Abstract
Purpose
Radiofrequency ablation technique for treatment of OO including ablation time and temperature vary greatly between and within reported studies. This study evaluates the immediate and long-term efficacy and complication rate of a two sequential RFA technique for OO.
Materials and methods
We retrospectively reviewed medical records and attempted interview follow-up for 25 patients treated with RFA for OO. Each treatment included 2 consecutive RFAs at 90 °C for 6 min with inter-ablation cooling to 40 °C and occasional inter-ablation probe adjustment. Additionally, we statistically compared the proportion of successful ablations using the DCRFA technique with published studies that utilized alternative OO ablation procedures.
Results
Long-term follow-up was obtained for 24 patients (96 %). Mean patient age at DCRFA was 17.2 years (range, 2.2–50.0 years). Mean time to follow-up was 60 ± 42 months (range 12–152 months). No acute DCRFA-related complications nor long-term recurrences were reported. All 24 interviewed patients reported partial relief of pre-procedural pain within 1 day of DCRFA and total relief within 1 week of DCRFA. One patient ultimately developed a major late complication (complex regional pain syndrome of the left ankle) after DCRFA of a cuboid lesion. Additionally, the DCRFA success rate was significantly higher when compared to two other published OO RFA treatment results.
Conclusion
DCRFA employing two sequential 6-min cycles is an effective treatment of OO. The 100 % primary success rate, 0 % long-term recurrence rate, and low complication rate compare favorably and may be superior to results of prior reports.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Percutaneous radiofrequency ablation (RFA) has largely supplanted surgical resection as first-line therapy for osteoid osteoma, a benign osseous neoplasm most commonly occurring in the long bones of the lower extremity and typically presenting with nonsteroidal anti-inflammatory (NSAID)-responsive nocturnal pain. Rosenthal et al. [1] described the first RFA of osteoid osteoma, where he used two sequential overlapping ablations in a 1.2-cm osteoid osteoma of the scapula. Rosenthal et al. [2] later described an 18-patient series employing a single 4-min ablation at 85–90 °C with a primary success rate, loosely defined in the literature as resolution of osteoid osteoma-related pain within days to several months following RFA, of 89 %. Multiple studies have since examined the primary success rate of RFA for osteoid osteoma, which are commonly above 90 % and range between 67–100 % [3–6]. While several recent studies use ablation times of at least 6 min, published studies demonstrate wide variation in RFA technique in terms of total ablation time and peak RFA temperature [3–5, 7–11]. Furthermore, fewer studies have assessed the long-term efficacy and complication rate of RFA in the treatment of osteoid osteoma, an important parameter considering delayed osteoid osteoma recurrences have been reported up to 13 and 3.5 years following surgical resection and RFA, respectively [5, 12, 13]. This study evaluates the immediate and long-term clinical outcomes following consistent use of a dual-cycle RFA (DCRFA) technique for treatment of osteoid osteoma that employs two sequential 6-min ablation sessions at 90 °C with interablation cooling to 40 °C.
Materials and methods
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional review board (IRB). After IRB approval, informed consent was obtained from all individual participants included in the study. The authors declare that they have no conflict of interest.
Patient data
We retrospectively reviewed the records of all patients treated with DCRFA for osteoid osteoma of the lower extremity between January 2000 and January 2013 at a single tertiary care institution. A picture archiving and communication system (PACS) query yielded 25 cases of osteoid osteoma treated with DCRFA. Diagnosis of osteoid osteoma prior to DCRFA was established using characteristic clinical features as well as typical cross-sectional imaging features [14]. All pre-treatment evaluations included radiographs and a CT examination, and some but not all patients were also evaluated with pre-procedure MRI (4 patients) and bone scintigraphy (1 patient). Lesion parameters were documented including lesion size and location. Biopsy rate at the time of DCRFA and histopathology results were documented.
Patient demographic data, early and late-onset complications, history of surgical or percutaneous intervention for the osteoid osteoma lesion, and symptom-free interval (mean ± standard deviation) after DCRFA were evaluated. Since many patients were referred from outside providers, and post-procedure clinical follow-up for many patients was performed at the referring outside institution, patients were contacted via telephone for current data regarding osteoid osteoma-related pain 1 month post DCRFA, current level of osteoid osteoma-related pain, and physician-documented osteoid osteoma recurrence and DCRFA-related complications as set forth by Callstrom et al. [14] (Table 1). For patient’s younger than 18 years of age at the time of follow-up, we interviewed the patient’s legal guardian.
Osteoid osteoma-related pain was defined as pain of similar location and quality compared to originally reported symptoms. Patients’ current pain levels were assessed using the verbal rating scale (VRS) [15]; patients were asked to rank their current pain level as (1) no pain, (2) mild pain, (3) moderate pain, and (4) severe pain. Primary clinical success was defined as resolution of osteoid osteoma-related pain 1 month after DCRFA without additional interventions including repeat RFA. Long-term treatment success was defined as no physician documented osteoid osteoma recurrence with no osteoid osteoma-related pain at time of follow-up. Symptom-free interval was defined as time between procedure and date of follow-up interview.
DCRFA technique
The DCRFA treatments were performed by two fellowship-trained musculoskeletal radiologists MR and CK (14 and 3 years of experience with osteoid osteoma DCRFA, respectively). All DCRFAs were performed under general anesthesia. No peri-procedural antibiotics where administered. Lesion access planning was performed with a pre-procedural thin-slice CT (max slice thickness = 2 mm) of the region of interest. Following a small skin incision, each osteoid osteoma nidus was accessed with either a 14-gauge Bonopty (Radi Medical Systems, Uppsala, Sweden) or an 11-gauge Laurane (Laurane Medical, Saint-Arnoult, France) coaxial bone biopsy system. Tissue sampling was attempted for most lesions at this time (Table 2). The internal drill or stylet was then removed and exchanged with the radiofrequency electrode. The cannula was subsequently retracted over the electrode in order to ensure that there was no contact between the cannula and electrode. A 17-gauge 5, 7, or 10-mm straight-needle Covidien Cool-Tip Radiofrequency electrode (Covidien, Dublin, Ireland) was used for each ablation depending on lesion size.
The electrode’s temperature was gradually increased by 1–2 °C/s until 90 °C was reached, and the temperature was maintained between 90 and 95 °C for 6 min. Impedance values as measured by the Covidien RFA system were also monitored throughout each ablation cycle in order to prevent excessive impedance increases that can result in tissue charring or an automatic system shut-off, and for a single case actual impedance values were documented during both RFA cycles every 10 s. The electrode tip was then allowed to cool to 40 °C. Infrequently, the electrode was repositioned during the inter-ablation period by either (1) adjusting the depth of the electrode via a single cortical access site or (2) accessing the lesion via a separate cortical access site if lesions were 1.2 cm or greater in any dimension due to expected incomplete coverage of a single zone of ablation (RF Cool-Tip User Guide, Covidient). The electrode’s temperature was then again raised to 90 °C and maintained for an additional 6 min. External cooling was not used in any case. The same goal temperature and ablation duration was used for all ablations in our study regardless of osteoid osteoma size or location. Statistical analysis compared the proportion of DCRFA initial successful cases to the proportion of initial successful cases of other studies published that utilized alternative osteoid osteoma ablation techniques using the Chi-square test [16] and post-hoc comparisons using the Marascuilo procedure [17]. The DCRFA success was compared to the successful results of Rehnitz et al., Rimondi et al., Rosenthal et al., and Sung et al. [3, 5, 8, 18]. P-values less than 0.05 were considered statistically significant.
All patients were discharged the same day of the procedure. Patients were instructed to use acetaminophen for routine pain control and were prescribed acetaminophen with codeine for PRN pain control during the first 48 h of the post-procedure period. All patients were instructed to avoid strenuous high-impact activity for 6 weeks following the procedure.
Results
Patient follow-up and osteoid osteoma lesion characteristics
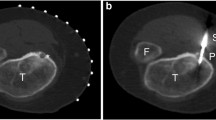
Long-term follow-up was obtained for 24 of 25 patients (96 %; Table 2). Lesion sites included metatarsal (n = 2), cuboid (n = 1), navicular (n = 1), talus (n = 1), tibia (n = 8), fibula (n = 2), femur (n = 8), and acetabulum (n = 1). Average nidus size was 7.2 mm ± 2.3 mm. One nidus was located in the medullary canal (fourth metatarsal lesion), and the remainder were located in the cortex. Mean time to follow-up was 60 months ± 42 months (range, 12–152 months). Time to follow-up was > 48 months in 14 patients. Mean patient age at DCRFA was 17.2 years (range, 2.2–50.0 years). Biopsy was obtained in 18 of 24 cases (75 %). Ten of 24 (42 %) patients had biopsies consistent with osteoid osteoma, yielding a 56 % positive diagnostic rate in patients that received biopsy; the remaining 46 % of biopsies were interpreted as indeterminate for osteoid osteoma. Inter-ablation probe depth adjustment without changing the cortical access site was employed in only four cases where the zone of initial ablation was not felt to cover the entire lesion due to lesion size or probe positioning, and inter-ablation probe adjustment employing a separate cortical access site was used in a single case demonstrating marked post-operative changes status-post failed surgical resection and RFA (Table 2; Fig. 1).
Examples of inter-ablation electrode position using two cortical access sites. a Initial RFA cycle in a patient with osteoid osteoma of the distal right fibula status-post failed surgical resection and RFA by another provider. b Second RFA cycle in the same patient as part (a) via a second, more distal cortical access site. c Coronal magnetic resonance (MR) STIR image from the same patient prior to our DCRFA protocol demonstrates the craniocaudal extent of the high-signal osteoid osteoma lesion is 3 cm. A rounded, low signal focus is seen at the superior aspect of the lesion. Superimposed post-surgical changes make identification of a discrete nidus difficult, however, and inter-ablation probe adjustment using separate cortical access sites was necessary to ensure total RFA coverage
Treatment outcomes and complications
All DCRFA treatments were primarily successful without technical or equipment failures. No immediate DCRFA-related complications as categorized by Callstrom et al. [14] or physician-documented osteoid osteoma recurrences were discovered on retrospective chart review or reported during the telephone interviews (Table 1). Follow-up telephone interviews confirmed long-term treatment success in all interviewed patients without evidence of any recurrences.
One patient (4 %) with osteoid osteoma of the left cuboid experienced a complex clinical course following DCRFA and ultimately developed a late major complication requiring additional therapy; the patient first developed pain in the midfoot 2 weeks following DCRFA coinciding with return to sports. This pain was of a different quality compared to pre-procedural osteoid osteoma-related pain and described as more constant in nature, exacerbated by activity, and only mildly relieved with NSAIDS. MRI 1 month post-DCRFA demonstrated marrow edema predominately of the left calcaneus, but also involving the talus, navicular bone, and metatarsals without evidence of fracture (Fig. 2a–c). Imaging demonstrated patchy edema in multiple osseous structures and in correlation with the clinical exam the patient was treated for stress reaction of the calcaneus with treatment including casting followed by a walking boot. After conservative treatment, MRI 15 months post-DCRFA showed near-resolution of marrow edema correlating with marked improvement of pain, as well as resorption of the cuboid osteoid osteoma nidus (Fig. 2d). Interestingly, approximately 2 years after DCRFA the patient developed left ankle skin discoloration, worsening pain, and chronic swelling during the beginning of a sports season, leading to a diagnosis of CRPS. The clinical course was complicated several months later by traumatic fracture of the left third metatarsal diaphysis and more recently by left ankle direct contusion without radiographic evidence of fracture.
Development of multi-tarsal osseous edema in a physically active 13-year-old female 2 weeks after DCRFA of left cuboid osteoid osteoma. a Sagittal CT image demonstrates well-circumscribed osteoid osteoma nidus (arrow) in the dorsal aspect of the left cuboid prior to DCRFA treatment. Two weeks after DCRFA treatment and early return to athletics, the patient experienced new-onset pain of different quality compared to her prior osteoid osteoma-associated pain. b Sagittal MR T2-weighted image (T2WI) with fat saturation (FS) obtained one month after DCRFA demonstrates the well-defined high-signal zone of ablation (arrow) surrounding the osteoid osteoma nidus. Diffuse high-signal bone marrow edema (stars) is also noted in the calcaneus and base of the fourth metatarsal. c A more medial sagittal MR T2WI with FS from the same examination demonstrates bone marrow edema (stars) also involving the navicular bone and talus. d Sagittal MR T2WI with FS 1 year after DCRFA following conservative treatment demonstrates marked improvement in diffuse bone marrow edema correlating with improvement in pain symptoms. There is RFA-related degenerative change involving the articular surface of the cuboid
Three patients treated by our DCRFA protocol received prior interventions with subsequent osteoid osteoma recurrences. One patient with osteoid osteoma of the left distal second metatarsal received DCRFA treatment employing a single 2-min session at 80 °C, a second patient experienced osteoid osteoma recurrence of an anterior right acetablular lesion after incomplete surgical resection, and a final patient experienced recurrence of a distal fibular osteoid osteoma after both a failed RFA treatment and a failed surgical resection. These patients were successfully treated per our protocol 7, 11, and 12 months, respectively, after their initial interventions by outside providers.
The proportion of initial successful cases for DCRFA (100 %/24 patients) was compared to the proportion of successful ablations at the end of follow-up times for osteoid osteoma ablation techniques used in other studies: Rehnitz (96.1 %/77 patients), Rimondi (95.7 %/557 patients), Rosenthal (91.5 %/117 patients) and Sung (82.1 %/28 patients) [3, 5, 8, 18]. The overall χ 2 = 14.16 and was highly significant (p = 0.0068), suggesting that some of the procedure successful proportions were significantly different. Post-hoc pair-wise comparisons made using the Marascuilo procedure [17] confirmed that the proportion of successful cases using DCRFA were significantly different when compared to the results of the studies by Rimondi and Rosenthal. No other comparisons were found to be significantly different (Table 3).
Intra- and inter-cycle impedance changes
We observed that tissue impedance levels tended to be lower during the second RFA cycle compared to the first cycle, which was reflected in our single case documenting impedance values (Fig. 3).
Discussion
RFA is the first-line treatment modality for most cases of osteoid osteoma; RFA technique parameters vary widely in prior reports, however, and relatively few studies have examined the long-term efficacy of RFA for osteoid osteoma [3, 5, 12, 13, 18]. Our retrospective review of 24 cases of osteoid osteoma treated with two sequential 6-min RFAs with inter-ablation cooling yielded a 100 % primary success rate and, therefore, compares well with the success rate of traditional RFA techniques for osteoid osteoma. The 0 % long-term recurrence rate also compares favorably with other recent reports, which demonstrate long-term retreatment rates between 3 to 33 % [3, 5, 6, 12, 13, 18].
The rationale for a second ablation cycle in our practice initially centered on allowing accurate electrode repositioning to ensure complete ablative coverage of the osteoid osteoma, particularly in patients with a history of prior failed surgical resection or RFA; this subset of patients has been reported to be at increased risk for RFA treatment failures [8], and post-surgical change can make identification of the osteoid osteoma nidus, and therefore determination of appropriate ablative coverage with a single electrode position, difficult (Fig. 1). Repositioning of the electrode, a technique performed by Rosenthal et al. [1] and, subsequently reported as an independent predictor of RFA treatment success in osteoid osteoma [3], was performed in only four cases in our study. Limited use of inter-ablation probe adjustment, either by depth adjustment, milling of the single cortical access site and subsequent probe angulation, or via a separate cortical access site may help ensure the best outcomes in select patients with larger lesions or when access to the lesion is difficult.
The primary benefit of a second ablation cycle, however, may relate to the interaction with intrinsic osseous tissue properties including impedance, defined as the electrical resistance to current flow in a tissue. While the osseous sclerosis surrounding the osteoid osteoma typically has high impedance, tissue impedance changes dynamically during the ablation process and decreases with tissue heating secondary to ion mobilization [3, 4, 11, 19]. Permanent reductions in tissue impedance also persist after the tissue temperature returns to baseline due to irreversible, albeit poorly understood, molecular changes in ablated tissues [19]. In our experience, we observed that the initial ablation cycle encountered high tissue impedance. Tissue impedance tended to be lower throughout the second ablation cycle compared to the first ablation cycle, and in general lower currents were therefore required on the second ablation to achieve and sustain goal temperatures. A favorable consequence of decreased impedance and voltage requirements is the reduced risk of local tissue charring, which prevents further increases in tissue impedance and yields more uniform tissue heating [11].
Rimondi et al. [3] performed the largest study of RFA for osteoid osteoma to date, a retrospective review encompassing 557 patients over an 11-year period and a mean follow-up period of 3.5 years. The first 68 patients in the series were treated with a 4-min RFA at 90–93 °C, while all remaining patients were treated with a single 14–15-min ablation at 90–93 °C preceded by a 2-min ablation at 60 °C. Reported variables between both patient groups, including the physicians performing the procedures, otherwise remained largely unchanged between groups. Of note, the recurrence rate reduced from 28 to 2 % after adoption of the longer ablation technique. While some authors advocate a single 4-min RFA cycle as adequate for osteoid osteoma [11], Rimondi et al. [3] and others have reported improved primary success rates by increasing total ablation times to 6 min or greater [4, 20]. This latter finding may reflect the biophysiologic underpinnings of the RFA process; while cellular coagulative necrosis occurs nearly instantaneously at temperatures between 60–100 °C in vivo, in situ RFA is a complex interaction of multiple variables related to tissue composition including thermal impedance and heat-sink effects from adjacent vascular structures that prevent uniform ablation and instantaneous cell death [11, 19]. We feel that maintaining ablation sessions for 6 min or more may allow for a more homogenous zone of ablation in part due to temporary intra-ablation impedance reductions, with subsequent potential for more effective killing of peripheral tumor cells. A second ablation cycle may further potentiate these effects of a homogenous zone of ablation via permanent tissue impedance reductions following the first ablation cycle.
This study’s single major complication following DCRFA of the cuboid reiterates the potential for osseous and soft tissue injury following ablation therapies (Fig. 2). Importantly, total ablation time does not appear correlated with risk of RFA complications. While Rimondi et al. [3] do report using a non-specified low-energy technique for foot and hand lesion, their technique employs some of the longest ablation times in the reported literature (14–15-min ablations at 90–93 °C) for 489 cases of their 557-case series. Rimondi et al. reported a decreased complication rate as well as recurrence rate after adoption of the 14–15 min ablation technique, and their < 1 % complication rate (including 1 thrombophlebitis, 1 skin burn, and 1 broken ablation probe) compares favorably with other studies using the more common, single 6-min ablation period. They also reported no subsequent development of CRPS or osseous stress-related injuries in any patients [5, 8, 13, 18, 21, 22]. A PubMed search (MeSH terms “osteoid osteoma” and “ablation” prior to November 15, 2015/English language) yielded one case of mild sympathetic dystrophy that resolved within several weeks following a standard 6-minute RFA [8]. Daniilidis et al. [13], who describe long-term follow-up after RFA in 29 cases of osteoid osteoma of the foot and ankle, also describe no RFA-related complications.
Despite our single complication, complications following RFA for osteoid osteoma do not appear correlated with total ablation time, and RFA of osteoid osteoma of the lower extremity and foot, in our experience and that described in the literature, therefore does appear safe. Given the asymmetric distribution of bone marrow edema primarily in the calcaneus, abrupt onset coinciding with early return to strenuous activity, and marked clinical improvement with immobilization, it is possible that this single major complication may have represented a component of acute osseous stress reaction (potentially precipitated by a rapid return to activity after an extended period of deceased weight bearing due to pre-procedural osteoid osteoma-related pain) superimposed on the CRPS that became more clinically apparent later in the patient’s clinical course. The proportion of successful procedures using DCRFA, when compared to other studies in the literature, suggested that using the DCRFA ablation technique in this study was significantly different when compared to the studies of Rimondi et al. and Rosenthal et al. [3, 8]. Post-hoc comparisons further suggested that there was no statistically significant difference in the proportions of successful ablations when results of the other four published studies listed in Table 3 were compared. While these results are encouraging, conducting a large-scale randomized clinical trial comparing different osteoid osteoma ablation techniques with DCRFA would be required to provide a more accurate comparison of the success of these different methods.
Among the limitations of our study is the small sample size of patients, although our group size is on par with other studies in the literature [5, 6, 12, 13]. Osteoid osteomas are reliably diagnosed based on imaging and clinical findings, and so our positive biopsy rate of 42 % is acceptable and also similar to other studies [3]. Recall bias of the participants is another study limitation given that (1) a specific pain monitoring scale was not documented in the medical record during the immediate pre- and post-operative period, and (2) pain data was collected retrospectively via phone with years-long time intervals from the RFA treatments. Since treatment success of osteoid osteoma is often purely evaluated clinically based on pain levels, we feel that defining long-term efficacy of treatment as “no pain” on VRS is valid. Also, our data regarding documented impedance changes during each ablation cycle is limited to a single case. Our follow-up rate for 24 of 25 patients (96 %) is satisfactory.
Conclusions
The 100 % primary success rate, 0 % long-term recurrence rate, and low complication rate of DCRFA compare favorably and may be superior to results of prior reports employing single ablations with shorter overall ablation times. Our study supports the long-term safety and efficacy of a dual-cycle RFA technique employing two sequential 6-min RFAs with a target temperature of 90 °C as a first-line treatment of osteoid osteoma.
Abbreviations
- RFA:
-
Radiofrequency ablation
- OO:
-
Osteoid osteoma
- DCRFA:
-
Dual-cycle radiofrequency ablation
References
Rosenthal DI, Alexander A, Rosenberg AE, Springfield D. Ablation of osteoid osteomas with a percutaneously placed electrode: a new procedure. Radiology. 1992;183:29–33.
Rosenthal DI, Springfield DS, Gebhardt MC, Rosenberg AE, Mankin HJ. Osteoid osteoma: percutaneous radio-frequency ablation. Radiology. 1995;197:451–4.
Rimondi E, Mavrogenis AF, Rossi G, et al. Radiofrequency ablation for non-spinal osteoid osteomas in 557 patients. Eur Radiol. 2012;22:181–8.
Vanderschueren GM, Taminiau AHM, Obermann WR, van den Berg-Huysmans AA, Bloem JL. Osteoid osteoma: factors for increased risk of unsuccessful thermal coagulation. Radiology. 2004;233:757–62.
Sung K-S, Seo J-G, Shim JS, Lee YS. Computed-tomography-guided percutaneous radiofrequency thermoablation for the treatment of osteoid osteoma: 2 to 5 years follow-up. Int Orthop. 2009;33:215–8.
Kjar RA, Powell GJ, Schilcht SM, Smith PJ, Slavin J, Choong PFM. Percutaneous radiofrequency ablation for osteoid osteoma: experience with a new treatment. Med J Aust. 2006;184:563–5.
Barei DP, Moreau G, Scarborough MT, Neel MD. Percutaneous radiofrequency ablation of osteoid osteoma. Clin Orthop. 2000;115–124.
Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology. 2003;229:171–5.
Torriani M, Rosenthal DI. Percutaneous radiofrequency treatment of osteoid osteoma. Pediatr Radiol. 2002;32:615–8.
Lindner NJ, Ozaki T, Roedl R, Gosheger G, Winkelmann W, Wörtler K. Percutaneous radiofrequency ablation in osteoid osteoma. J Bone Joint Surg (Br). 2001;83:391–6.
Pinto CH, Taminiau AHM, Vanderschueren GM, Hogendoorn PCW, Bloem JL, Obermann WR. Technical considerations in CT-guided radiofrequency thermal ablation of osteoid osteoma: tricks of the trade. AJR Am J Roentgenol. 2002;179:1633–42.
Neumann D, Berka H, Dorn U, Neureiter D, Thaler C. Follow-up of thirty-three computed-tomography-guided percutaneous radiofrequency thermoablations of osteoid osteoma. Int Orthop. 2012;36:811–5.
Daniilidis K, Martinelli N, Gosheger G, et al. Percutaneous CT-guided radio-frequency ablation of osteoid osteoma of the foot and ankle. Arch Orthop Trauma Surg. 2012;132:1707–10.
Callstrom MR, York JD, Gaba RC, et al. Research reporting standards for image-guided ablation of bone and soft tissue tumors. J Vasc Interv Radiol JVIR. 2009;20:1527–40.
Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005;14:798–804.
Conover W. Practical nonparametric statistics, 2nd edn. New York: Wiley; 1980.
Marascuilo LA. Large-sample multiple comparisons. Psychol Bull. 1966;65:280–90.
Rehnitz C, Sprengel SD, Lehner B, et al. CT-guided radiofrequency ablation of osteoid osteoma: correlation of clinical outcome and imaging features. Diagn Interv Radiol Ank Turk. 2013;19:330–9.
Haemmerich D. Biophysics of radiofrequency ablation. Crit Rev Biomed Eng. 2010;38:53–63.
Rosenthal DI, Hornicek FJ, Wolfe MW, Jennings LC, Gebhardt MC, Mankin HJ. Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg Am. 1998;80:815–21.
Earhart J, Wellman D, Donaldson J, Chesterton J, King E, Janicki JA. Radiofrequency ablation in the treatment of osteoid osteoma: results and complications. Pediatr Radiol. 2013;43:814–9.
Bourgault C, Vervoort T, Szymanski C, Chastanet P, Maynou C. Percutaneous CT-guided radiofrequency thermocoagulation in the treatment of osteoid osteoma: a 87 patient series. Orthop Traumatol Surg Res OTSR. 2014;100:323–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All authors report “No conflict of interests”.
Rights and permissions
About this article
Cite this article
Abboud, S., Kosmas, C., Novak, R. et al. Long-term clinical outcomes of dual-cycle radiofrequency ablation technique for treatment of osteoid osteoma. Skeletal Radiol 45, 599–606 (2016). https://doi.org/10.1007/s00256-015-2321-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-015-2321-3