Abstract
Objective
To compare and evaluate T2 values of compartmental femorotibial cartilage and subregional menisci in patients with hyperuricemia at 3T.
Materials and methods
Thirty-two subjects were included in this study and subdivided into two subgroups: 15 healthy controls (3 females, 12 males; mean age = 45.3 ± 10.9 years), 17 patients with hyperuricemia (2 females, 15 males; mean age = 44.4 ± 12.7 years). All subjects were assessed on a 3T MR scanner using an 8-channel phased-array knee coil (transmit-receive). Wilcoxon rank sum test and analysis of covariance (ANCOVA) were performed to determine whether there were any statistically significant differences in T2 values of compartmental femorotibial cartilage and subregional menisci between the two subgroups.
Results
Lateral tibial cartilage (48.6 ± 3.5 ms) in healthy subgroup had significantly lower (p < 0.05) T2 values than all subcompartments of femorotibial cartilage in hyperuricemia subgroup. Medial tibial cartilage (56.5 ± 4.3 ms) in hyperuricemia subgroup had significantly higher (p < 0.05) T2 values than all subcompartments of femorotibial cartilage except medial tibial cartilage in healthy subgroup. Medial anterior horn of meniscus (39.4 ± 2.9 ms) in healthy subgroup had significantly lower (p < 0.05) T2 values than all subregional menisci except both medial anterior horn and medial body segment of meniscus in hyperuricemia subgroup.
Conclusion
T2 values in certain compartmental femorotibial cartilage and subregional menisci in patients with hyperuricemia are evidently and abnormally heightened compared with those in healthy subjects, to which special attention should be paid when diagnosing and treating the patients with hyperuricemia in the clinical setting.
Graphical abstract

The LT cartilage had significantly lower T2 values (48.6 ± 3.5 ms) in healthy subgroup compared to all compartmental femorotibial cartilage in cohort with HU. MF cartilage had significantly lower T2 values (51.6 ± 2.9 ms) in healthy subgroup compared to both LF (54.4 ± 4.1 ms) and MT (56.5 ± 4.3 ms) in cohort with HU. MT cartilage had significantly higher T2 values (56.5 ± 4.3 ms) in cohort with HU subgroup compared to LF (52.5 ± 3.0 ms) in healthy subgroup. T2 mapping may be promising and potential sensitive discriminator of understanding and examining the early compositional and structural change in proteoglycan–collagen matrix of human femorotibial cartilage in patients with hyperuricemia.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The pathogenesis of osteoarthritis (OA) is complex and multi-factorial, and the etiologies of which are quite a few: sports injuries such as anterior cruciate ligament (ACL) strain or tear and meniscal tear, knee malalignment, over-weight or obesity, age, female gender, increased body mass index (BMI), and metabolic or genetic factors, among others, are all well-known risk factors or possible initiations of knee OA [1,2,3,4,5,6,7,8,9,10,11]. Particularly, both hyperuricemia and gout may be potential onsets of knee OA as well [12, 13].
Hyaline cartilage consists of chondrocytes scattered in the extracellular matrix (ECM), which is mainly composed of water, proteoglycans (PGs), and collagen (primarily type II) fibers. The macromolecules restrict the motion of water in the ECM [11]. The adjacent menisci are predominantly made up of water, collagen (primarily type I), and PGs as well [14, 15], and the ECM of intact meniscus is comprised of roughly 72% water, 22% collagen, and 0.8% glycosaminoglycans (GAGs) [15, 16]. Pathophysiological processes of early cartilage or meniscus degeneration are characterized by GAG loss and deterioration of the PG-collagen matrix network integrity, which may give rise to increased mobility of water and consequentially elevated water content within the cartilage or meniscus ECM [17, 18].
Only a few previous studies had been performed to determine whether joints affected by asymptomatic hyperuricemia (HU) or gout (symptomatic HU) were also affected by OA using radiographs of the hip, hand wrist, and knee joint [12, 13, 19, 20]. Roddy et al. [12] suggested that patients with OA may be prone to the localized deposition of monosodium urate (MSU) crystals and partially determine which joints are affected by acute attacks of gout. Bevis and colleagues [13] indicated that no significant association was identified between gout and radiographic OA, although there was a trend that people with gout may be more likely to have small joint OA. Ding and co-workers [19] indicated that the serum uric acid concentration and prevalence of HU are positively associated with knee joint osteophytes more preferentially in the female population. Kuo et al. [20] implied that chronic hyperuricaemia and asymptomatic MSU crystal deposition prior to the first clinical presentation of gout may result in joint damage. Specifically, Neogi and colleagues [21] reviewed the evidence linking gout and osteoarthritis and indicated that the overlap between OA and gout—and in particular, OA and hyperuricemia—suggests that a co-occurrence that had been recognized clinically may not be only coincidental.
T2 relaxation reflects the dipole–dipole interaction between bulk water and PG-collagen matrix within the cartilage or meniscus, and damage to the ECM and the elevation of water content in degenerating cartilage or meniscus may increase T2 relaxation times [22,23,24]. Many studies have used T2 mapping of cartilage or meniscus to explore cartilage or meniscus deterioration even without distinct radiographic changes yet or in morphologically normal knees on conventional MRI and in OA-associated cartilage [15, 23, 25,26,27]. While generally increased T2 values are associated with cartilage or meniscus degradation, one study did imply that no appreciable change at all in T2 values with cartilage degeneration relative to normal cartilage in prior publication [28]. Prior radiographic studies on OA had examined the advanced joint degeneration like the emergence of osteophytes or joint space narrowing (JSN) in HU or gout (symptomatic HU) population [12, 13, 19, 24]. However, to the best of our knowledge, investigations using quantitative T2 mapping of compartmental femorotibial cartilage and subregional menisci in patients with HU to examine and assess the pre-structural and pre-radiographic changes of cartilage and menisci have not been performed as yet. Therefore, the purpose of this work was to compare and assess T2 values of compartmental femorotibial cartilage and subregional menisci in HU population. Our hypothesis in this work was that the T2 values in certain compartmental femorotibial cartilage and subregional menisci in patients with HU may be significantly different than those of healthy subjects, and MR T2 mapping may be a potential biomarker and sensitive discriminator of understanding and examining the early structural change in the PG-collagen matrix integrity of human femorotibial cartilage and menisci in patients with HU.
Materials and methods
Study population
The procedure and nature of this prospective study were fully explained to all the participated volunteers. All recruited subjects signed written informed consent to be involved in the study, which was approved by the local institutional review board (IRB). All healthy subjects were recruited via the advertisement extensively posted in both a few living communities nearby and our university campus. The control group was selected based on matched age and the BMI (Table 1) [29]. For all recruited healthy subjects, the inclusion criteria were in healthy conditions, and no existing medical conditions based on medical history, physical checkup, and no contraindications to MR examination. Meanwhile, the enrollment criteria for the cohort with healthy knee joint were as follows: no history of chronic or frequent knee pain, absence of clinical symptoms, no hyperglycemia or diabetes mellitus (DM) (both based on the periodic physical examinations in our university-affiliated hospital), and normal knee radiographs prior to MR scans. Exclusion criteria were inflammatory arthritis, knee OA, and knee OA secondary to some other causes (i.e., acute or chronic infection, prior surgery or fracture). All volunteers with HU were recruited from those taking periodic medical examinations in our university-affiliated hospital. The inclusion criteria were based on their physical checkup results with the blood samples assayed as uric acid ≥ 416 μmol/L for male and ≥ 360 μmol/L for female [19]. The exclusion criteria for HU cohort were frequent knee joint ache, gout (symptomatic HU), history of hyperglycemia or DM, or currently using hypoglycemic agent or diuretics (drugs to treat hyperuricemia), chronic kidney disease, hypothyroidism, hypercholesterolemia, and any contraindications to MR imaging. Meanwhile, all enrolled subjects had no history of malignancy and chemo- or radiotherapy.
Thirty-two subjects were included in this study and subdivided into two subgroups: 15 healthy controls (3 females, 12 males; mean age = 45.3 ± 10.9 years), 17 patients with hyperuricemia (2 females, 15 males; mean age = 44.4 ± 12.7 years) (Table 1). The subjects’ body height and weight were obtained for computing the BMI. The demographics of the participated study population were listed in Table 1.
MR protocol
All MR imaging experiments were conducted on a 3.0 Tesla clinical MR scanner (Ingenia, Philips, The Netherlands). An 18-cm diameter, quadrature transmit/8-channel phased-array (PA) receive knee coil (dS Knee 8ch 3.0 T, Invivo Corporation, USA) was employed for all the MR imaging measurements. The knee was fixed with foam padding for immobilizing during acquisition.
All MR examinations were performed in the sagittal plane. The following imaging protocol was applied: a sagittal 2D multi-echo turbo spin-echo (TSE) pulse sequence with fat saturation and with a repetition time (TR) of 4000 ms; echo times (TEs) of 16.5, 33, 49.5, 66, and 82.5 ms; a field of view (FOV) of 150 × 150 mm; a matrix of 188 × 185 (interpolated to 256 × 256); a receive bandwidth of 122 Hz/pixel; a number of signal averages (NSA) of 1; an in-plane spatial resolution of 0.59 × 0.59 mm2; and a slice thickness of 3 mm (inter-slice gap = 0). Total acquisition time (TA) for all 5 echo times (28 slices for each TE) was roughly 10 min. The data acquisition and analyses for all the included subjects were performed from December 2016 through January 2020.
Image analysis
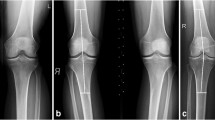
For quantitative T2 assessment of femorotibial cartilage and meniscus, all MR images were analyzed based on the compartmental femorotibial cartilage as lateral femoral (LF), lateral tibial (LT), medial femoral (MF), and medical tibial (MT), respectively (Fig. 1(a, b)), and segmented subregional meniscus regions of interest (ROIs) as lateral anterior horn (La), lateral central (body segment) (Lc), lateral posterior horn (Lp), medial anterior horn (Ma), medial central (body segment) (Mc), and medial posterior horn (Mp), respectively also as defined in Fig. 1 (c, d, e, f) or Refs [30,31,32].
Exemplary sagittal fat-saturated T2-weighted 2D Turbo spin-echo (TSE) images with echo time (TE) = 16.5 ms illustrating how the regions of interest (ROIs) of the femorotibial cartilage and menisci subregions in the lateral (Fig. 1(a); Fig. 1(c, e)) and medial (Fig. 1(b); Fig. 1(d, f)) compartments were defined, obtained from a 25-year old female healthy subject. White arrowheads mark the location of the femorotibial cartilage and menisci subregions
The in-house developed routines in MATLAB (version R2017a; The Mathworks, Natick, MA, USA) and C + + were used for offline processing of the acquired MR images including femorotibial cartilage and menisci segmentation, and T2 value calculation of compartmental femorotibial cartilage and subregional meniscus. The signal intensities of the serial T2-weighted images were fitted on a pixel-wise basis using a linear least-squares method as shown in Eq. 1 [22, 29, 32]:
The detailed description on how to calculate the T2 values is the same as in Refs [29, 32]. For the analysis of the T2 values of the compartmental femorotibial cartilage and subregional menisci, ROIs were manually drawn (by L.W.) as LF, LT, MF, MT for the femorotibial cartilage, and La, Lc, Lp, Ma, Mc, and Mp for menisci as illustrated in Fig. 1 or Refs 30, 31, and 32 using a custom-built MATLAB routine. The custom-built MATLAB routine ultimately yielded color-coded T2 maps, in which the manually segmented cartilage and meniscal ROIs were overlaid on the shortest echo time (namely, TE = 16.5 ms) images.
Statistical analysis
Statistical analysis was performed (by X.L.) using a SPSS Statistics software tool (version 22.0, IBM, Armonk, NY, USA). As non-parametric analyses were conducted, results are summarized as mean ± SD and median ± inter-quartile range (IQR) (not listed). The inter-subject variation of cartilage and meniscus T2 value measurement was quantified using root mean square coefficient of variation percentage (RMS-CV%). Meanwhile, to document the intra-operator variation for both cartilage and meniscal T2, the LF and LT cartilage and La and Lp subregional menisci from 11 healthy controls were measured twice, respectively, for roughly calculating the intra-observer reproducibility of cartilage and meniscus T2 value measurement.
All subjects were divided into two subgroups, i.e., healthy controls and cohort with HU. All comparisons were stratified by compartment and/or subregion. One-way analysis of covariance (ANCOVA) was utilized to obtain the p values comparing T2 measurements by regressing out confounding covariates, i.e., age, gender, and BMI in each of the subcompartments for femorotibial cartilage and subregions for menisci between the two subgroups (healthy controls vs. HU cohort). T2 values recorded for a given region were first converted to ranks that were used as the dependent variable. ANCOVA analyses for each compartment and region included age and BMI as numeric covariates, gender as a nominal covariate, and subject subgroups as the between-subject nominal factor of interest. Meanwhile, Wilcoxon rank sum test was also performed to calculate p values when comparing mean T2 values for compartmental femorotibial cartilage and subregional menisci between the two subject subgroups for reference. All the reported p values were deemed statistically significant when p < 0.05.
Results
As displayed in Table 1, 32 subjects were involved in the study and subdivided into two subgroups: 15 healthy controls (3 females, 12 males; mean age = 45.3 ± 10.9 years, age range 25–72 years), 17 subjects with HU (2 females, 15 males; mean age = 44.4 ± 12.7 years, age range 26–77 years), respectively. There is no statistically significant difference in gender (p = 0.55), BMI (p = 0.57), and age (p = 0.79), respectively, between healthy controls and cohort with HU.
The T2 values (Mean ± SD) (ms) of cartilage and menisci in healthy controls and cohort with HU were listed in Table 2. The CV% of compartmental femorotibial cartilage T2 measurement from LF, LT, MF, and MT were 5.79, 7.16, 5.64, and 10.1 for healthy controls and 7.56, 7.85, 9.02, and 7.53 for cohort with HU, respectively. The CV% of subregional meniscus T2 measurement from La, Lc, Lp, Ma, Mc, and Mp were 6.95, 10.8, 9.07, 7.33, 8.57, and 8.36 for healthy controls and 6.96, 5.37, 6.92, 7.05, 9.38, and 6.25 for cohort with HU, respectively. The twice measured CV% for reporting the intra-observer variation of the LF and LT cartilage, and La and Lp subregional menisci T2 measurement from 11 healthy controls were as follows: 6.16 versus (vs.) 6.83, 7.84 vs. 8.67, and 6.71 vs. 7.74, 9.91 vs. 9.16, respectively, and the average CV% of the intra-observer variation of the LF and LT cartilage, and La and Lp menisci T2 measurement from 11 healthy controls were 6.5, 8.26, and 7.23, 9.54, respectively. Representative T2 maps of cartilage on the lateral (a, c) and medial (b, d) side, obtained from a 38-year old healthy male volunteer (a, b) and a 26-year old male patient with hyperuricemia (c, d), respectively, were shown in Fig. 2. Similarly, representative T2 maps of menisci on the lateral (a, c, e, g) and medial (b, d, f, h) side from healthy control (a, b, c, d) and the patient with hyperuricemia (e, f, g, h), respectively, (c, g) show the meniscal body segment on the lateral side, and (d, h) on the medial side, respectively, were shown in Fig. 3. Figure 4 shows the box and whisker plots comparing the cartilage median T2 values among the four compartments as LF, LT, MF, and MT in healthy controls and cohort with HU, respectively. Likewise, Fig. 5 shows the box and whisker plots comparing the menisci median T2 values among the six subregions as La, Lc, Lp, Ma, Mc, and Mp in healthy controls and cohorts with HU, respectively. Particularly, the p values from ANCOVA to compare cohorts of healthy controls and patients with hyperuricemia in terms of T2 values of cartilage subcompartments and menisci compartmental subregions adjusted for age, gender, and BMI when significant at the 5% significance level adjusted for multiple comparisons were shown simultaneously in Fig. 4 and Fig. 5, respectively.
Exemplary T2 maps of menisci from the same two subjects as in Fig. 2. T2 maps of menisci on the lateral (a, c, e, g) and medial (b, d, f, h) side from healthy control (a, b, c, d) and the patient with hyperuricemia (e, f, g, h), respectively. c, g Show the meniscal body segment on the lateral side, and (d, h) on the medial side, respectively. The color bar scale on the right shows the distribution of T2 values
Box and whisker plots comparing the cartilage median T2 values among the four compartments [lateral femoral (LF), lateral tibial (LT), medial femoral (MF), and medial tibial (MT)] in healthy controls and patients with hyperuricemia, respectively. The horizontal-dashed lines on the boxes show the corresponding mean T2 values. The box and whisker plots show the five statistics (minimum, first quartiles, median, third quartiles, and maximum), and the p values from ANCOVA to compare cohorts of healthy controls and patients with hyperuricemia in terms of T2 values of cartilage subcompartments adjusted for age, gender, and BMI when significant at the 5% significance level adjusted for multiple comparisons were shown simultaneously
Box and whisker plots comparing the menisci median T2 values among the six subregions (lateral anterior horn (La), lateral body segment (Lc), lateral posterior horn (Lp), medial anterior horn (Ma), medial body segment (Mc), and medial posterior horn (Mp)) in healthy controls and patients with hyperuricemia, respectively. The horizontal-dashed lines on the boxes show the corresponding mean T2 values. The box and whisker plots show the five statistics (minimum, first quartiles, median, third quartiles, and maximum), and the p values from ANCOVA to compare cohorts of healthy controls and patients with hyperuricemia in terms of T2 values of menisci compartmental subregions adjusted for age, gender, and BMI when significant at the 5% significance level adjusted for multiple comparisons were shown as well
The comparisons among compartmental femorotibial cartilage in terms of T2 values between healthy controls and cohort with HU without controlling for age, gender, and BMI effects were shown for reference in Tables 2, 3, and Fig. 4.
When the comparisons were made among compartmental femorotibial cartilage in terms of T2 values by controlling for age, gender, and BMI effects, the results (Table 2 and Fig. 4) show the following significant findings: the LT cartilage had significantly lower T2 values (48.6 ± 3.5 ms) in healthy subgroup compared to all compartmental femorotibial cartilage in cohort with HU. MT cartilage had significantly higher T2 values (56.5 ± 4.3 ms) in cohort with HU subgroup compared to LF (52.5 ± 3.0 ms) and MF (51.6 ± 2.9 ms) in healthy subgroup.
The comparisons among subregional menisci in terms of T2 values between healthy controls and cohort with HU without regressing out confounding covariates, such as age, gender, and BMI were shown for reference in Tables 2, 4, and Fig. 5.
Furthermore, comparisons among subregional menisci in terms of T2 values between healthy controls and cohort with HU were also conducted by regressing out confounding covariates, such as age, gender, and BMI. Such comparisons show significant findings (Table 2 and Fig. 5): Both Lp and Mp menisci had significantly lower T2 values (40.1 ± 3.6 ms, 39.6 ± 3.3 ms) in healthy subgroup compared to both La and Lc menisci (43.1 ± 3.0 ms, 43.4 ± 2.3) in cohort with HU. Ma meniscus had significantly lower T2 values (39.4 ± 2.9 ms) in healthy subgroup compared to all subregional menisci except both Ma and Mc menisci in cohort with HU. Lc had significantly higher T2 values (43.4 ± 2.3 ms) in subgroup of cohort with HU compared to both La and Mc menisci (41.1 ± 2.9 ms, 40.7 ± 3.5 ms) in healthy subgroup.
Discussion
Very few studies in the literature dealt with using MR imaging technique to explore the possible correlation between asymptomatic hyperuricemia (HU) and associated cartilage or meniscus damage [12, 13, 19,20,21]. Most prior work had predominantly applied radiographic rather than MR imaging technique to investigate the relationship between HU or gout (i.e., symptomatic HU) and OA mainly according to the knee joint pain in association with joint space narrowing (JSN) or osteophyte [12, 13, 19,20,21]. In this prospective study, T2 maps for the first time were applied to perform quantitative analyses and to assess the possible association in T2 values of femorotibial cartilage and subregional menisci on both lateral and medial sides between healthy controls and subjects with asymptomatic HU (namely, HU in the absence of established gout), and the current work is definitely an initial report of potential differences in T2 values of femorotibial cartilage and subregional menisci on both compartments between the two subgroups.
Much published work has suggested that T2 mapping of cartilage and menisci may be appropriate for being a non-invasive biomarker to diagnose early stages of knee OA and to monitor treatment response of OA, and elevated T2 values may be sensitive to increased water mobility and water content resulted from disrupted collagen network and the ECM in cartilage or menisci [8, 11, 15, 33,34,35]. Of note, our current initial findings underscore that T2 mapping may be a potentially promising sensitive discriminator of understanding and examining the early-stage compositional and even structural variation of human femorotibial cartilage and menisci in patients with hyperuricemia.
The femorotibial cartilage and the adjoining menisci in human diarthrodial joints act as important functions for body weight bearing, joint shock absorption, joint stability, and joint lubrication [15, 36,37,38]. The injuries or degeneration of both femorotibial cartilage and menisci may be possible risk factors for the initiation of knee OA [5, 7, 29, 39, 40]. Quantitative MR T2 mapping offers important information concerning the variations of the integrity of PG-collagen matrix network and the mobility of unbound water in cartilage or meniscus as observed in prior ex vivo or in vivo reports [8, 15, 25, 29, 40,41,42]. T2 values of cartilage and menisci in our healthy subjects were slightly higher than previously reported in the literature [15, 22, 25, 29, 32, 42, 43], with a range roughly from 48.6 to 53.7 ms in cartilage and from 39.4 to 41.6 ms, respectively (Table 2). However, this elevation of T2 values in both cartilage and menisci may actually be due to many different influencing factors such as the fitting computing methods of T2 relaxation times, different vendors of MR scanners and pulse sequences for T2-weighted MR images acquisition [33, 44,45,46,47,48,49]. The general consensus acknowledged that there may be roughly 25 ~ 38% difference in T2 values of cartilage and menisci computed from different vendors of MR scanners and pulse sequences in the literature [47,48,49]. One distinct factor of contributing the inaccuracy of T2 measurements of both cartilage and menisci results from the stimulated echoes by using a multi-echo sequence for in vivo cartilage imaging [50, 51]. The computed T2 values of both cartilage and menisci in our current study are approximately 26% higher than those of previous work [8, 22, 25, 29, 32, 42, 43]. Such relatively high meniscus T2 values obtained may be caused by the acquisition protocol and data fitting method-related issues. Ideally, gradient echo-based T2 sequence is preferred for meniscus quantification because it can achieve much shorter TE. In contrast, turbo spin-echo (TSE) has relatively long TE (> 10 ms), making it not ideal to apply the TSE-based T2 sequence to quantitate meniscus; it was shown in previous studies that the signal was low from meniscus in TSE [8, 15, 25, 32, 41,42,43,44, 46, 48, 50]. TSE is more suitable for cartilage just as shown in Table 2 and Fig. 3. Taken together, the slight increase in T2 values of cartilage and menisci in particular is reasonable and acceptable in practice. Particularly, the average measured CV% for documenting the intra-observer variation of the LF and LT cartilage, and La and Lp subregional menisci T2 measurement from 11 healthy controls was between 6.5 and 9.54, which indicates a good intra-observer variation of the compartmental cartilage and subregional menisci T2 measurement [48]. In general, the less the CV%, the better the intra-observer variation for the compartmental cartilage and subregional menisci T2 measurement; a CV% value of less than 10 is considered a good one [11, 52].
The etiopathogenesis of knee OA is complicated and multi-factorial, which is initiated from many risk factors [26]. Hyperuricemia (HU) or gout (symptomatic HU) is merely possible cause that induces OA, which even remains indeterministic and controversial [12, 13, 19]. The mechanisms or correlations why T2 values in certain compartmental femorotibial cartilage and subregional menisci in patients with hyperuricemia are evidently and abnormally heightened compared with those in healthy subjects are unclear. Previous studies on the relationship between knee OA and hyperuricemia (HU) or gout mostly applied radiographic method characterized by the showing up of joint space narrowing (JSN) and osteophyte. This kind of radiographic detection approach can only apply to advanced degradation or morphologically apparent deterioration of femorotibial cartilage or menisci in human knee [12, 13, 19]. In other words, it has the shortcoming of inability to detect the early-stage compositional degeneration of cartilage and menisci without the appearance of distinct radiographic changes. Radiographs examine the association of knee joint pain with joint space narrowing and osteophyte formation [12, 13, 19]. As a more appropriate alternative, quantitative MR T2 mapping may be a preferred option to investigate the possible association of the degeneration of femorotibial cartilage and menisci in human knee with HU. In fact, our current work did observe that lateral tibial cartilage (LT) in healthy subgroup had significantly lower (p < 0.05) T2 values than all subcompartments of femorotibial cartilage in HU subgroup. Medial tibial cartilage (MT) in HU subgroup had significantly higher (p < 0.05) T2 values than all subcompartments of femorotibial cartilage except medial tibial cartilage in healthy subgroup. Lateral body segment of meniscus (Lc) in HU subgroup had significantly higher (p < 0.05) T2 values than all subregional menisci except lateral body segment of meniscus in healthy subgroup. Medial anterior horn of meniscus (Ma) in healthy subgroup had significantly lower (p < 0.05) T2 values than all subregional menisci except both medial anterior horn and medial body segment of meniscus in HU subgroup (Tables 2, 3, 4, Figs. 4, 5). As is well-known in the research community of quantitative MR T2 mapping technique [5, 6, 8, 15, 22, 23, 25, 26, 29, 32, 41,42,43], Just as abovementioned, T2 mapping is sensitive to the changes of water content in cartilage or menisci, and elevated T2 values may be suggestive of the increased mobility of unbound or bulk water in the ECM or PG-collagen matrix network of cartilage or menisci, which may further result from the early disorganization or disruption of the collagen fibril integrity and the ECM network [33, 43]. As to the findings in the current work, MT cartilage T2 value shows statistically significant abnormal elevation compared with all compartmental femorotibial cartilage other than MT cartilage in healthy subgroup, and T2 values of all compartmental femorotibial cartilage in HU subgroup are significantly higher than that of LT cartilage in healthy subgroup. Of special note, this abnormal elevation of T2 values in HU cohort is entirely in tibial plateau instead of femoral condyle, which may imply that the disruption of the collagen fibril integrity and the ECM network perhaps happens more possibly in tibial plateau rather than in femoral condyle of joint cartilage in HU cohort compared with healthy controls (Tables 2, 3, Fig. 4). Similarly, menisci on the lateral side generally have significantly higher T2 values compared with those on the medial side. In particular, T2 values of Lc meniscus in HU subgroup are significantly higher than those of all subregional menisci except Lc meniscus in healthy controls; also, T2 values of La meniscus in HU subgroup are significantly higher than those of Lp, Ma, and Mp menisci in healthy controls, and T2 values of Lp meniscus in HU subgroup are significantly higher than those of Ma meniscus in healthy subgroup. Likewise, the statistical increase of T2 values in HU cohort is predominantly on the lateral rather than medial side, and only T2 values of Mp meniscus in HU cohorts are significantly higher than those of Ma in healthy controls, which suggest that subregional menisci on the lateral side may have early ECM disruption more easily than on the medial side in HU cohort when compared with healthy controls (Tables 2, 4, Fig. 5). All these findings in the current work and possible explanations for them remain preliminary and tentative, and a deep understanding why T2 values in certain compartmental femorotibial cartilage and subregional menisci in patients with HU are abnormally elevated compared with those of healthy subjects should benefit diagnosis and treatment in clinical settings. Therefore, more work and further investigation to clarify the aforementioned hypotheses remain advisable and warranted.
Surely, the present study has limitations concerning measurement errors coming from the manually-drawn ROIs among the different compartmental femorotibial cartilage and subregions in the anterior horn, central (body segment), and posterior horn meniscus on lateral and medial sides. Given these measurement limitations in T2 values, further investigation is warranted. Another shortcoming in our current study lies that the confounding factor of the magic angle effects on the computing of cartilage T2 values was not considered, because there remain controversial and indetermined, or even mutually contradictive conclusions in previous publications [32, 53, 54]. Other drawbacks include a relatively small sample size of both healthy human subjects and cohort with HU, especially very few female subjects involved in our current study which may have some influence on the ultimate research conclusions [19]. Future enrollment of more female subjects for both healthy controls and cohort with HU is warranted. In addition, there exists a disadvantage that the examination and comparison of T2 values of patella cartilage between healthy human subjects and cohort with HU are not conducted. Our current initial findings are merely preliminary, the progressive examination and investigation to enroll the subjects with established gout are under consideration, and in planning, the ultimate goal of our investigation is to determine if T2 mapping is able to effectively discriminate among healthy human subjects, cohort with HU (asymptomatic hyperuricemia), and the subjects with gout (symptomatic HU). Further and future work remains necessary and advisable to fix all these deficiencies. A larger sample size is indispensable as well.
Conclusion
This preliminary work has suggested that T2 values in certain compartmental femorotibial cartilage and subregional menisci in patients with hyperuricemia are evidently and abnormally elevated compared with those in healthy subjects, to which special attention should be paid when diagnosing and treating the patients with hyperuricemia in clinical settings. These initial findings imply that T2 mapping may be a potentially promising biomarker and possible sensitive discriminator of understanding and examining the compositional and structural variation of human femorotibial cartilage and menisci in patients with hyperuricemia as early as possible.
References
Roberts J, Burch TA. Osteoarthritis prevalence in adults by age, sex, race, and geographic area. Vital Health Stat. 1966;11(15):1–27.
Vos T, Allen C. Arora M and GBD 2015 Disease and injury incidence and prevalence Collaborators (630), Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study. Lancet. 2015;388(10053):1545–602.
Wang L, Chang G, Bencardino J, Babb JS, Rokito A, Jazrawi L, et al. T1rho MRI at 3T of menisci in patients with acute anterior cruciate ligament (ACL) injury. J Magn Reson Imaging. 2015;41(2):544–9.
Amano K, Pedoia V, Su F, Souza RB, Li X, Ma CB. Persistent biomechanical alterations after ACL reconstruction are associated with early cartilage matrix changes detected by quantitative MR. Orthop J Sports Med. 2016;4(4):2325967116644421.
Wang L, Vieira RL, Rybak LD, Babb JS, Chang G, Krasnokutsky S, et al. Relationship between knee alignment and T1ρ values of articular cartilage and menisci in patients with knee osteoarthritis. Eur J Radiol. 2013;82(11):1946–52.
Gong J, Pedoia V, Facchetti L, Link TM, Ma CB, Li X. Bone marrow edema-like lesions (BMELs) are associated with higher T1ρ and T2 values of cartilage in anterior cruciate ligament (ACL)-reconstructed knees: a longitudinal study. Quant Imaging Med Surg. 2016;6(6):661–70.
Wang L, Chang G, Xu J, Vieira RL, Krasnokutsky S, Abramson S, et al. T1rho MRI of menisci and cartilage in patients with osteoarthritis at 3T. Eur J Radiol. 2012;81(9):2329–36.
Rauscher I, Stahl R, Cheng J, Li X, Huber MB, Luke A, et al. Meniscal measurements of T1rho and T2 at MR imaging in healthy subjects and patients with osteoarthritis. Radiology. 2008;249(2):591–600.
Dijkgraaf LC, de Bont LG, Boering G, Liem RS. The structure, biochemistry, and metabolism of osteoarthritic cartilage: a review of the literature. J Oral Maxillofac Surg. 1995;53(10):1182–92.
Abramson SB, Attur M. Developments in the scientific understanding of osteoarthritis. Arthritis Res Ther. 2009;11(3):227–35.
Baum T, Joseph GB, Karampinos DC, Jungmann PM, Link TM, Bauer JS. Cartilage and meniscal T2 relaxation time as non-invasive biomarker for knee osteoarthritis and cartilage repair procedures. Osteoarthr Cartilage. 2013;21(10):1474–84.
Roddy E, Zhang W, Doherty M. Are joints affected by gout also affected by osteoarthritis? Ann Rheum Dis. 2007;66(10):1374–7.
Bevis M, Marshall M, Rathod T, Roddy E. The association between gout and radiographic hand, knee and foot osteoarthritis: a cross-sectional study. BMC Musculoskelet Disord. 2016;17:169.
Mosher TJ, Zhang Z, Reddy R, Boudhar S, Milestone BN, Morrison WB, et al. Knee articular cartilage damage in osteoarthritis: analysis of MR image biomarker reproducibility in ACRIN-PA 4001 multicenter trial. Radiology. 2011;258(3):832–42.
Takao S, Nguyen TB, Yu HJ. T1rho and T2 relaxation times of the normal adult knee meniscus at 3T: analysis of zonal differences. BMC Musculoskelet Disord. 2017;18(1):202.
Herwing J, Egner E, Buddecke E. Chemical changes of human knee joint menisci in various stages of degeneration. Ann Rheum Dis. 1984;43:635–40.
Felson DT, McLaughlin S, Goggins J, LaValley MP, Gale ME, Totterman S, et al. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med. 2003;139(5 Pt 1):330–6.
Brouwer GM, van Tol AW, Bergink AP, Belo JN, Bernsen RM, Reijman M, et al. Association between valgus and varus alignment and the development and progression of radiographic osteoarthritis of the knee. Arthritis Rheum. 2007;56(4):1204–11.
Ding X, Zeng C, Wei J, Li H, Yang T, Zhang Y, et al. The associations of serum uric acid level and hyperuricemia with knee osteoarthritis. Rheumatol Int. 2016;36(4):567–73.
Kuo CF, Chou IJ, See LC, Chen JS, Yu KH, Luo SF, et al. Urate-lowering treatment and risk of total joint replacement in patients with gout. Rheumatology (Oxford). 2018;57(12):2129–39.
Neogi T, Krasnokutsky S, Pillinger MH. Urate and osteoarthritis: evidence for a reciprocal relationship. Joint Bone Spine. 2019;86(5):576–82.
Li X, Benjamin MC, Link TM, Castillo DD, Blumenkrantz G, Lozano J, et al. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthr Cartilage. 2007;15(7):789–97.
Liebl H, Joseph G, Nevitt MC, Singh N, Heilmeier U, Subburaj K, et al. Early T2 changes predict onset of radiographic knee osteoarthritis: data from the osteoarthritis initiative. Ann Rheum Dis. 2015;74(7):1353–9.
Muraki S, Akune T, En-Yo Y, Yoshida M, Suzuki T, Yoshida H, et al. Joint space narrowing, body mass index, and knee pain: the ROAD study (OAC1839R1). Osteoarthr Cartilage. 2015;23(6):874–81.
Tsai PH, Chou MC, Lee HS, Lee CH, Chung HW, Chang YC, et al. MR T2 values of the knee menisci in the healthy young population: zonal and sex differences. Osteoarthr Cartilage. 2009;17(8):988–94.
Guermazi A, Alizai H, Crema MD, Trattnig S, Regatte RR, Roemer FW. Compositional MRI techniques for evaluation of cartilage degeneration in osteoarthritis. Osteoarthr Cartilage. 2015;23(10):1639–53.
Smith GN, Mickler EA, Albrecht ME, Myers SL, Brandt KD. Severity of medial meniscus damage in the canine knee after anterior cruciate ligament transection. Osteoarthr Cartilage. 2002;10(4):321–6.
Hirose J, Nishioka H, Nakamura E, Oniki Y, Yamashita Y, Mizuta H. T1rho and T2 mapping of the proximal tibiofibular joint in relation to aging and cartilage degeneration. Eur J Radiol. 2012;81:2776–82.
Friedrich KM, Shepard T, de Oliveira VS, Wang L, Babb JS, Schweitzer M, et al. T2 Measurements of cartilage in osteoarthritis patients with meniscal tears. Am J Roentgenol. 2009;193(5):W411–5.
Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthr Cartilage. 2004;12(3):177–90.
Eckstein F, Ateshian G, Burgkart R, Burstein D, Cicuttini F, Dardzinski B, et al. Proposal for a nomenclature for Magnetic Resonance Imaging based measures of articular cartilage in osteoarthritis. Osteoarthr Cartilage. 2006;14:974–83.
Zhu J, Hu N, Liang X, Li X, Guan J, Wang Y, et al. T2 mapping of cartilage and menisci at 3T in healthy subjects with knee malalignment: initial experience. Skeletal Radiol. 2019;48(5):753–63.
Vitorino C, Ribeiro M, Carolino E, Costa JP, Ferreira A. (2019) Evaluation of knee joint cartilage through T2 mapping technique by magnetic resonance. In Proceedings of the IEEE; April.
Wang A, Pedoia V, Su F, Abramson E, Kretzschmar M, Nardo L, et al. MR T1ρ and T2 of meniscus after acute anterior cruciate ligament injuries. Osteoarthr Cartilage. 2016;24(4):631–9.
Eijgenraam SM, Bovendeert FAT, Verschueren J, van Tiel J, Bastiaansen-Jenniskens YM, Wesdorp MA, et al. T2 mapping of the meniscus is a biomarker for early osteoarthritis. Eur Radiol. 2019;29(10):5664–72.
Seedhom BB, Dowson D, Wright V. Proceedings: functions of the menisci. A preliminary study Ann Rheum Dis. 1974;33(1):111.
Shoemaker SC, Markolf KL. The role of meniscus in the anterior-posterior stability of the loaded anterior cruciate-deficient knee Effects of partial versus total excision. J Bone Joint Surg Am. 1986;68(1):71–9.
Macconaill MA. The function of intra-articular fibrocartilages, with special reference to the knee and inferior radio-ulnar joints. J Anat. 1932;66:210–27.
Nebelung S, Tingart M, Pufe T, Kuhl C, Jahr H, Truhn D. Ex vivo quantitative multiparametric MRI mapping of human meniscus degeneration. Skeletal Radiol. 2016;45(12):1649–60.
Lohmander LS, Englund PM, LDahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35:1756–69.
Son M, Goodman SB, Chen W, Hargreaves BA, Gold GE, Levenston ME. Regional variation in T1ρ and T2 times in osteoarthritic human menisci: correlation with mechanical properties and matrix composition. Osteoarthr Cartilage. 2013;21(6):796–805.
Friedrich KM, Shepard T, Chang G, Wang L, Babb JS, Schweitzer M, et al. Does joint alignment affect the T2 values of cartilage in patients with knee osteoarthritis? Eur Radiol. 2010;20:1532–8.
Wang L, Regatte RR (2014) Quantitative mapping of human cartilage at 3.0T: parallel changes in T2, T1ρ, and dGEMRIC. Acad Radiol 21 (4): 463–71.
Surowiec RK, Lucas EP, Ho CP. Quantitative MRI in the evaluation of articular cartilage health: reproducibility and variability with a focus on T2 mapping. Knee Surg Sports Traumatol Arthrosc. 2014;22(6):1385–95.
Balamoody S, Williams TG, Wolstenholme C, Waterton JC, Bowes M, Hodgson R, et al. Magnetic resonance transverse relaxation time T2 of knee cartilage in osteoarthritis at 3-T: a cross-sectional multicentre, multivendor reproducibility study. Skeletal Radiol. 2013;42(4):511–20.
Pai A, Li X, Majumdar S. A comparative study at 3T of sequence dependence of T2 quantitation in the knee. Magn Reson Imaging. 2008;26(9):1215–20.
Matzat SJ, McWalter EJ, Kogan F, Chen W, Gold GE. T2 Relaxation time quantitation differs between pulse sequences in articular cartilage. J Magn Reson Imaging. 2015;42(1):105–13.
Kim J, Mamoto K, Lartey R, Xu K, Nakamura K, Shin W, et al. Multi-vendor multi-site T1ρ and T2 quantification of knee cartilage. Osteoarthr Cartilage. 2020;28(12):1539–50.
MacKay JW, Roemer FW, Kogan F. Standardized multi-vendor compositional MRI of knee cartilage: a key step towards clinical translation? Osteoarthr Cartilage. 2020;28(12):1497–500.
Takashima H, Takebayashi T, Yoshimoto M, Terashima Y, Tsuda H, Ida K, Yamashita T. Correlation between T2 relaxation time and intervertebral disk degeneration. Skeletal Radiol. 2012;41(2):163–7.
Maier CF, Tan SG, Hariharan H, Potter HG. T2 quantitation of articular cartilage at 1.5 T. J Magn Reson Imaging. 2003;17(3):358–64.
Wang L, Schweitzer ME, Padua A, Regatte RR. Rapid 3D–T1 mapping of cartilage with variable flip angle and parallel imaging at 3.0T. J Magn Reson Imaging. 2008;27(1):154–61.
Mosher TJ, Smith H, Dardzinski BJ, Schmithorst VJ, Smith MB. MR imaging and T2 mapping of femoral cartilage: in vivo determination of the magic angle effect. Am J Roentgenol. 2001;177(3):665–9.
Wang L, Regatte RR. Investigation of regional influence of magic-angle effect on T2 in human articular cartilage with osteoarthritis at 3T. Acad Radiol. 2015;22(1):87–92.
Acknowledgements
Dr. Xiaoyun Liang from Mary Mackillop Institute for Health Research, Australian Catholic University, Melbourne, Australia, is in particular thanked for discussion and help in statistics analyses and processing. Zhen Jiang, M.D., Junkang Shen, M.D., and Caiyuan Zhang, MSc from Department of Radiology, The Second Affiliated Hospital of Soochow University, Suzhou, China, are acknowledged for full support to this research work. The authors are very much indebted to all the recruited volunteers participating in this study.
Funding
This study is funded by the Scientific Research Foundation for the Returned Overseas Chinese Scholars from the Ministry of Education (K512801315), China, the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), China, the Suzhou Science and Technology Development Project (SZS201721), China, and the Suzhou Municipal Science and Technology Bureau Project (SYS2018058), China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• T2 values in certain compartmental femorotibial cartilage and subregional menisci in patients with asymptomatic hyperuricemia are evidently and abnormally elevated compared with those in healthy subjects
• T2 mapping may be promising and potential sensitive discriminator of understanding and examining the early compositional and structural change of human femorotibial cartilage and menisci in patients with asymptomatic hyperuricemia
• Patients with asymptomatic hyperuricemia may have early elevated water content in both femorotibial cartilage and menisci, which should be in particular paid attention to when diagnosing and treating the patients with hyperuricemia in the absence of established gout in clinical settings.
Rights and permissions
About this article
Cite this article
Hu, N., Zhu, J., Liang, X. et al. T2 MRI at 3T of cartilage and menisci in patients with hyperuricemia: initial findings. Skeletal Radiol 51, 607–618 (2022). https://doi.org/10.1007/s00256-021-03861-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-021-03861-1