Acceleration techniques such as compressed sensing, with pseudo-random under-sampling of k-space, can be implemented, reducing SEMAC acquisition times by 60–70% [36, 49, 51]. Additionally, while STIR sequences can provide homogeneous periprosthetic fat suppression, they have lower SNR compared with frequency-selective fat suppression techniques and preclude the use of intravenous contrast as the signal of enhancing tissue will be nulled [46]. If post-contrast sequences are desired, non-fat suppressed post-contrast sequences can be subtracted from pre-contrast T1 sequences to evaluate for tissue enhancement [48].
Image-guided procedures
Percutaneous image-guided prosthetic joint aspirations are essential in the workup of suspected PJI. Synovial fluid markers are included in multiple different societies’ diagnostic criteria for PJI, as joint fluid analysis has higher diagnostic accuracy than serum analysis [4, 6,7,8, 52]. Preoperative prosthetic joint aspirations allow for laboratory analysis of synovial fluid, including leukocyte count, neutrophil percentage, leukocyte esterase, alpha-defensin, and c-reactive protein (CRP) [53]. Two recent meta-analyses concluded that alpha-defensin, an anti-microbial peptide secreted by neutrophils, has the highest diagnostic performance for PJI with a sensitivity of 97% and specificity of 96–97% [53,54,55]. Synovial fluid bacterial cultures can also be performed, and a recent meta-analysis found that preoperative synovial fluid cultures have a sensitivity of 68.6% and specificity of 96.4% [53]. Although the sensitivity of synovial culture is insufficient to definitively exclude PJI, obtaining preoperative synovial fluid samples percutaneously allows for appropriate selection of antibiotic therapy based on cultured sensitivities and selection of appropriate antibiotic laden cement for revision surgery [56, 57]. More recently, next-generation gene sequencing assays have been found to outperform culture in accurate detection of PJI, with reported sensitivity, specificity, and positive predictive value of 94.8%, 89.2%, and 93.2%, respectively, as well as a shorter reporting time of 1.3 days [58,59,60].
Although the diagnostic benefit of synovial fluid analysis in patients with high suspicion for PJI is well-documented, there is less consensus on the role of aspiration in equivocal cases. Some institutions will perform preoperative aspirations to exclude infection in all patients with planned arthroplasty revision, but this practice is not universal. The AAOS 2011 guidelines for diagnosis of PJI recommended aspiration for all patients with high clinical suspicion, as well as those with low clinical suspicion for PJI but with elevated erythrocyte sedimentation rate (ESR) or CRP [61]. Regardless of clinical concern, if both ESR and CRP were normal, aspiration was not recommended. In 2019, the International Consensus on Orthopedic Infections concluded that joint aspiration was an important initial step in the workup of PJI, with “no clearly identified contraindications” [62]. A recent study by Staphorst et al. concluded that patients undergoing revision surgery for mechanical failure with normal ESR/CRP probably do not need preoperative synovial analysis, but aspiration is justifiable in all patients with loosening [63].
While aspiration of a knee arthroplasty can be performed without imaging guidance using palpable bony landmarks, the use of ultrasound guidance allows the performing radiologist to target small pockets of fluid in joints where only trace effusions are present. In deeper joints such as the hip, a lack of palpable landmarks and proximity of regional neurovascular structures obligate the need for image guidance when performing joint aspirations. Hip aspirations are frequently performed with either fluoroscopic or ultrasound guidance (Fig. 8). With fluoroscopic guidance, the aspiration needle can be advanced into the intra-articular space, allowing for fluid aspiration and culture. Fluoroscopically guided aspirations of the hip joint can also serve a diagnostic purpose, as injection of iodinated contrast into the hip joint after aspiration can delineate communication from the hip joint to adjacent soft tissue collections or sinus tracts (Fig. 9). While communication with the adjacent greater trochanteric or iliopsoas bursae of the hip may be an expected post-surgical finding, communication of an irregular soft tissue collection with the prosthetic hip joint is more suggestive of infection [18]. With ultrasound guidance, the operator can directly target fluid within the prosthetic hip joint, as well as within adjacent soft tissue collections, if present. Perhaps owing to the ability to directly target loculated fluid within the joint, ultrasound-guided hip aspirations have reported to have higher sensitivity and specificity compared with fluoroscopically guided hip aspirations [64]. Less commonly, CT can be utilized for image-guided prosthetic joint aspirations; however, CT lacks the real-time guidance of ultrasound and has higher radiation dose than fluoroscopy [65, 66]. While this technique has been shown to be an accurate method of diagnosing PJI [29, 67], it is rarely utilized at the authors’ institution, except in cases were joint access is limited by overlying bony proliferative changes, or extreme cases of osteolysis with a displaced prosthesis. Ultimately, multiple factors including provider comfort, patient body habitus, and availability of equipment all play a role in which modality is appropriate for an image-guided prosthetic joint aspiration.
Image-guided hip arthroplasty aspiration. A A 69-year-old man with hip pain after hip arthroplasty was referred for fluoroscopic-guided hip aspiration to rule out infection. Fluoroscopy image demonstrates an 18-gauge needle within the prosthetic hip joint. Prosthetic hip joint aspirations can also be performed under ultrasound guidance (B), as seen in an intra-procedural image of an 80-year-old woman who was also referred for aspiration given concern for infection. An 18-gauge needle (arrowheads) can be seen within a markedly distended anterior recess of the hip joint, with the neck (arrow) and femoral head (star) components of the arthroplasty visualized deep to the joint effusion
A 59-year-old man with right hip PJI. Initial radiograph (A) demonstrates a right total hip arthroplasty in anatomic position (periacetabular lucency reflects subchondral cysts that were present preoperatively). No periprosthetic osteolysis was appreciated. The patient subsequently underwent fluoroscopic-guided right hip aspiration, with an intra-procedural image (B) demonstrating an 18-gauge needle overlying the neck of the arthroplasty (arrow). No native fluid was obtained (“dry tap”). Subsequently, iodinated contrast was injected (C), with an intra-procedural image demonstrating contrast extension through a posterior defect in the pseudocapsule, communicating with an adjacent sinus tract (arrowheads)
A “dry tap” can be encountered if there is insufficient aspirate from a prosthetic joint for laboratory analysis in the setting of suspected infection (typically less than 0.5 mL) [68]. Saline lavage, or injection of non-bacteriostatic sterile saline into the joint and subsequent re-aspiration, may be performed following a dry tap, although this technique remains controversial. Some studies have demonstrated that dilution of synovial fluid in saline decreases sensitivity of cell counts [69] or increases rates of intraoperative and preoperative culture discordance [68]. However, other studies have shown that neutrophil percentage and cultured organisms from lavage samples remain similar to native fluid aspiration [56, 70, 71]. The International Consensus on Orthopedic Infections (2019) recommended against saline lavage, with an exception made for aspirations performed by dedicated radiologists in a sterile manner [62]. In our clinical practice, we have found that surgeons’ preferences regarding lavage can vary depending on their pre-test probability for infection and consultation prior to aspiration is recommended. If small amounts of native fluid are obtained, cell count is often prioritized, given higher sensitivity and shorter reporting time compared with synovial culture. A recent study reviewing the outcomes of patients whose image-guided hip aspiration for suspected PJI resulted in a dry tap found that 84% of these patients were ultimately not diagnosed with PJI [71]. Among those who were diagnosed with PJI, greater than one third had dehiscence of the hip prosthesis pseudocapsule, with extra-articular extension of joint fluid into a dependent or posterior location, likely contributing to the dry tap. In these cases, pre-aspiration MRI or ultrasound could help delineate the presence of joint effusion or pseudocapsule dehiscence with extra-articular fluid collection and help to guide a targeted aspiration (Fig. 10) [71]. Finally, synovial biopsies can also be performed preoperatively, with multiple techniques described in the literature, ranging from fine needle aspiration to sampling with various biopsy devices [72,73,74,75]. Studies have shown that synovial biopsy can have diagnostic benefit when joint fluid is absent, but have no more diagnostic yield than synovial aspiration alone when joint fluid is present [72,73,74,75].
A 48-year-old man with right hip resurfacing arthroplasty and concern for infection. An ultrasound-guided aspiration was attempted, with pre-procedural image (A) demonstrating the femoral neck (arrow) and resurfaced femoral head (star). Synovial thickening was noted anterior to the arthroplasty (arrowheads); however, there was no fluid distention of the anterior recess of the joint, and the subsequent aspiration yielded no fluid. Coronal and axial STIR images from an MRI performed the following day (B and C) demonstrate periprosthetic bone marrow edema pattern (star), anterior dehiscence of the pseudocapsule with lateral escape of fluid (arrow), and edema of the adjacent musculature (arrowheads)
Conclusion
Diagnostic imaging can play an important role in the workup of PJI. Radiographs are a useful screening tool, but more advanced imaging or image-guided joint aspiration is usually required. MRI is the superior diagnostic imaging modality for characterizing the prosthetic joint and its surrounding bone and soft tissue, but requires modification of standard acquisition techniques to be clinically valuable. Image-guided joint aspirations remain crucial component in the workup of PJI, especially with advent of more efficient and accurate diagnostic tests such as next-generation gene sequencing. In the case of insufficient fluid for aspiration, further diagnostic imaging can identify extracapsular decompression of fluid that can be specifically targeted. Although dry taps are less likely to be associated with PJI, lack of fluid on aspiration, or even negative synovial fluid culture, should not be considered sufficient to definitively exclude PJI. With the emergence of more evidence-based diagnostic algorithms for imaging utilization in the workup of PJI, as well as technical advances in CT and MR image acquisition, diagnostic imaging and image-guided intervention can help guide clinical management of patients with suspected prosthetic joint infection.
References
Maradit Kremers H, Larson DR, Crowson CS, Kremers WK, Washington RE, Steiner CA, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97:1386–97.
Singh JA, Yu S, Chen L, Cleveland JD. Rates of total joint replacement in the United States: future projections to 2020-2040 using the national inpatient sample. J Rheumatol. 2019;46:1134–40.
Tubb CC, Polkowksi GG, Krause B. Diagnosis and prevention of periprosthetic joint infections. J Am Acad Orthop Surg. 2020;28:e340–8.
Parvizi J, Tan TL, Goswami K, Higuera C, Della Valle C, Chen AF, et al. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty. 2018;33:1309–1314.e2.
Romanò CL, Petrosillo N, Argento G, Sconfienza LM, Treglia G, Alavi A, et al. The role of imaging techniques to define a peri-prosthetic hip and knee joint infection: multidisciplinary consensus statements. J Clin Med. 2020;9:2548.
Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, et al. diagnosis and management of prosthetic joint infection: Clinical Practice Guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56:e1–25.
Renz N, Yermak K, Perka C, Trampuz A. Alpha defensin lateral flow test for diagnosis of periprosthetic joint infection: not a screening but a confirmatory test. J Bone Jt Surg. 2018;100:742–50.
Romanò CL, Khawashki HA, Benzakour T, Bozhkova S, Del Sel H, Hafez M, et al. The W.A.I.O.T. definition of high-grade and low-grade peri-prosthetic joint infection. J Clin Med. 2019;8:650.
Parvizi J, Gehrke T. Proceedings of the second international consensus meeting on musculoskeletal infection. Data Trace Publishing Company; 2018.
Parvizi J, Gehrke T. International consensus group on periprosthetic joint infection. Definition of periprosthetic joint infection. J Arthroplasty. 2014;29:1331.
Signore A, Sconfienza LM, Borens O, Glaudemans AWJM, Cassar-Pullicino V, Trampuz A, et al. Consensus document for the diagnosis of prosthetic joint infections: a joint paper by the EANM, EBJIS, and ESR (with ESCMID endorsement). Eur J Nucl Med Mol Imaging. 2019;46:971–88.
Sconfienza LM, Signore A, Cassar-Pullicino V, Cataldo MA, Gheysens O, Borens O, et al. Diagnosis of peripheral bone and prosthetic joint infections: overview on the consensus documents by the EANM, EBJIS, and ESR (with ESCMID endorsement). Eur Radiol. 2019;29:6425–38.
Hochman MG, Melenevsky YV, Metter DF, Roberts CC, Bencardino JT, Cassidy RC, et al. ACR Appropriateness Criteria ® imaging after total knee arthroplasty. J Am Coll Radiol. 2017;14:S421–48.
Thejeel B, Endo Y. Imaging of total hip arthroplasty: part II – imaging of component dislocation, loosening, infection, and soft tissue injury. Clin Imaging. 2022;92:72–82.
Harmer JL, Pickard J, Stinchcombe SJ. The role of diagnostic imaging in the evaluation of suspected osteomyelitis in the foot: a critical review. The Foot. 2011;21:149–53.
Walde TA, Weiland DE, Leung SB, Kitamura N, Sychterz CJ, Engh CA, et al. Comparison of CT, MRI, and radiographs in assessing pelvic osteolysis: a cadaveric study. Clin Orthop. 2005;NA:138–44.
Cyteval C, Hamm V, Sarrabère MP, Lopez FM, Maury P, Taourel P. Painful infection at the site of hip prosthesis: CT imaging. Radiology. 2002;224:477–83.
Miller TT. Imaging of hip arthroplasty. Eur J Radiol. 2012;81:3802–12.
van Holsbeeck MT, Eyler WR, Sherman LS, Lombardi TJ, Mezger E, Verner JJ, et al. Detection of infection in loosened hip prostheses: efficacy of sonography. AJR Am J Roentgenol. 1994;163:381–4.
Li N, Kagan R, Hanrahan CJ, Hansford BG. Radiographic evidence of soft-tissue gas 14 days after total knee arthroplasty is predictive of early prosthetic joint infection. Am J Roentgenol. 2020;214:171–6.
Expert Panel on Musculoskeletal Imaging, Weissman BN, Palestro CJ, Fox MG, Bell AM, Blankenbaker DG et al. ACR Appropriateness Criteria® imaging after total hip arthroplasty. J Am Coll Radiol. 2023;20:S413–32.
Klauser AS, Tagliafico A, Allen GM, Boutry N, Campbell R, Court-Payen M, et al. Clinical indications for musculoskeletal ultrasound: a Delphi-based consensus paper of the European society of musculoskeletal radiology. Eur Radiol. 2012;22:1140–8.
Soliman SB, Davis JJ, Muh SJ, Vohra ST, Patel A, van Holsbeeck MT. Ultrasound evaluations and guided procedures of the painful joint arthroplasty. Skeletal Radiol. 2022;51:2105–20.
Craig JG. Ultrasound of the postoperative hip. Semin Musculoskelet Radiol. 2013;17:49–55.
Weybright PN, Jacobson JA, Murry KH, Lin J, Fessell DP, Jamadar DA, et al. Limited effectiveness of sonography in revealing hip joint effusion: preliminary results in 21 adult patients with native and postoperative hips. Am J Roentgenol. 2003;181:215–8.
Douis H, Dunlop DJ, Pearson AM, O’Hara JN, James SLJ. The role of ultrasound in the assessment of post-operative complications following hip arthroplasty. Skeletal Radiol. 2012;41:1035–46.
Math KR, Berkowitz JL, Paget SA, Endo Y. Imaging of musculoskeletal infection. Rheum Dis Clin North Am. 2016;42:769–84.
Reish TG, Clarke HD, Scuderi GR, Math KR, Scott WN. Use of multi-detector computed tomography for the detection of periprosthetic osteolysis in total knee arthroplasty. J Knee Surg. 2006;19:259–64.
Isern-Kebschull J, Tomas X, García-Díez AI, Morata L, Ríos J, Soriano A. Accuracy of computed tomography–guided joint aspiration and computed tomography findings for prediction of infected hip prosthesis. J Arthroplasty. 2019;34:1776–82.
Taljanovic MS, Gimber LH, Omar IM, Klauser AS, Miller MD, Wild JR, et al. Imaging of postoperative infection at the knee joint. Semin Musculoskelet Radiol. 2018;22:464–80.
Wellenberg RHH, Hakvoort ET, Slump CH, Boomsma MF, Maas M, Streekstra GJ. Metal artifact reduction techniques in musculoskeletal CT-imaging. Eur J Radiol. 2018;107:60–9.
Katsura M, Sato J, Akahane M, Kunimatsu A, Abe O. Current and novel techniques for metal artifact reduction at CT: practical guide for radiologists. RadioGraphics. 2018;38:450–61.
Huang JY, Kerns JR, Nute JL, Liu X, Balter PA, Stingo FC, et al. An evaluation of three commercially available metal artifact reduction methods for CT imaging. Phys Med Biol. 2015;60:1047–67.
Andersson KM, Norrman E, Geijer H, Krauss W, Cao Y, Jendeberg J, et al. Visual grading evaluation of commercially available metal artefact reduction techniques in hip prosthesis computed tomography. Br J Radiol. 2016;89:20150993.
Pessis E, Sverzut J-M, Campagna R, Guerini H, Feydy A, Drapé J-L. Reduction of metal artifact with dual-energy CT: virtual monospectral imaging with fast kilovoltage switching and metal artifact reduction software. Semin Musculoskelet Radiol. 2015;19:446–55.
Khodarahmi I, Fishman E, Fritz J. Dedicated CT and MRI techniques for the evaluation of the postoperative knee. Semin Musculoskelet Radiol. 2018;22:444–56.
Huang Z, Zhang G, Lin J, Pang Y, Wang H, Bai T, et al. Multi-modal feature-fusion for CT metal artifact reduction using edge-enhanced generative adversarial networks. Comput Methods Programs Biomed. 2022;217:106700.
Schwaiger BJ, Gassert FT, Suren C, Gersing AS, Haller B, Pfeiffer D, et al. Diagnostic accuracy of MRI with metal artifact reduction for the detection of periprosthetic joint infection and aseptic loosening of total hip arthroplasty. Eur J Radiol. 2020;131:109253.
Goodman SB, Gallo J. Periprosthetic osteolysis: mechanisms, prevention and treatment. J Clin Med. 2019;8:2091.
Plodkowski AJ, Hayter CL, Miller TT, Nguyen JT, Potter HG. Lamellated hyperintense synovitis: potential MR imaging sign of an infected knee arthroplasty. Radiology. 2013;266:256–60.
Gao Z, Jin Y, Chen X, Dai Z, Qiang S, Guan S, et al. Diagnostic value of MRI lamellated hyperintense synovitis in periprosthetic infection of hip. Orthop Surg. 2020;12:1941–6.
Fritz J, Meshram P, Stern SE, Fritz B, Srikumaran U, McFarland EG. Diagnostic performance of advanced metal artifact reduction MRI for periprosthetic shoulder infection. J Bone Jt Surg. 2022;104:1352–61.
Jiang M, He C, Feng J, Li Z, Chen Z, Yan F, et al. Magnetic resonance imaging parameter optimizations for diagnosis of periprosthetic infection and tumor recurrence in artificial joint replacement patients. Sci Rep. 2016;6:36995.
Galley J, Sutter R, Stern C, Filli L, Rahm S, Pfirrmann CWA. Diagnosis of periprosthetic hip joint infection using MRI with metal artifact reduction at 1.5 T. radiology. 2020;296:98–108.
Fritz J, Lurie B, Miller TT, Potter HG. MR imaging of hip arthroplasty implants. RadioGraphics. 2014;34:E106–32.
Berkowitz JL, Potter HG. Advanced MRI techniques for the hip joint: focus on the postoperative hip. Am J Roentgenol. 2017;209:534–43.
Khodarahmi I, Khanuja HS, Stern SE, Carrino JA, Fritz J. Compressed Sensing SEMAC MRI of hip, knee, and ankle arthroplasty implants: A 1.5-T and 3-T intrapatient performance comparison for diagnosing periprosthetic abnormalities. AJR Am J Roentgenol. 2023;221:661–72.
Fritz J, Lurie B, Potter HG. MR imaging of knee arthroplasty implants. Radiogr Rev Publ Radiol Soc N Am Inc. 2015;35:1483–501.
Murthy S, Fritz J. Metal artifact reduction MRI in the diagnosis of periprosthetic hip joint infection. Radiology. 2023;306:e220134.
Choi S-J, Koch KM, Hargreaves BA, Stevens KJ, Gold GE. Metal artifact reduction with MAVRIC SL at 3-T MRI in patients with hip arthroplasty. AJR Am J Roentgenol. 2015;204:140–7.
Khodarahmi I, Nittka M, Fritz J. Leaps in technology: advanced MR imaging after total hip arthroplasty. Semin Musculoskelet Radiol. 2017;21:604–15.
Shahi A, Tan TL, Kheir MM, Tan DD, Parvizi J. Diagnosing periprosthetic joint infection: and the winner is? J Arthroplasty. 2017;32:S232–5.
Carli AV, Abdelbary H, Ahmadzai N, Cheng W, Shea B, Hutton B, et al. Diagnostic accuracy of serum, synovial, and tissue testing for chronic periprosthetic joint infection after hip and knee replacements: a systematic review. J Bone Jt Surg. 2019;101:635–49.
Lee YS, Koo K-H, Kim HJ, Tian S, Kim T-Y, Maltenfort MG, et al. Synovial fluid biomarkers for the diagnosis of periprosthetic joint infection: a systematic review and meta-analysis. J Bone Jt Surg. 2017;99:2077–84.
Bonanzinga T, Ferrari MC, Tanzi G, Vandenbulcke F, Zahar A, Marcacci M. The role of alpha defensin in prosthetic joint infection (PJI) diagnosis: a literature review. EFORT Open Rev. 2019;4:10–3.
Partridge DG, Winnard C, Townsend R, Cooper R, Stockley I. Joint aspiration, including culture of reaspirated saline after a “dry tap”, is sensitive and specific for the diagnosis of hip and knee prosthetic joint infection. Bone Joint J. 2018;100-B:749–54.
Kanthawang T, Bodden J, Joseph GB, Vail T, Ward D, Patel R, et al. Diagnostic value of fluoroscopy-guided hip aspiration for periprosthetic joint infection. Skeletal Radiol. 2021;50:2245–54.
Li M, Zeng Y, Wu Y, Si H, Bao X, Shen B. Performance of sequencing assays in diagnosis of prosthetic joint infection: a systematic review and meta-analysis. J Arthroplasty. 2019;34:1514–1522.e4.
Flierl MA, Sobh AH, Culp BM, Baker EA, Sporer SM. Evaluation of the painful total knee arthroplasty. J Am Acad Orthop Surg. 2019;27:743–51.
Hao L, Wen P, Song W, Zhang B, Wu Y, Zhang Y, et al. Direct detection and identification of periprosthetic joint infection pathogens by metagenomic next-generation sequencing. Sci Rep. 2023;13:7897.
Della Valle C, Parvizi J, Bauer TW, DiCesare PE, Evans RP, Segreti J, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: the diagnosis of periprosthetic joint infections of the hip and knee. J Bone Joint Surg Am. 2011;93:1355–7.
Abdel Karim M, Andrawis J, Bengoa F, Bracho C, Compagnoni R, Cross M, et al. Hip and knee section, diagnosis, algorithm: proceedings of international consensus on orthopedic infections. J Arthroplasty. 2019;34:S339–50.
Staphorst F, Jutte PC, Boerboom AL, Kampinga GA, Ploegmakers JJW, Wouthuyzen-Bakker M. Should all hip and knee prosthetic joints be aspirated prior to revision surgery? Arch Orthop Trauma Surg. 2021;141:461–8.
Battaglia M, Vannini F, Guaraldi F, Rossi G, Biondi F, Sudanese A. Validity of preoperative ultrasound-guided aspiration in the revision of hip prosthesis. Ultrasound Med Biol. 2011;37:1977–83.
Li X, Hirsch JA, Rehani MM, Yang K, Liu B. Effective dose assessment for patients undergoing contemporary fluoroscopically guided interventional procedures. Am J Roentgenol. 2020;214:158–70.
Yang K, Ganguli S, DeLorenzo MC, Zheng H, Li X, Liu B. Procedure-specific CT dose and utilization factors for CT-guided interventional procedures. Radiology. 2018;289:150–7.
Tomas X, Bori G, Garcia S, Garcia-Diez AI, Pomes J, Soriano A, et al. Accuracy of CT-guided joint aspiration in patients with suspected infection status post-total hip arthroplasty. Skeletal Radiol. 2011;40:57–64.
Christensen TH, Ong J, Lin D, Aggarwal VK, Schwarzkopf R, Rozell JC. How does a “dry tap” impact the accuracy of preoperative aspiration results in predicting chronic periprosthetic joint infection? J Arthroplasty. 2022;37:925–9.
Deirmengian C, Feeley S, Kazarian GS, Kardos K. Synovial fluid aspirates diluted with saline or blood reduce the sensitivity of traditional and contemporary synovial fluid biomarkers. Clin Orthop. 2020;478:1805–13.
Heckmann ND, Nahhas CR, Yang J, Della Valle CJ, Yi PH, Culvern CN, et al. Saline lavage after a “dry tap.” Bone Joint J. 2020;102-B:138–44.
Serfaty A, Jacobs A, Gyftopoulos S, Samim M. Likelihood of hip infection with image-guided hip aspiration dry tap: a 10-year retrospective study. Skeletal Radiol. 2022;51:1947–58.
Sconfienza LM, Albano D, Messina C, D’Apolito R, De Vecchi E, Zagra L. Ultrasound-guided periprosthetic biopsy in failed total hip arthroplasty: a novel approach to test infection in patients with dry joints. J Arthroplasty. 2021;36:2962–7.
Cross MC, Kransdorf MJ, Chivers FS, Lorans R, Roberts CC, Schwartz AJ, et al. Utility of percutaneous joint aspiration and synovial biopsy in identifying culture-positive infected hip arthroplasty. Skeletal Radiol. 2014;43:165–8.
Coiffier G, Ferreyra M, Albert J-D, Stock N, Jolivet-Gougeon A, Perdriger A, et al. Ultrasound-guided synovial biopsy improves diagnosis of septic arthritis in acute arthritis without enough analyzable synovial fluid: a retrospective analysis of 176 arthritis from a French rheumatology department. Clin Rheumatol. 2018;37:2241–9.
Rajakulasingam R, Cleaver L, Khoo M, Pressney I, Upadhyay B, Palanivel S, et al. Introducing image-guided synovial aspiration and biopsy in assessing peri-prosthetic joint infection: an early single-centre experience. Skeletal Radiol. 2021;50:2031–40.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• Diagnostic imaging is frequently utilized in the workup of prosthetic joint infection (PJI), despite lack of clear consensus on the specific role of each modality.
• MRI is the optimal diagnostic modality for PJI, as it has high sensitivity and specificity for PJI when metal artifact reduction techniques are utilized.
• Image-guided prosthetic joint aspirations allow for synovial fluid analysis that is critical to diagnosing PJI, with newer synovial laboratory markers demonstrating higher diagnostic performance than culture.
• Although “dry” image-guided joint aspirations are more common in patients without PJI, dry taps in the setting of hip PJI are commonly due to dehiscence of the prosthesis pseudocapsule.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jardon, M., Fritz, J. & Samim, M. Imaging approach to prosthetic joint infection. Skeletal Radiol 53, 2023–2037 (2024). https://doi.org/10.1007/s00256-023-04546-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-023-04546-7