Abstract
Objective
To review the efficacy of percutaneous thermal ablation (TA) of bone metastases (radiofrequency ablation [RFA], microwave ablation [MWA], cryoablation [CA], and MR-guided focused ultrasound [MRgFUS]) in reducing pain in patients with advanced stage cancer.
Materials and Methods
We searched MEDLINE/PubMed, MEDLINE In-Process, BIDS ISI, Embase, CINAHL, and the Cochrane database using the keywords “ablation,” “painful,” “bone,” and “metastases” combined in multiple algorithms. Inclusion criteria were: original clinical studies published between 2001 and 2018; performance of RFA, MWA, CA or MRgFUS; and quantitative pain assessment before/after TA of bone metastasis.
Results
Eleven papers (3 on RFA, 1 on MWA, 2 on CA, and 5 on MRgFUS) involving 364 patients were reviewed. A technical success rate of 96–100% was reported, with follow-up for up to 6 months. At baseline, pain scores ranged from 5.4 to 8, at 1–4 weeks from 0.5 to 5, and at 12 weeks from 0.3 to 4.5. Mean pain reduction compared with baseline ranged from 26 to 91% at 4 weeks and from 16% to 95% at 12 weeks. MWA treatments caused no complications, whereas MRgFUS showed the highest complication rate. The number of minor complications observed ranged from 0 to 59 (complication ratio 0–1.17), whereas the number of significant adverse effects ranged from 0 to 4 (complication ratio 0–0.04).
Conclusion
All techniques achieved pain relief after 1 and 3 months, in up to 91% and 95% of patients respectively. MWA showed a negligible complication rate, whereas MRgFUS is associated with a noteworthy rate of adverse events. Future studies should adopt a standardized pain reporting scale to allow for meta-analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metastatic disease involving the bone is common and frequently originates from breast, prostate, and lung cancers. Bone lesions may lead to severe complications such as fractures, spinal compression, malignant hypercalcemia, and chronic pain resistant to medical therapy [1]. Their management is of paramount importance to sustaining the quality of life in these fragile patients [2].
Radiation therapy (RT) is usually used to treat pain and performs well in patients with radiosensitive diseases [3]. Nonresponsive tumors may instead undergo percutaneous ablation using different techniques, all of which aim to achieve thermal necrosis [4, 5]. These include radiofrequency ablation (RFA), microwave ablation (MWA), cryoablation (CA), laser ablation (LA), and magnetic resonance-guided focused ultrasound (MRgFUS) ablation. All are minimally invasive interventions that have been proven to be safe and effective, but reliable data regarding long-term efficacy and complications are scarce.
The aim of this systematic review is thus to provide an overview and comparison of the currently available data on the efficacy of thermal ablation techniques.
Materials and methods
Search strategy and selection criteria
This study followed the guidelines included in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement [6] and Cochrane’s guidelines for systematic reviews of interventions [7]. We searched the MEDLINE/PubMed, MEDLINE In-Process (US National Library of Medicine), BIDS ISI, Embase (Elsevier), CINAHL, and the Cochrane database for original clinical studies regarding thermal ablation (TA) in the setting of solitary or multiple bone metastases. Keywords included “radiofrequency,” “microwave,” “cryoablation,” “laser,” “thermal,” “ablation,” “painful,” “bone,” “metastases,” “pain management,” and their expansions. Both medical subject heading terms (MeSH term for MEDLINE; Emtree for Embase), and free text strategies were used to perform the research. We also included on-topic papers found in the citation list of retrieved articles. Gray literature was also screened using the Google search engine.
We included only original articles written in English between January 2001 and March 2018 and excluded clinical studies on less than five patients to avoid a small study effect. Papers reporting molecular, focused, in vitro, or animal studies, or patients who also underwent other therapies (e.g., RT, cementoplasty) were excluded. We set pain evaluation after TA of bone metastases as our primary endpoint. All selected papers reported follow-up at least 1 and 3 months after intervention.
Analysis of the studies
For every article included, we extracted data regarding technical success, technique efficacy, type of anesthesia, number of patients and lesions treated, the maximum follow-up available, the scale used to assess pain, pain data at different time points, and the number and occurrence of minor and major complications. We evaluated technical success as the ability to complete the treatment as initially planned [8].
Pain improvement was evaluated at four time intervals: baseline (before treatment), 0–1 week, 1–4 weeks, and 4–12 weeks. If an author reported more than one pain evaluation falling into the same time interval (e.g., 5 and 7 weeks), only the latest was considered (e.g., 7-week assessment reported in the 4- to 12-week time interval). Complications were assessed and classified according to the unified, standardized grading system developed by the Society of Interventional Radiology (SIR) [8, 9].
Statistical analysis
We used descriptive and basic statistics in Microsoft Excel 2010. All but one paper included in the present study assessed pain using different ten-point scales. For comparison purposes, we transformed the data of one paper from a 100-point scale into a ten-point scale. We calculated the differential pain between baseline assessment and the different time intervals. The complication ratio was calculated for both minor and major complications as the ratio between the number of minor or major complications and the number of treated patients.
Methodological quality assessment
A modified version of the Newcastle–Ottawa scale (NOS), already used in previous reviews [10,11,12,13], was applied to assess the risk of bias. This tool evaluates the quality of nonrandomized studies to be included in a systematic review, using a “star system” from 0 to 6 to judge three aspects of the study groups: selection, comparability, and ascertainment of either the intervention or the outcome of interest for case–control or cohort studies respectively. Owing to the absence of a control group in the cohorts included in our review, the adapted version of NOS takes into consideration the following: the representativeness of the cohort, the ascertainment of the intervention, the outcome of interest, the assessment of outcome, the adequate duration of follow-up, and a proper follow-up of the cohort. Each paper was awarded a grade A*, B*, C, or D for each feature. Only grades A* and B* were considered worthy of a star. Articles scoring at least five stars were retained in the analysis.
Results
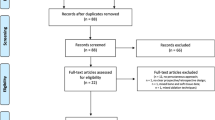
The database search retrieved 513 articles. Upon initial screening, we excluded 434 studies, and 79 were considered eligible. After analyses, we included 29 original studies. From the initial group, we ruled out 6 papers because of the short follow-up and 4 because of the lack of clinical data (at baseline, 1 month, and 3 months); 8 additional articles were eliminated as they considered combined treatments, including drug therapy, internal or external RT, vertebroplasty, and bone cement injection. One article reported the results separately from two different institutions; hence, we analyzed its data as two different entries [14]. A flow chart illustrating the inclusion and exclusion process is shown in Fig. 1. We finally included 11 studies in the analysis.
The risk of bias was found to be negligible in all studies, as they were all awarded six stars using the modified NOS scale.
Regarding the ablation technique, RFA was used in 3 studies, MRgFUS in 5, MWA in 1, and CA in 2. We found no articles about LA.
Regarding the type of anesthesia, 2 studies used general anesthesia only, 2 studies used conscious sedation, 3 studies used a combination of general anesthesia and conscious sedation, and 4 studies used both conscious sedation and locoregional anesthesia.
The number of patients per study ranged from 5 [14] to 112 [15] and the number of lesions from 5 [14] to 112 [15]. Overall, we counted a total of 423 lesions treated in 364 patients, with a follow-up of 3 months in six studies and of 6 months in five.
Two studies used the Brief Pain Inventory (short form, BPI-SF) to assess pain scores, 5 adopted the Visual Analog Scale (VAS), 3 a numerical rating scale (NRS), and 1 a 100-point pain scale, which we converted to a ten-point scale for the purposes of this review.
At baseline, the average reported pain scores ranged from 5.4 to 8, after 0–1 weeks from 1.6 to 5.8, at 1–4 weeks from 0.5 to 5, and at 4–12 weeks from 0.3 to 4.5. Pain reduction compared with baseline ranged from 26% to 91% at 4 weeks and from 16% to 95% at 12 weeks (Fig. 2).
Technical success was reported in seven studies, ranging from 96% to 100%. Technique efficacy was rated as high in all studies but one, where it was considered as moderate [16].
The complication ratio with respect to minor complications ranged between 0 and 1.17, with absolute numbers ranging from 0 to 59. The main reported minor adverse effects of RFA were second-degree skin burn (n = 3), early postprocedural pain (n = 9), and postprocedural pain after discharge (n = 5). In the study on MWA, no minor complications were reported. In studies on MRgFUS, the main minor complications were sonication pain (n = 36), position pain (n = 9), and early postprocedural pain (n = 5). In one case, unbearable sonication pain [14] caused premature interruption of the procedure, which was performed successfully at a later stage. In studies regarding CA, early postprocedural nerve pain (n = 2) and a minimally displaced fracture were listed.
The complication ratio with respect to major complications ranged between 0 and 0.04, with absolute values ranging from 0 to 4. Major complications observed in MRgFUS studies comprised two fractures, a third-degree skin burn, and a hip flexor neuropathy. In RFA studies, one acetabular fracture and one “foot drop” were observed. No major complications arose in the MWA studies. Two cases of hemothorax and one of “foot drop” were listed as significant complications of CA. Details of the included papers are summarized in Table 1. Complications are reported in Table 2. Examples of two ablation procedures are reported in Figs. 3 and 4.
A 52-year-old male patient suffering from bladder cancer. a The patient was referred for the palliative management of a painful metastasis of the left iliac bone (arrow). b, c Four cryoprobes (dots in b) were deployed percutaneously under CT guidance (an additional 20G needle was deployed in the retroperitoneal space to protect the sciatic and the obturator nerves; arrow in c) to achieve d a large hypodense ice-ball (dashed line). Case courtesy of Dr Roberto Luigi Cazzato, CHRU de Strasbourg, France
A 73-year-old male patient affected by lung cancer. a, b The patient was referred for the palliative management of a painful lytic L3 metastasis (asterisk), which disrupted the posterior wall. c Bipolar radiofrequency ablation was performed using a bilateral approach. d Temperature monitoring was applied at the level of the posterior (arrow). e The procedure was completed with cement injection (i.e., vertebroplasty). Case courtesy of Dr Roberto Luigi Cazzato, CHRU de Strasbourg, France
Discussion
Bone metastases occur in approximately 80% of patients affected by cancer and are the cause of significant pain in more than half of them [24, 25]. Traditionally, palliative treatment has been performed using RT [26,27,28,29]; however, many lesions are nonresponsive and are treated with percutaneous ablation [30].
Use of TA to treat bone metastases was first reported in 2002 by Callstrom et al. [17]. TA is now widely performed in patients unfit for surgery, and reported success rates in reducing pain are as high as 95% at 12 weeks after treatment [31]. The common endpoint to all ablation techniques is induction of the largest possible thermal necrosis of the target lesion to destroy periosteal nociceptors and reduce cancer size. There is no current evidence favoring the use of one ablation technique over another. CA may have the advantage of being less painful in the postoperative course, safer in respect of surrounding structures, and unaffected by the lytic or blastic consistency of lesions. Also, using multiple cryoprobes simultaneously allows for the treatment of large and irregular masses (Fig. 3). Additionally, a possible additive effect due to a CA-stimulated systemic antitumor immune response has been described [32]. The major drawback of CA is the high cost. RFA is usually used in lesions up to 3 cm with soft-tissue density, mostly with osteolytic or mixed osteolytic/osteoblastic features (Fig. 4) [33]. In water-poor environments, RFA is unable to reach the temperatures needed to induce coagulative necrosis [34]. MWA can often overcome these limitations [35]. MRgFUS has the advantage of being non-invasive, similar to RT. It involves the use of high-intensity focused ultrasound energy to generate lethal heat in a limited area, with no need for percutaneous applicator placement and no radiation. It is currently employed in the treatment of uterine fibroids, bone metastases, and essential tremors [36]. However, this technique shows limitations when the target is too superficial or is shielded by hollow viscera or other bones [37].
Although numerous articles have been published on the subject in the last two decades, we could identify only 11 studies meeting our inclusion criteria, mainly due to the lack of much-needed standardization of follow-up schedules [38]; in addition, some studies involved TA procedures in the setting of combined treatment strategies [39,40,41,42,43,44] (e.g., RT plus TA; TA plus cementoplasty). Therefore, understanding the real impact of TA alone was challenging. Still, we were able to perform a narrative analysis on a considerable number of patients (n = 364), which showed consistent and prolonged pain reduction at 4 and 12 weeks (by up to 91% and 95% respectively), likely attributable to TA.
Complication rates were low overall. It is interesting that the complication with the highest incidence was sonication pain when using MRgFUS, despite being less invasive. However, this is to be expected and should more appropriately be regarded as a side effect [8]. The low incidence of complications confirms the high safety of percutaneous TA procedures.
Thermal ablation should be compared with external RT, which represents the gold standard for palliative care of painful bone metastases [45, 46]. However, half of patients do not respond adequately to RT, and among the responders, the prevalence of relapses is reported to be as high as 30% [47]. Most responders do not show a benefit after RT for at least 2 weeks, with any further improvement in pain relief unlikely to occur after the first 6 weeks [48]. The efficacy of pain palliation from RT correlates with the biology of the lesions. Secondary neoplasms from sarcoma, melanoma, gastrointestinal carcinoma, nonsmall cell lung carcinoma, and renal cell carcinoma are known to be frequently radioresistant [49]. Moreover, RT can be performed only in anatomical districts that have not exceeded the limit of radiation tolerance for normal tissues [50]. Given the above-mentioned limitations, and considering the results of the present analysis, TA may play a more significant role alongside RT than has been recommended by recent guidelines [14].
However, it is currently unclear which patients benefit most from RT, TA, or both. Promising results have been published regarding combined procedures, including TA [27, 29, 51]. Di Staso and co-workers showed how RFA followed by radiotherapy can achieve better outcomes than RT alone in patients with osteolytic bone metastases [52]. A phase II clinical trial evaluating the efficacy of combining TA and spine stereotactic radiosurgery for patients with spine metastases and epidural involvement is currently recruiting and will provide the first results in 2022 [53]. Cementoplasty in combination with TA is a well-known strategy, especially if the risk of pathological fracture is considerable [31, 39,40,41,42,43,44, 54]. Further confirmation of the efficacy of these combined efforts in achieving pain control is required from clinical trials to endorse findings, supporting an increasing role for TA [55].
We support a multidisciplinary team composed of radiologists, oncologists, and radiotherapists in choosing the best treatment for each patient, in the setting of what has been named “osteoncology” [53, 55,55,56,57,58,60].
A shortcoming of this paper is that only 11 papers using four different techniques were included and therefore the generalizability of the data is unclear. We also acknowledge that the main limitation of this work is the impossibility of performing a meta-analysis of the collected data owing to the heterogeneity of study designs and assessment scales used by the authors. Further, we excluded studies in which patients underwent not only TA, but also other concurrent treatments. However, we have highlighted promising results regarding the efficacy and safety of MW, RF, MRgFUS, and CA. All techniques seem capable of achieving pain reduction in the short and medium term, with a low incidence of minor and major complications. We have found no compelling indication of the superiority of one technique over the others.
In conclusion, compared with baseline, TA for palliation of bone metastases has been reported to achieve pain reduction rates ranging from 26% to 91% at 4 weeks and from 16% to 95% at 12 weeks. Future studies should be aimed at more detailed reporting to allow for a meta-analysis. Trials comparing TA with RT and also measuring their combined effects are still needed and could help to improve guidelines for the palliation of metastatic bone pain.
Abbreviations
- RFA:
-
Radiofrequency ablation
- MWA:
-
Microwave ablation
- MRgFUS:
-
Magnetic resonance-guided focused ultrasound
- CA:
-
Cryoablation
- SD:
-
Standard deviation
- SE:
-
Standard error
- AD:
-
Absolute deviation
- IQR:
-
Interquartile range
References
Li S, Peng Y, Weinhandl ED, Blaes AH, Cetin K, Chia VM, et al. Estimated number of prevalent cases of metastatic bone disease in the US adult population. Clin Epidemiol. 2012;4:87–93.
Yong M, Jensen AÖ, Jacobsen JB, Nørgaard M, Fryzek JP, Sørensen HT. Survival in breast cancer patients with bone metastases and skeletal-related events: a population-based cohort study in Denmark (1999-2007). Breast Cancer Res Treat. 2011;129:495–503.
Zeng L, Chow E, Bedard G, Zhang L, Fairchild A, Vassiliou V, et al. Quality of life after palliative radiation therapy for patients with painful bone metastases: results of an international study validating the EORTC QLQ-BM22. Int J Radiat Oncol Biol Phys. 2012;84:e337–42.
Nielsen OS, Munro AJ, Tannock IF. Bone metastases: pathophysiology and management policy. J Clin Oncol. 1991;9:509–24.
Kurup AN, Callstrom MR. Image-guided percutaneous ablation of bone and soft tissue tumors. Semin Intervent Radiol. 2010;27:276–84.
Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
Higgins JP, Green S, The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions v 5.1.0 [Internet]. 2011 [cited 12 June 2016]. Available from: http://handbook.cochrane.org
Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria—a 10-year update. J Vasc Interv Radiol. 2014;25:1691–705.e4.
Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14:S199–202.
Lanza E, Palussiere J, Buy X, Grasso RF, Beomonte Zobel B, Poretti D, et al. Percutaneous image-guided cryoablation of breast cancer: a systematic review. J Vasc Interv Radiol. 2015;26:1652–7.e1.
Lanza E, Banfi G, Serafini G, Lacelli F, Orlandi D, Bandirali M, et al. Ultrasound-guided percutaneous irrigation in rotator cuff calcific tendinopathy: what is the evidence? A systematic review with proposals for future reporting. Eur Radiol. 2015;25:2176–83.
Cazzato RL, Palussière J, Buy X, Denaro V, Santini D, Tonini G, et al. Percutaneous long bone cementoplasty for palliation of malignant lesions of the limbs: a systematic review. Cardiovasc Intervent Radiol. 2015;38:1563–72.
Lanza E, Thouvenin Y, Viala P, Sconfienza LM, Poretti D, Cornalba G, et al. Osteoid osteoma treated by percutaneous thermal ablation: when do we fail? A systematic review and guidelines for future reporting. Cardiovasc Intervent Radiol. 2014;37:1530–9.
Catane R, Beck A, Inbar Y, Rabin T, Shabshin N, Hengst S, et al. MR-guided focused ultrasound surgery (MRgFUS) for the palliation of pain in patients with bone metastases—preliminary clinical experience. Ann Oncol. 2007;18:163–7.
Hurwitz MD, Ghanouni P, Kanaev SV, Iozeffi D, Gianfelice D, Fennessy FM, et al. Magnetic resonance-guided focused ultrasound for patients with painful bone metastases: phase III trial results. J Natl Cancer Inst. 2014;106:dju082.
Dupuy DE, Liu D, Hartfeil D, Hanna L, Blume JD, Ahrar K, et al. Percutaneous radiofrequency ablation of painful osseous metastases: a multicenter American College of Radiology Imaging Network trial. Cancer. 2010;116:989–97.
Callstrom MR, Charboneau JW, Goetz MP, Rubin J, Wong GY, Sloan JA, et al. Painful metastases involving bone: feasibility of percutaneous CT- and US-guided radio-frequency ablation. Radiology. 2002;224:87–97.
Goetz MP, Callstrom MR, Charboneau JW, Farrell MA, Maus TP, Welch TJ, et al. Percutaneous image-guided radiofrequency ablation of painful metastases involving bone: a multicenter study. J Clin Oncol. 2004;22:300–6.
Kastler A, Alnassan H, Aubry S, Kastler B. Microwave thermal ablation of spinal metastatic bone tumors. J Vasc Interv Radiol. 2014;25:1470–5.
Gianfelice D, Gupta C, Kucharczyk W, Bret P, Havill D, Clemons M. Palliative treatment of painful bone metastases with MR imaging-guided focused ultrasound. Radiology. 2008;249:355–63.
Liberman B, Gianfelice D, Inbar Y, Beck A, Rabin T, Shabshin N, et al. Pain palliation in patients with bone metastases using MR-guided focused ultrasound surgery: a multicenter study. Ann Surg Oncol. 2009;16:140–6.
Tomasian A, Wallace A, Northrup B, Hillen TJ, Jennings JW. Spine cryoablation: pain palliation and local tumor control for vertebral metastases. AJNR Am J Neuroradiol. 2016;37:189–95.
Wallace AN, McWilliams SR, Connolly SE, Symanski JS, Vaswani D, Tomasian A, et al. Percutaneous image-guided cryoablation of musculoskeletal metastases: pain palliation and local tumor control. J Vasc Interv Radiol. 2016;27:1788–96.
Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–9s.
van den Beuken-van Everdingen MHJ, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18:1437–49.
Carrafiello G, Laganà D, Pellegrino C, Mangini M, Fontana F, Piacentino F, et al. Ablation of painful metastatic bone tumors: a systematic review. Int J Surg. 2008;6(Suppl 1):S47–52.
Choi J, Raghavan M. Diagnostic imaging and image-guided therapy of skeletal metastases. Cancer Control. 2012;19:102–12.
Callstrom MR, Charboneau JW, Goetz MP, Rubin J, Atwell TD, Farrell MA, et al. Image-guided ablation of painful metastatic bone tumors: a new and effective approach to a difficult problem. Skeletal Radiol. 2006;35:1–15.
Lee JH, Stein M, Roychowdhury S. Percutaneous treatment of a sacral metastasis with combined embolization, cryoablation, alcohol ablation and sacroplasty for local tumor and pain control. Interv Neuroradiol. 2013;19:250–3.
Botsa E, Mylona S, Koutsogiannis I, Koundouraki A, Thanos L. CT image guided thermal ablation techniques for palliation of painful bone metastases. Ann Palliat Med. 2014;3:47–53.
Pusceddu C, Sotgia B, Fele RM, Melis L. Treatment of bone metastases with microwave thermal ablation. J Vasc Interv Radiol. 2013;24:229–33.
Mauri G, Orsi F, Sconfienza LM. Systemic effects of local tumor ablation: oncogenesis and antitumor induced immunity. Radiology. 2017;283:923.
Callstrom MR, Charboneau JW. Image-guided palliation of painful metastases using percutaneous ablation. Tech Vasc Interv Radiol. 2007;10:120–31.
Hinshaw JL, Lubner MG, Ziemlewicz TJ, Lee FT, Brace CL. Percutaneous tumor ablation tools: microwave, radiofrequency, or cryoablation—what should you use and why? Radiographics. 2014;34:1344–62.
Lubner MG, Brace CL, Hinshaw JL, Lee FT. Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol. 2010;21:S192–203.
Dababou S, Marrocchio C, Scipione R, Erasmus H-P, Ghanouni P, Anzidei M, et al. High-intensity focused ultrasound for pain management in patients with cancer. Radiographics. 2018;38:603–23.
Napoli A, Anzidei M, Marincola BC, Brachetti G, Noce V, Boni F, et al. MR imaging-guided focused ultrasound for treatment of bone metastasis. Radiographics. 2013;33:1555–68.
Mauri G, Pisani Mainini A, Monaco C, Pescatori LC, De Angelis C, Sconfienza LM. Urgent need to apply a common language in image-guided thermal ablations. J Ultrasound. 2018;21:77–8.
David E, Kaduri S, Yee A, Chow E, Sahgal A, Chan S, et al. Initial single center experience: radiofrequency ablation assisted vertebroplasty and osteoplasty using a bipolar device in the palliation of bone metastases. Ann Palliat Med. 2017;6:118–24.
Zhang Q, Zhang K, Xie B, Ren Y, Li G, Zhang L, et al. Analysis of curative effect of I 125 implantation combined with radiofrequency ablation in treating bone metastases. J Bone Oncol. 2018;11:23–6.
Yang P-L, He X-J, Li H-P, Zang Q-J, Wang G-Y. Image-guided minimally invasive percutaneous treatment of spinal metastasis. Exp Ther Med. 2017;13:705–9.
Ma Y, Wallace AN, Waqar SN, Morgensztern D, Madaelil TP, Tomasian A, et al. Percutaneous image-guided ablation in the treatment of osseous metastases from non-small cell lung cancer. Cardiovasc Intervent Radiol. 2018;41:726–33.
Li F, Wang W, Li L, Chang Y, Su D, Guo G, et al. An effective therapy to painful bone metastases: cryoablation combined with zoledronic acid. Pathol Oncol Res. 2014;20:885–91.
Pusceddu C, Sotgia B, Fele RM, Ballicu N, Melis L. Combined microwave ablation and cementoplasty in patients with painful bone metastases at high risk of fracture. Cardiovasc Intervent Radiol. 2016;39:74–80.
Wu JS-Y, RKS W, Lloyd NS, Johnston M, Bezjak A, Whelan T, et al. Radiotherapy fractionation for the palliation of uncomplicated painful bone metastases—an evidence-based practice guideline. BMC Cancer. 2004;4:71.
Lutz S, Balboni T, Jones J, Lo S, Petit J, Rich SE, et al. Palliative radiation therapy for bone metastases: update of an ASTRO evidence-based guideline. Pract Radiat Oncol. 2017;7:4–12.
Tong D, Gillick L, Hendrickson FR. The palliation of symptomatic osseous metastases: final results of the study by the radiation therapy oncology group. Cancer. 1982;50:893–9.
Lutz S, Chow E. A review of recently published radiotherapy treatment guidelines for bone metastases: contrasts or convergence? J Bone Oncol. 2012;1:18–23.
Laufer I, Rubin DG, Lis E, Cox BW, Stubblefield MD, Yamada Y, et al. The NOMS framework: approach to the treatment of spinal metastatic tumors. Oncologist. 2013;18:744–51.
Lutz S, Berk L, Chang E, Chow E, Hahn C, Hoskin P, et al. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;79:965–76.
Gennaro N, Mauri G, Della Vigna P. IR combined with other treatment modalities in bone tumors. In: Cazzato RL. Interventional bone tumor management. Torino: Minerva Medica Publishing; 2019. In press.
Di Staso M, Zugaro L, Gravina GL, Bonfili P, Marampon F, Di Nicola L, et al. A feasibility study of percutaneous radiofrequency ablation followed by radiotherapy in the management of painful osteolytic bone metastases. Eur Radiol. 2011;21:2004–10.
Thermal ablation and stereotactic spine radiosurgery (SSRS). Accessed 12 February 2018. Available from: https://www.clinicaltrials.gov/ct2/show/NCT02713269?cond=NCT02713269&rank=1
De Felice F, Piccioli A, Musio D, Tombolini V. The role of radiation therapy in bone metastases management. Oncotarget. 2017;8:25691–9.
Lane MD, Le HBQ, Lee S, Young C, Heran MKS, Badii M, et al. Combination radiofrequency ablation and cementoplasty for palliative treatment of painful neoplastic bone metastasis: experience with 53 treated lesions in 36 patients. Skeletal Radiol. 2011;40:25–32.
Overduin CG, Jenniskens SFM, Sedelaar JPM, Bomers JGR, Fütterer JJ. Percutaneous MR-guided focal cryoablation for recurrent prostate cancer following radiation therapy: retrospective analysis of iceball margins and outcomes. Eur Radiol. 2017;27:4828–36.
Crabtree T, Puri V, Timmerman R, Fernando H, Bradley J, Decker PA, et al. Treatment of stage I lung cancer in high-risk and inoperable patients: comparison of prospective clinical trials using stereotactic body radiotherapy (RTOG 0236), sublobar resection (ACOSOG Z4032), and radiofrequency ablation (ACOSOG Z4033). J Thorac Cardiovasc Surg. 2013;145:692–9.
Hadji P, Aapro M, Costa L, Gnant M. Antiresorptive treatment options and bone health in cancer patients—safety profiles and clinical considerations. Cancer Treat Rev. 2012;38:815–24.
Pilot study of stereotactic ablation for oligometastatic breast neoplasia in combination with the anti-PD-1 antibody MK-3475. Accessed 12 February 2018. Available from:https://www.clinicaltrials.gov/ct2/show/NCT02303366?cond=NCT02303366&rank=1
Ibrahim T, Mercatali L, Amadori D. Bone and cancer: the osteoncology. Clin Cases Miner Bone Metab. 2013;10:121–3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gennaro, N., Sconfienza, L.M., Ambrogi, F. et al. Thermal ablation to relieve pain from metastatic bone disease: a systematic review. Skeletal Radiol 48, 1161–1169 (2019). https://doi.org/10.1007/s00256-018-3140-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-018-3140-0