Abstract
Objective
Describe the imaging appearance of well-differentiated liposarcoma with myxoid stroma (WDLMS) and correlate with histopathology.
Materials and methods
A keyword search of the institution medical records was performed from 1 January 2000 to 30 June 2017. The histopathology slides of cases identified in this fashion were then reviewed by a pathologist. Additional cases were prospectively collected from extramural referrals and tumor boards. Diagnostic imaging studies of pathologically proven cases of WDLMS were then reviewed in consensus and correlated with pathology.
Results
Ten cases of pathologically proven WDLMS were identified (7 men, 3 women, ages 26–81). Tumor location included the retroperitoneum (n = 5), thigh (n = 4), and the shin (n = 1). Nine patients had macroscopic fat on imaging. The nonlipomatous components had a variable appearance, including septal, nodular, and lacelike patterns. Two cases included two distinct areas that were predominantly myxoid or lipomatous (“bi-morphic”). One tumor had no macroscopic fat on imaging. On CT, the nonlipomatous nodular components were hypodense/had hypodense areas. On MRI, the nodular components had intermediate/bright T2W signal. Interval nonlipomatous nodular growth was identified in 3 cases.
Conclusion
WDLMS may present on imaging as a mass with variable morphology and amounts of nonlipomatous components. Histopathological diagnosis of WDLMS is challenging and imaging correlation may be helpful, as this tumor may have ≥50% fatty volume, may have a myxoid nodular component or bi-morphic appearance, or may be located in the retroperitoneum, features that are unusual for myxoid liposarcoma. WDLMS with a nodular component cannot be distinguished from dedifferentiated liposarcoma based on imaging alone.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Well-differentiated liposarcoma with myxoid stroma (WDLMS) is a difficult tumor to prospectively diagnose on percutaneous core needle biopsy. Small tissue samples may not include the sparse atypical pleomorphic cells indicative of a well-differentiated liposarcoma (WDL) on all histology slides. This rare tumor was previously classified by the World Health Organization as a “mixed” subtype of liposarcoma and was most often thought to be composed of both WDL and myxoid liposarcomas. However, in 2013, the WHO revised its classification system to eliminate the “mixed” category. Based on immunohistochemistry, cytogenetics, and fluorescence in situ hybridization (FISH), it was shown that some of these “mixed” types of liposarcomas were actually WDLMS [1]. Misdiagnosis of WDLMS as a myxoid liposarcoma has major treatment implications [2]. In these cases, diagnostic imaging may play an important role in preventing misdiagnosis.
Myxoid liposarcomas are multinodular masses with a high water component that present as hypodense masses on computed tomography (CT) with a low signal on T1W sequences and increased signal on T2W sequences on magnetic resonance imaging (MRI) [3]. A fatty component typically encompasses less than 10–25% of the tumor volume [4]. By contrast, WDLs usually have a predominant mature fatty component. However, up to one third of WDLs may have variable amounts of myxoid stroma [3]. Although myxoid liposarcoma can be distinguished from WDL based upon molecular findings (specifically DDIT3-FUS or DDIT3-EWS translocations in myxoid liposarcoma, and MDM2 amplification in WDL), the distinction of myxoid liposarcoma and WDLMS on histopathology by morphology alone can be challenging as the nuclear atypia and pleomorphism needed to exclude myxoid liposarcoma may not be present in small tissue samples [1, 5].
Although the presence of myxoid stroma within WDL has been described in the pathology literature, there are only sparse descriptions of the imaging presentation of this specific tumor and, to our knowledge, a study dedicated to the imaging presentation of pathologically proven WDL with myxoid stroma has not been published. Although the fraction of macroscopic fat is relatively low in myxoid liposarcoma [4], this may not be the case in WDLMS. Thus, diagnostic imaging may be helpful in making the distinction between these two types of tumors. Given the challenge in discriminating between myxoid liposarcomas and WDLMS based on the histopathological appearance alone, and their divergent treatment implications, we propose to characterize the imaging appearance of WDLMS.
Materials and methods
This retrospective study was approved by our Institutional Review Board. A search was performed for pathology-proven cases of WDLMS from 1 January 2000 to 30 June 2017 using EMERSE [6], a program that searches the electronic medical record of the entire institution for word associations and identifies in which system they appear (radiology, pathology, clinical notes). A search was performed for the terms “myxoid,” “lipomatous,” and “differentiated.” Additional cases were prospectively identified during outside hospital consultation and at the weekly multidisciplinary sarcoma conference held at our institution. All cases were then reviewed by a pathologist with fellowship training in bone and soft-tissue pathology and 24 years as an expert consultant in this specialty. The imaging studies were reviewed separately but in consensus by two fellowship trained musculoskeletal radiologists (15 years and 13 years of experience respectively). Imaging assessment included: size and location of the tumors, morphology of the tumors including the presence of macroscopic fat, the presence and morphology of nonlipomatous components such as nodules, septations (septations ≤2 mm were considered “thin septations”), lacelike/hazy (if not completely replacing the fatty stroma), and the density (relative to muscle) and signal characteristics of the nonlipomatous components on CT and MRI respectively. Demographic factors, including age at presentation and gender, were noted. The imaging findings correlated with demographic factors, pathology, and clinical and surgical findings.
Results
Ten cases of pathologically proven WDLMS were identified (7 men, 3 women, median age 64.5 years, range 26–81 years). Six patients had both CT and MR imaging, 2 patients had CT evaluation whereas 2 patients had MRI evaluation. Five tumors were located in the retroperitoneum, 4 in the thigh, and 1 in the leg (Shin) (Table 1).
Imaging
Computed tomography was performed with intravenous contrast medium in 5 cases and without intravenous contrast medium in 4 cases (including CT-guided biopsies). MRI following intravenous contrast medium administration was available in 8 cases. MRI vendors (Phillips, Siemens, GE) and technique varied as many of the cases were outside referrals with accompanying imaging: spin echo (SE) T1 (repetition time [TR] 450–700, echo time [TE] 8–12), SE T2 (TR 1,050–6,750, TE 40–106), ultrafast spoiled gradient-echo (TR 3.7–5.6, TE 1.8–2.4), inversion recovery (TR 1,481–5,900, TE 20–31). Information regarding the strength of the magnetic field was not available for all studies.
Nine cases had prominent macroscopic fat on imaging with a variable appearance of the nonlipomatous components including nodular, septal, and hazy/lacelike patterns. In 2 cases, the tumor was composed of 2 distinct areas, 1 predominantly myxoid and the other containing a predominant lipomatous component (termed “bi-morphic” in this study). One case (Fig. 1) showed no macroscopic fat at all on imaging. In 7 of the cases with macroscopic fat, 50% or more of the tumor volume was fatty on initial imaging. Six of the 9 cases with macroscopic fat had a nodular or mass-like nonlipomatous component. This tumor presentation included two patterns: the bi-morphic pattern (n = 2) or a nodule(s) or mass surrounded by fatty tumor (n = 4). In the bi-morphic presentation (Figs. 2, 3), the area with prominent fat was intermixed with myxoid tissue, either in a nodular form or interleaved fat and myxoid stroma. The fat component occupied approximately 50% and 40% of the tumor volume in these 2 cases.
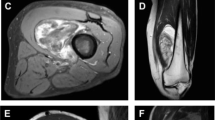
A 68-year-old woman with a retroperitoneal WDL with myxoid stroma without macroscopic fat on imaging. a Axial CT following intravenous and oral contrast medium administration depicts a retroperitoneal mass with hypodense areas measuring fluid density without macroscopic fat. b High-power micrograph depicts abundant pale-blue myxoid and collagenous fibrillary matrix (star) and anastomosing capillary (“chickenwire”) vessels (right side) mimicking the vascular pattern of a true myxoid liposarcoma. Note the multinucleated stromal cells (broad arrow) and multivacuolated lipoblasts (narrow arrow) which confirm the diagnosis of well-differentiated liposarcoma with myxoid stroma. (Hematoxylin and eosin, original magnification ×400)
A 37-year-old woman with a bi-morphic appearing well-differentiated liposarcoma with myxoid stroma (WDLMS) in the thigh. a Sagittal short tau inversion recovery (STIR) and b axial T1-weighted (T1W) sequences depict an inter-muscular posterior thigh mass with myxoid-rich stroma superiorly (asterisk) and lipomatous component inferiorly (arrows). c Low-power histopathological image shows a sharp interface between a myxoid-rich area superiorly and a lipomatous area inferiorly. Inset depicts a large, atypical lipoblast within the lipomatous area. (Hematoxylin and eosin, original magnification ×100)
A 68-year-old woman with a bi-morphic-appearing well-differentiated liposarcoma (WDL) in the hamstring compartment with a well-demarcated myxoid-rich component superiorly. Sagittal a STIR, b T1W and c, d axial CT images depict a myxoid-rich component in the superior aspect of the mass (asterisk in a–c) with an abrupt transition to a mixed component with intermixed fat and myxoid stroma inferiorly (arrows). e Scanning power micrographs depict the highly myxoid component comprising abundant pale-blue matrix with only scattered adipocytes (narrow arrows) and rare atypical stroma cells (broad arrow). (Hematoxylin and eosin, original magnification ×100) and f long, wispy fibrous bands (arrow) traversing the predominantly lipomatous component. (Hematoxylin and eosin, original magnification ×100). g Further magnification of the lipomatous (nonmyxoid) areas demonstrates atypical stromal cells. (Hematoxylin and eosin, original magnification ×400)
When present, the nodular/mass-like components (Fig. 4) were either present initially (n = 2), or appeared at imaging follow-up (n = 2, 27, and 29 months after initial imaging).
A 58-year-old man with a large retroperitoneal WDL including a very discrete small myxoid nodule. Axial T2W images with fat saturation depict a an extensive retroperitoneal WDL (arrows) with b a single small discrete myxoid nodule (asterisk). c Corresponding low-power micrograph depicts this circumscribed myxoid nodule (arrow) with aggregates of lymphocytes (narrow arrow) in a large retroperitoneal WDL. Insert depicts an atypical stromal cell with hyperlobated nucleus. (Hematoxylin and eosin, original magnification ×20)
Interval growth of nodules present on initial imaging was noted in 1 case (Fig. 5). Interval growth of the nodular/mass component was often disproportionate to the growth of the fatty component, progressively comprising more of the tumor volume up to 20x14x20 cm (Fig. 6). On imaging presentation, the smallest nodule measured 0.7 × 0.7x1 cm (the only case where the nodule measured less than 2 cm in maximal dimension; Fig. 7), whereas the mass-like myxoid component in the bi-morphic tumor measured 14.5x12x17 cm (Fig. 2). When present, most tumors had more than one nonlipomatous nodule or mass and all nodules/masses had a hypodense or intermediate to bright T2W signal component (relative to muscle) on CT/MRI accordingly (Figs. 2, 3, 4, 6, 7).
A 61-year-old woman with WDL with interval growth of a myxoid nodule. a Axial post-contrast CT shows a large retroperitoneal fat-contacting mass (arrows) with a nonlipomatous nodule (asterisk) and an additional nodule (double asterisk) seen in a which was resected at an outside hospital and diagnosed with sclerosing WDL without myxoid stroma. b Bright signal on an axial T2W image with fat saturation and c interval growth of the nodule (asterisk) over a 3-month period. d Gross photograph of the resected specimen illustrates the cut surface of a solitary, 14-cm, gelatinous, yellow-green, hemorrhagic, myxoid mass located within an otherwise typical lipomatous WDL. e The myxoid mass comprises mature adipocytes and multivacuolated lipoblasts (broad arrow) embedded within pale blue myxoid matrix containing interconnecting capillaries (narrow arrows). (Hematoxylin and eosin, original magnification ×400). f Atypical stromal cell found elsewhere in the tumor. (Hematoxylin and eosin, original magnification ×400)
An 80-year-old man with WDL with interval growth of a myxoid nodule/mass. a Axial CT following administration of intravenous and oral contrast medium depicts a WDL (arrows) with b interval growth of myxoid nodule (asterisk) over an 8-month period. The nodule, which was not present on imaging 2 years earlier, was sampled under ultrasound guidance. c High-power micrograph depicts a well-differentiated liposarcoma with edematous pale-blue myxoid fibrillary matrix (star), mature adipocytes (narrow arrows) and scattered large, atypical stromal cells (broad arrow). (Hematoxylin and eosin, original magnification ×400)
A 60-year-old man with WDL with peripheral myxoid nodules. Axial a T1W, b T2W with fat saturation, and c T1W with fat saturation following intravenous gadolinium administration depict a WDL (arrows) with peripheral enhancing myxoid nodules (asterisk). d Corresponding scanning power micrograph depicts a focal small myxoid nodule (arrows) within a typical WDL. (Hematoxylin and eosin, original magnification ×10). e Arrows highlight atypical stromal cells readily visualized in this low-power magnification, typical of well-differentiated liposarcoma. (Hematoxylin and eosin, original magnification ×40)
Thin well-defined septations were common, occurring in 7 of the 9 cases with macroscopic fat and these septations were often intermixed with other patterns (Fig. 8). Although a hazy/ill-defined nonlipomatous component was found in many of the tumors to some degree, 2 cases presented with a dominant hazy/lacelike pattern (1 with intermixed fine septations; Fig. 9). A relatively monomorphic interleaved/laminated appearance was present in 1 case (Fig. 10).
A 26-year-old man with an WDLMS with multiple thin septations. a Axial CT image and b axial T1W image depict a fatty mass (arrowheads) with multiple thin septa (arrows) without nodularity. c Scanning power micrograph depicts parallel fibrous band within a well-differentiated liposarcoma with myxoid stroma (hematoxylin and eosin, original magnification ×40). d Closer inspection reveals spiculated pale-blue myxoid matrix (narrow arrow) insinuating between adipocytes and adherent to a fibrous band (broad arrow). Atypical stromal cells are depicted in the insert. (Hematoxylin and eosin, original magnification ×100, insert ×400)
An 80-year-old man with a retroperitoneal WDL (asterisk) with an ill-defined lace-like nonfatty component. a Coronal CT following oral and intravenous contrast medium administration depict a retroperitoneal fatty mass (arrows) with ill-defined, lace-like nonfatty component without nodules or septations and without enhancement on a b coronal 3D GRE T1W post-contrast image. c Low-power micrograph depicts a tumor characterized by diffuse infiltration of pale-blue myxoid matrix surrounding adipocytes. Insert depicts two atypical stromal cells with hyperchromatic, lobulated nuclei in a myxoid matrix. (Hematoxylin and eosin, original magnification ×100)
An 81-year-old man with an adductor muscle WDLMS with an interleaved pattern of fat and nonlipomatous components. a Axial T1W, b axial T2W with fat saturation and c axial T1W with fat saturation following intravenous administration of gadolinium depict an adductor muscle mass with interleaved and intermixed layers of fat and nonlipomatous stroma that undergoes patchy enhancement. d Scanning power micrograph depicts a WDLMS containing parallel bands of fibrosis (broad arrows) and a diffuse, honeycomb-like texture of myxoid stroma insinuating within adipose tissue (narrow arrow). Insert depicts hyperchromatic spindle cells within a fibrous septum. (Hematoxylin and eosin, original magnification ×20, insert ×400)
The single case without evidence of macroscopic fat (Fig. 1) was heterogeneous in appearance on post-contrast CT with intermixed isodense and hypodense areas. In cases in which MRI was performed, bright T2W signal was present in some, but not all, of the nonlipomatous components. Post-contrast enhancement could be evaluated in 7 cases: variable enhancement characteristics included no enhancement, diffuse heterogeneous enhancement, septal, nodular, and capsular enhancement. Of note, the 2 cases with a dominant hazy/lace-like pattern did not undergo enhancement (Fig. 9).
Histopathology
In all cases but one, tissue from surgical excision was available for final histological assessment in addition to the pre-operative core biopsies. All these cases were resection specimens sampled at one section per centimeter of greatest tumor dimension. In the single case without surgical excision, image-guided core biopsies were performed on two separate occasions, allowing ample tissue sampling. Microscopically, the myxoid stroma consisted of pale-blue mucoid matrix associated with variable amounts of fibrillary collagen. The extent of myxoid change was highly variable among tumors, ranging from wispy matrix insinuating among adipocytes to large, confluent areas of myxoid stroma nearly devoid of adipocytes. Plexiform capillary vascular stroma was prominent in several cases, mimicking the vascular pattern of myxoid liposarcoma. In all cases, histological features excluding myxoid liposarcoma, such as nuclear pleomorphism, were identified, allowing the final diagnosis of WDLMS.
In case number 4, the initial core biopsy was diagnosed as suspicious for myxoid liposarcoma. In case number 3, initial core biopsy was described as “a low-grade spindle cell neoplasm, low-grade sarcoma not excluded”. The final diagnosis in both cases was changed to WDL based on the surgically resected specimens.
In case number 9, the initial interpretation of the resected specimen was consistent with WDL; however, the nodular component was described as having mixed morphological features including myxoid areas reminiscent of low-grade myxoid liposarcoma. In case number 6, the resected specimen was thought to represent a dedifferentiated liposarcoma. In our review of all initially discordant cases (by a fellowship trained pathologist), the final diagnosis was determined to be WDLMS. Sampling error was a concern following the initial diagnosis of an intra-muscular lipoma based on core biopsy in 1 case. Given interval enlargement on short-term imaging follow-up, resection of this mass was undertaken with a final diagnosis of WDLMS.
Follow-up
All but one of the patients (case 6) underwent excision of the mass. Post-operative CT or MRI follow-up of the surgical area was available for 7 patients (interval follow-up from initial excision 4–62 months). There was one incidence of local recurrence 46 months following initial excision. The recurrence was excised with a diagnosis of recurrent WDLMS confirmed on histology. Continued imaging follow-up was suggested in 2 patients given soft-tissue changes favored to represent post-surgical changes. In 2 patients, imaging follow-up raised concern for local residual disease, which was not confirmed or addressed surgically for the duration of this study. Follow-up imaging in 2 patients (4 and 25 months after excision respectively) showed no evidence of local recurrence. In the single case that was not excised, follow-up imaging showed initial interval growth of the mass and of the nonlipomatous nodule. Latest available follow-up studies up (32 months following diagnostic biopsy) showed that the mass had stabilized in size. The patient’s care was then transferred to an outside facility; however, a later clinical note stated that the patient had passed away approximately 42 months after the first diagnostic biopsy (cause of death was not specified).
Of the 9 patients who had undergone excision of the mass, 3 had no chest or abdomen/pelvis imaging to evaluate for osseous metastases. In 4 patients, there was no evidence of osseous metastases on CT of the chest (performed from 2.5 weeks before surgical excision to 7 months following surgical excision of the mass). CT of the abdomen and pelvis was available for 4 patients, without evidence of osseous metastatic disease (one CT was the initial diagnostic study; the other CTs were performed 4–8 months following excision of the mass). Two patients had both a chest and an abdomen CT performed and 4 patients underwent either a chest or an abdomen/pelvis CT. In the single patient who was treated conservatively, there was no evidence of osseous metastases on CT of the chest and CT of the abdomen and pelvis performed 30 and 42 months following the initial diagnostic biopsy respectively.
Discussion
In the past, a small group of liposarcomas that did not seem to fit into 1 of the 4 World Health Organization categories of liposarcoma (well-differentiated [WDL], dedifferentiated, myxoid/round cell, and pleomorphic liposarcoma) were categorized as a fifth “mixed” type group that was most often thought to be composed of both WDL and myxoid liposarcomas. In the 2013 WHO classification, the “mixed” classification was removed based on the consensus view that these rare tumors were likely not a separate entity [7]. Using immunohistochemistry, cytogenetics, and FISH, Sioletic et al. [1] showed that some of these “mixed” types of liposarcoma were actually WDL with myxoid stroma (WDLMS). In addition, these investigators also noted that myxoid liposarcoma-like plexiform vessels were present in 50% of the cases of WDL with myxoid stroma, thereby leading to a possible misdiagnosis [1, 2].
In this study, we characterize the variable imaging appearance of WDLMS, which includes two contrasting presentations: tumors with variable amounts of macroscopic fat (n = 9) and tumors without macroscopic fat (n = 1). This finding corresponds to previous histopathological studies that described either a lipoma-like presentation or a predominantly nonfatty tumor [1]. On MRI and CT, the bright T2W signal and relative hypodensity were consistent with the myxoid component seen on histology. The myxoid component in WDLMS had a variable configuration on imaging corresponding to the histopathological appearance of the matching specimens reminiscent of the varied distribution of the myxoid component described in previous histopathological studies [1]. The nodular/mass or bi-morphic configurations correspond to the large confluent pools of mucin described on histological studies [1]. To our knowledge, the myxoid nodular/mass and bi-morphic configuration has not been described in previous imaging studies. Disproportionate growth of the nodular/myxoid component may transform a nodular configuration to a bi-morphic configuration.
The WDLMS poses a diagnostic challenge on histopathology, as the well-differentiated adipocytic component may be inconspicuous [1]. In our case series, 1 case of WDLMS was initially mistaken for a myxoid liposarcoma based on histological assessment of a core biopsy. In another case, there were components reminiscent of low-grade myxoid liposarcoma in the resected specimen. Mistaking WDL for a myxoid liposarcoma, which has been described [1], is a misdiagnosis that may have a negative impact on treatment. WDLMS and myxoid liposarcoma have divergent behavior and require different treatments. For example, WDL does not metastasize or respond to radiation or chemotherapy. However, myxoid liposarcoma can metastasize and, unlike WDL, is highly radio- and chemo-sensitive [2]. In a study by Jones et al., although 48% of myxoid liposarcomas responded to chemotherapy, none of the WDL responded [8]. Radiologists should be aware of the potential for misdiagnosing WDLMS as myxoid liposarcoma, as imaging may help to correct the diagnosis: myxoid liposarcomas have been described as typically having a fat content of <10–25% of the tumor volume [3, 4, 9]. By contrast, most of the WDLMS in this study had a volume of fatty tissue of 50% or greater. Therefore, a greater fat content is suggestive of a WDL. However, 1 of our cases of WDLMS had no macroscopic fat on imaging and 1 patient had a myxoid component of slightly more than 50%. It is not only the relative fraction of macroscopic fat in the tumor, but also the distribution pattern of macroscopic fat and myxoid tissue as seen on imaging, which may be helpful in making the distinction between WDLMS and myxoid liposarcomas. The macroscopic fat, as seen on imaging in myxoid liposarcomas, is typically described as lace-like, linear, nodular, marbled or amorphous rather than solid, and is interspersed between the bright T2 signal myxoid component [4, 10,11,12]. Myxoid liposarcomas containing a myxoid component of less than 65% tend to lack a large fatty component, but rather have a large necrotic component [4]. To our knowledge, myxoid nodules surrounded by macroscopic fat or a bi-morphic appearance, as seen in this study, have not been described as a typical presentation of myxoid liposarcoma. A biphasic appearance akin to the bi-morphic appearance has been attributed to a “mixed” type liposarcoma containing both WDL and myxoid liposarcomas [4, 13]. These studies date (2000, 2003) back to before the new WHO classification and these “mixed” tumors may represent WDLMS as well [1]. Other morphological criteria could not be used to discern between WDLMS and myxoid liposarcomas; the presence of heterogeneous enhancement or lack of enhancement seen in our study has also been described in myxoid liposarcoma and the presence of thin septa [4, 14].
The location of the tumor may also help to establish a diagnosis. A retroperitoneal location is considered an unusual location for myxoid liposarcoma, which often present in the deep soft tissues of the extremities [1]. Because myxoid liposarcoma in the retroperitoneum is rare, some authors venture to say that primary retroperitoneal myxoid liposarcoma does not exist [2]. However, well-documented cases of retroperitoneal myxoid liposarcomas have been recently reported [15]. In locations other than the retroperitoneum, immunohistochemical cytogenetic or molecular studies may be helpful when the well-differentiated component is inconspicuous and not identified on careful inspection of the histology [1]. It is important to note that not all these adjunctive tests perform comparably; MDM2 immunohistochemistry has a sensitivity of 85% and specificity of 89% compared with FISH (100%, 100%) in the diagnoses of well-differentiated liposarcomas on core biopsy specimens [16].
Myxoid liposarcoma has been described as often having a pathognomonic appearance on imaging [3]. However, based on results from the current study, WDL with myxoid stroma may mimic other myxoid tumors, including true myxoid liposarcoma and a myxoid variant of dedifferentiated liposarcoma with macroscopic fat not present, present in less than 50% of the tumor volume, or demonstrating a septal or lacelike/hazy presentation on diagnostic imaging.
Dedifferentiated liposarcoma represents progression of WDL to a more aggressive form with acquisition of a metastatic risk. These tumors have nonlipomatous sarcomatous areas with a variable appearance [1, 3]. Although more common in the retroperitoneum, dedifferentiated liposarcomas also occur in the extremities. On imaging, the dedifferentiated area appears as nonlipomatous nodules within a fatty tumor. In our study, nonlipomatous nodules were commonly present in WDL with myxoid stroma. The size of these nodules was frequently greater than 2 cm, in contradiction to a previous description of nodules within WDL being usually smaller than 2 cm [3]. In addition, these nodules/myxoid components may progressively enlarge. In these cases, the imaging findings may raise the concern of a dedifferentiated liposarcoma and tissue sampling is needed to discern between these two entities [3]. It should be noted that the histopathological diagnosis in these cases may be difficult as one of the retroperitoneal tumors in our case series was initially misdiagnosed as a dedifferentiated liposarcoma. It is especially important for the pathologist to be aware of myxoid stroma in WDL because the nonlipomatous sarcomatous component of a dedifferentiated liposarcoma can be highly myxoid in appearance [1].
Although the clinical follow-up varied and was not available for all patients, the findings were similar to those described in the study by Sioletic et al. [1]. Local recurrence, confirmed in 1 patient in our study, is not uncommon in WDLMS. Sioletic et al. [1] described at least one local recurrence in all but one of the patients with available follow-up. A single patient within our study who did not have the tumor excised died more than 4 years after the initial diagnostic study. This is also consistent with the natural history of this disease; Sioletic et al. [1] reported the death of 1 of 9 patients with WDLMS and also noted that mortality was typically associated with uncontrolled local disease. In our case, the cause of death was unknown.
The limitations of this study are its retrospective nature, the lack of confirmatory immunohistochemistry, cytogenetics or FISH, and the relatively small number of cases presented. Regarding the lack of confirmatory studies: Sioletic et al. had emphasized the importance of thorough tissue sampling in these cases to demonstrate the nuclear atypia and pleomorphism needed to exclude myxoid liposarcoma. Sioletic et al. [1] also emphasized that immunohistochemistry, cytogenetics, and FISH may be useful in the absence of these typical morphological changes on biopsy [1]. In this study, tissue from surgical excision was available for histological assessment in addition to the core biopsies in all but a single case. In the single case without surgical excision, image-guided core biopsies were performed on two separate occasions allowing ample tissue sampling. In all cases, histological features excluding myxoid liposarcoma were identified, obviating the need for additional testing in making the final diagnosis. This approach is concordant with the approach suggested by Sioletic et al. [1]. Regarding the small sample size: WDLMS is a rare tumor and even the article by Sioletic et al. included only 22 cases of WDLMS [1].
Conclusion
The WDLMS is distinct from myxoid liposarcoma based on cytogenetics, biology, and treatment. Histopathological diagnosis is key to the correct treatment and prognostic stratification. However, the diagnosis is prone to misinterpretation based on histomorphology alone, especially in small core biopsy samples of tumors where the nuclear atypia and pleomorphism needed to exclude myxoid liposarcoma is sparse [7]. There is a variable appearance of WDLMS on imaging with two contrasting presentations; tumors with variable amounts of macroscopic fat and tumors without macroscopic fat. In cases with macroscopic fat, diagnostic imaging may help to guide the correct diagnosis based on tumor location, the relative extent and distribution pattern of fatty tissue in addition to the presence, extent, and distribution of the myxoid component. However, when the imaging presentation is not specific or contradictory to the histopathology, additional tissue sampling may be indicated. If tissue sampling is noncontributory, molecular testing may be helpful. Imaging alone cannot distinguish WDLMS from dedifferentiated liposarcoma, and sampling of the nodule is warranted.
References
Sioletic S, Dal Cin P, Fletcher CD, Hornick JL. Well-differentiated and dedifferentiated liposarcomas with prominent myxoid stroma: analysis of 56 cases. Histopathology. 2013;62(2):287–93.
De Vreeze RS, de Jong D, Tielen IH, Ruijter HJ, Nederlof PM, Haas RL, et al. Primary retroperitoneal myxoid/round cell liposarcoma is a nonexisting disease: an immunohistochemical and molecular biological analysis. Mod Pathol. 2009;22(2):223–31.
Murphey MD, Arcara LK, Fanburg-Smith J. From the archives of the AFIP: imaging of musculoskeletal liposarcoma with radiologic-pathologic correlation. Radiographics. 2005;25(5):1371–95.
Sung MS, Kang HS, Suh JS, Lee JH, Park JM, Kim JY, et al. Myxoid liposarcoma: appearance at MR imaging with histologic correlation. Radiographics. 2000;20(4):1007–19.
Antonescu CR, Elahi A, Humphrey M, Lui MY, Healey JH, Brennan MF, et al. Specificity of TLS-CHOP rearrangement for classic myxoid/round cell liposarcoma: absence in predominantly myxoid well-differentiated liposarcomas. J Mol Diagn. 2000;2(3):132–8.
Hanauer DA, Mei Q, Law J, Khanna R, Zheng K. Supporting information retrieval from electronic health records: a report of University of Michigan's nine-year experience in developing and using the electronic medical record search engine (EMERSE). J Biomed Inform. 2015;55:290–300.
Matthyssens LE, Creytens D, Ceelen WP. Retroperitoneal liposarcoma: current insights in diagnosis and treatment. Front Surg. 2015;2:4.
Jones RL, Fisher C, Al-Muderis O, Judson IR. Differential sensitivity of liposarcoma subtypes to chemotherapy. Eur J Cancer. 2005;41(18):2853–60.
Kransdorf MJ, Bancroft LW, Peterson JJ, Murphey MD, Foster WC, Temple HT. Imaging of fatty tumors: distinction of lipoma and well-differentiated liposarcoma. Radiology. 2002;224(1):99–104.
Wortman JR, Tirumani SH, Jagannathan JP, Tirumani H, Shinagare AB, Hornick JL, et al. Primary extremity liposarcoma: MRI features, histopathology, and clinical outcomes. J Comput Assist Tomogr. 2016;40(5):791–8.
El Ouni F, Jemni H, Trabelsi A, Ben Maitig M, Arifa N, Ben Rhouma K, et al. Liposarcoma of the extremities: MR imaging features and their correlation with pathologic data. Orthop Traumatol Surg Res. 2010;96(8):876–83.
Barile A, Zugaro L, Catalucci A, Caulo M, Di Cesare E, Splendiani A, et al. Soft tissue liposarcoma: histological subtypes, MRI and CT findings. Radiol Med. 2002;104(3):140–9.
Irie T, Hatori M, Watanabe M, Ehara S, Kokubun S. Radiologically and histologically mixed liposarcoma: a report of two biphasic cases. Jpn J Clin Oncol. 2003;33(9):482–5.
Lowenthal D, Zeile M, Niederhagen M, Fehlberg S, Schnapauff D, Pink D, et al. Differentiation of myxoid liposarcoma by magnetic resonance imaging: a histopathologic correlation. Acta Radiol. 2014;55(8):952–60.
Setsu N, Miyake M, Wakai S, Nakatani F, Kobayashi E, Chuman H, et al. Primary retroperitoneal myxoid liposarcomas. Am J Surg Pathol. 2016;40(9):1286–90.
Weaver J, Rao P, Goldblum JR, Joyce MJ, Turner SL, Lazar AJ, et al. Can MDM2 analytical tests performed on core needle biopsy be relied upon to diagnose well-differentiated liposarcoma? Mod Pathol. 2010;23(10):1301–6.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morag, Y., Yablon, C., Brigido, M.K. et al. Imaging appearance of well-differentiated liposarcomas with myxoid stroma. Skeletal Radiol 47, 1371–1382 (2018). https://doi.org/10.1007/s00256-018-2940-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-018-2940-6