Abstract
Objective
To assess diffusion tensor imaging (DTI) parameters in cervical compressive myelopathy (CCM) patients compared to normal volunteers, to relate them with myelopathy severity, and to relate tractography patterns with postoperative neurologic improvement.
Subjects and methods
Twenty patients suffering from CCM were prospectively enrolled (M:F = 13:7, mean age, 49.6 years; range 22–67 years) from September 2009 to March 2010. Sensitivity encoding (SENSE) single-shot echo-planar imaging (EPI) was used for the sagittal DTI. Twenty sex- and age-matched normal volunteers underwent the same scanning procedure. Fractional anisotropy (FA) and apparent diffusion coefficient (ADC) values in the spinal cord were compared between the patients and normal volunteers and were related to myelopathy severity based on Japanese Orthopedic Association (JOA) scores. Tractography patterns were related to myelopathy severity and postoperative improvement.
Results
There were significant differences between patients and normal volunteers in terms of FA (0.498 ± 0.114 vs. 0.604 ± 0.057; p = 0.001) and ADC (1.442 ± 0.389 vs. 1.169 ± 0.098; p = 0.001). DTI parameters and tractography patterns were not related to myelopathy severity. In ten patients in the neurologically worse group, postoperative neurologic improvement was seen in four of five patients with intact fiber tracts, but only one of five patients with interrupted fiber tracts exhibited neurologic improvement.
Conclusion
DTI parameters in CCM patients were significantly different from those in normal volunteers but were not significantly related to myelopathy severity. The patterns of tractography appear to correlate with postoperative neurologic improvement.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diffusion tensor imaging (DTI) is a magnetic resonance imaging (MRI) technique that evaluates the scalar properties of the diffusion of extracellular water molecules within white matter fibers. DTI provides fractional anisotropy (FA) and apparent diffusion coefficient (ADC) values. The FA values reflect the global anisotropy of the analyzed structure. In addition, fiber tractography can be obtained using FA values [1–5].
Applications of DTI to the spinal cord have been reported in cases of spinal cord compression, cervical spondylosis, multiple sclerosis, neuromyelitis optica, acute transverse myelitis, spinal cord arteriovenous malformation, spinal cord injury, and astrocytoma [5–14]. Although DTI is not in routine clinical use, it has proven to be an invaluable tool for detecting subtle damage to white matter that appears normal on conventional T2-weighted MR images [1, 5, 6, 8, 10, 15, 16].
Demir et al. [6] reported that ADC values and diffusion tensor measurements showed better sensitivity than T2-weighted imaging in detecting myelopathy in cervical spondylotic myelopathy. DTI may have a role in grading neurologic severity in cervical compressive myelopathy (CCM) because it is known to provide objective measures in terms of microstructural change. To our knowledge, no report has correlated DTI parameters and different tractography patterns with clinical severity and postoperative results in CCM.
Several recently published reports about DTI in cervical myelopathy have concluded that DTI parameters were significantly different in compressive myelopathy [17–20]. However, few reports [21, 22] have compared DTI parameters and clinical severity, and there are no reports about the relationship between DTI and postoperative results.
Our hypothesis is that DTI parameters and different tractography patterns correlate with clinical severity in patients with CCM. The purpose of this study was to assess DTI parameters in CCM patients compared to normal volunteers, to relate them to myelopathy severity, and to relate tractography patterns to postoperative neurologic improvement.
Subjects and methods
Subjects
This study was approved by an institutional review board. Informed consent was obtained. Patients scheduled for cervical operations in the Department of Orthopedic Surgery at our hospital during the period from September 2009 to March 2010 were prospectively enrolled in this study. Inclusion criteria were (1) the patient’s willingness to enroll in the study and (2) clear evidence of cord compression on a cervical spine MRI, such as cervical spondylosis, an ossified posterior longitudinal ligament, or os odontoideum. One radiologist determined spinal cord compression when the cord surface was clearly indented or the cord diameter was narrowed by compression as a result of one of the above-mentioned causes. Exclusion criteria were (1) refusal by the patient or (2) trauma or infection-related cord compression. There were 13 male and 7 female patients, whose ages ranged from 22–67 years (mean age, 49.6 years). Twenty sex- and age-matched normal volunteers with no past history of neurological disorders and with normal results of neurological examinations underwent the same scanning procedure as the patients.
DTI technique
A 3-T MR scanner (Achieva, Philips Medical Systems, Best, the Netherlands) was used. Sixteen-channel neurovascular coils were applied to all subjects. Sensitivity encoding (SENSE) single-shot echo planar imaging (EPI) with a SENSE factor of 4 was used for the sagittal DTI of the cervical spinal cord with a b-value of 600 s/mm2, 15 diffusion gradient directions, four excitations, and a slice thickness of 2 mm [23]. The diffusion gradient strength was 40 mT/m, the foldover direction was anterior-posterior, and the fat shift direction was posterior. The TR/TE was 3,380/56 ms, the EPI factor was 19, the matrix was 128 × 128, and the FOV was 250 × 224 mm. The scan time was 3 min 43 s. The voxel size was 1.95 × 1.95 × 2 mm.
Measurements
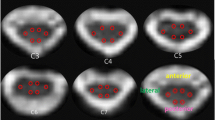
After sending all source DTI images to a personal computer, one technician who was blind to the clinical conditions of each patient measured FA and ADC in the cervical spinal cord at each spine segment level. Each spine segment was selected for each disc level from C1/2 to C7/T1, in reference to the midsagittal T1-weighted image. Next, FA and ADC measurements were taken for each single voxel inside the spinal cord in the axial image (Fig. 1). The average FA and ADC values for all voxels inside the spinal cord at each spine segment level were calculated.
Fiber tractography of the spinal cord in a 67-year-old male (patient number 1). We selected two slices, one at the C1 level and one at the C7 level. On the selected slice, we drew a circular region of interest (ROI) to include the whole spinal cord in the axial image and performed fiber tracking. Fiber tractography was displayed as itself and overlapped with the T1-weighted image. Partially interrupted fiber tracts were noted
DTI post-processing with fiber tracking
After sending all source DTI images to a personal computer, one technician performed fiber tracking for the cervical spinal cord using PRIDE software (Philips Medical Systems) based on the FACT method [24]. From the axial b = 0 image, we selected two slices, one at the C1 level and one at the C7 level. On the selected slice, we drew a circular region of interest (ROI), including the whole spinal cord and performed fiber tracking (Fig. 2). Only the fibers passing through the ROI at both the C1 and C7 positions for each subject were displayed. The thresholds for tracking termination were 0.1 for FA and 27° for the angle between two contiguous eigenvectors in all subjects, which were set as default parameters for spinal cord tractography by Philips Medical Systems.
Clinical assessment
The clinical grade of cervical myelopathy was evaluated in accordance with the Japanese Orthopedic Association (JOA) score by one orthopedic surgeon [22, 25]. Patients were placed in two groups according to their preoperative JOA score: the neurologically better group (a score of 11 or higher) vs. the neurologically worse group (a score of 10 or less). The JOA scores were also evaluated 3 months after surgery. For patients in the neurologically worse group, postoperative neurologic improvement was defined as a JOA score increase of more than three over the preoperative JOA score at 3 months after surgery.
MR assessment
One radiologist who was blind to clinical information reviewed the MR images, focusing on the level of spinal cord compression, the number of levels of spinal cord compression, and the presence of intramedullary high signal intensity on T2-weighted MR images near the compression level. If there were multiple levels of spinal cord compression, the most severe compression level was also determined by the radiologist.
Tractography evaluation
A radiologist who was blind to the patients’ clinical information qualitatively described the tractography pattern as completely intact, waist, partially interrupted, or completely interrupted. “Waist” was defined as fiber indentation at the compression level without interruption. “Partially interrupted” was defined as fiber interruption across parts of the axial plane. “Completely interrupted” was defined as fiber interruption across the entire axial plane [12].
Statistical analysis
Statistical analyses were performed by one radiologist (J.W.L.). The Wilcoxon signed rank test was used to compare the average FA and ADC values of the spinal cord at the most severe compression level in the patients and at the same level in the matched normal volunteers. Only for those who showed no signal change in the spinal cord on T2-weighted image were Wilcoxon signed rank tests also used to evaluate differences of FA and ADC values of the spinal cord at each matched level between patients (at the most severe compression level) and normal volunteers.
Average FA and ADC values of the spinal cord at the most severe compression level were compared between the neurologically better group and the neurologically worse group using the Mann-Whitney U test.
Four different tractography patterns were related to the two groups (neurologically better vs. neurologically worse) using the chi-squared test. Two separate patterns of tractography [interrupted (both completely and partially) vs. intact (completely intact and waist)] were also related to the two groups (neurologically better vs. neurologically worse) using the Fisher exact test.
Age differences were evaluated between the two groups (neurologically better vs. neurologically worse) using the Mann-Whitney U test. The presence of cord atrophy and intramedullary high signal intensity on T2-weighted MR images was related to the two groups (neurologically better vs. neurologically worse) using the Fisher exact test. The number of levels of cord compression was related to the two groups (neurologically better vs. neurologically worse) using the chi-squared test.
The PASW statistical package (17.0; SPSS, Chicago, IL) was used for statistical calculations.
Results
The clinical details of the patients are shown in Table 1. Average FA and ADC values of the spinal cord at the most severe compression levels in the patients are compared with those of the age- and sex-matched normal volunteers in Table 2. The FA values for the most severe compression levels in patients were significantly lower than those of normal volunteers (0.498 ± 0.114 vs. 0.604 ± 0.057; p = 0.001). The ADC values for the most severe compression levels in patients were significantly higher than those of normal volunteers (1.442 ± 0.389 vs. 1.169 ± 0.098; p = 0.001). Four patients showed no abnormal signal change in the spinal cord on T2-weighted images. For those four patients, FA values for the most severe compression levels were lower than those of normal volunteers (0.584 ± 0.055 vs. 0.708 ± 0.089; p = 0.144) and ADC values for the most severe compression levels were higher than those of normal volunteers (1.201 ± 0.149 vs. 1.076 ± 0.193; p = 0.144). However, there was no statistical significance, which may be due to the small sample size.
The MR features, DTI parameters, and neurologic status of the patients are shown in Table 3. Table 4 shows how DTI, clinical, and MR variables are related with neurologic status. There was no significant difference of FA in the spinal cord between the neurologically better and neurologically worse groups (0.517 ± 0.097 vs. 0.479 ± 0.131; p = 0.684). There was also no significant difference of ADC values in the spinal cord at the most severe compression level between the two groups (1.481 ± 0.479 vs. 1.403 ± 0.295; p = 0.971). Tractography patterns were not significantly related with neurologic status (Figs. 3, 4). Although there was no statistical significance, one patient who showed complete interruption of the fiber tract had a neurologically worse status (JOA score = 3.5). Of six patients who showed intact fiber tracts, four (67%) had neurologically better status. Whether fiber tracts were interrupted (completely or partially) or were intact (waist or completely intact) was not significantly related with neurologic status. Five of 8 patients (62.5%) with interrupted tractography had neurologically worse status, but 5 of 12 patients (41.7%) with intact tractography had neurologically worse status. There was also no significant difference observed for correlations among age, sex, levels of cord compression, presence of cord atrophy, or intramedullary high signals on the T2-weighted images for the two groups. Although there was no statistical significance, 5 of 7 patients (71.4%) with cord atrophy showed neurologically worse status, but 5 of 13 patients (18.5%) without cord atrophy showed neurologically worse status. Of four patients who did not show an intramedullary signal change on T2-weighted MR images of the spinal cord, only one showed neurologically worse status.
A 22-year-old female (patient number 15) with partially interrupted fiber tracts and a JOA score of 15. On T2-weighted sagittal images, the cervical canal was narrowed at the C2/3, C3/4, C4/5, C5/6, and C6/7 levels due to skeletal dysplasia (a). The fiber tract was partially interrupted on tractography (b, arrows), but the patient was included in the neurologically better group with a JOA score of 15
A 46-year-old male (patient number 19) with completely intact fiber tracts. Spinal cord compressions were seen at multiple cervical levels and mostly at the C3/4 level by the ossified posterior longitudinal ligaments (arrow) on T2-weighted sagittal image (a). The fiber tract was completely intact on tractography (b). This patient showed neurologically worse status preoperatively, with a JOA score of 7, and postoperative improvement, with a JOA score of 16.5
Of ten patients who showed neurologically worse status, four of five patients (80%) with intact tractography showed neurologic improvement after surgery, but only one of five patients (20%) with interrupted tractography showed postoperative neurologic improvement (Table 5) (Fig. 5 ).
A 50-year-old female (patient number 2) with partially interrupted fiber tracts. Spinal cord compression was seen at the C4/5 and C5/6 levels by herniated discs (arrows) on the T2-weighted sagittal image (a). The fiber tracts were partially interrupted on tractography (b). This patient showed neurologically worse status preoperatively, with a JOA score of 10, and no improvement postoperatively, with a JOA score of 10
Discussion
According to our study, there were significant differences between patients and normal volunteers in terms of FA and ADC, as has been found in previous reports [5, 6, 17–20]. Tractography patterns were not related to myelopathy severity, but postoperative neurologic improvement was more common in patients with intact fiber tractography than in those with interrupted fiber tractography, which has not been previously reported.
Several reports about DTI in cervical myelopathy have been recently published and reported that DTI parameters were significantly different in compressive myelopathy and in normal volunteers, and that DTI was more sensitive than conventional T2-weighted images [17–20]. These results were similar to previous reports [5, 6], which concluded that DTI parameters were better than conventional MR images. In our study, we saw similar results. We observed that FA and ADC values of the spinal cords of patients were significantly different from those of normal volunteers. In addition, in four patients who showed no abnormal signal change in the spinal cord on T2-weighted images, FA values were lower than those of normal volunteers and ADC values were higher, which meant that DTI was more sensitive than conventional MR images.
Aota et al. [22] studied the correlation of diffusion-weighted MR in CCM with neurologic severity. They concluded that ADC maps could show internal changes in the early stages of chronic spinal cord compression but had limitations in detecting intramedullary changes in late-stage myelopathy. In our study, we compared these parameters with myelopathy severity. According to our study, there was no significant difference in DTI parameters between the neurologically worse and neurologically better groups.
Kara et al. [20] reported there was no significant difference between the duration of myelopathy and DTI parameters. Budzik et al. [21] reported that FA values were significantly correlated with some of the patients’ clinical scores. According to our results, there was no significant difference in DTI parameters or type of tractography between neurologically worse and neurologically better groups, which was unexpected. We think this is a limitation of current DTI techniques and suggest that current DTI techniques cannot reveal the full microstructural damage of the spinal cord. Although many articles have reported positive results from DTI applications for the spinal cord, we believe the clinical usefulness of DTI parameters should be considered carefully. However, tractography may have a role because it may have a correlation with postoperative neurology recovery. According to our results, there were many nonsignificant results, which may be due to limitations of current DTI techniques.
According to our results, there was quite significant intersubject variability in both the patient and control groups. This is often seen in DTI studies. DTI can be affected by many factors, such as age and spinal level [7, 10]. Thus, age and level-matched data for normal volunteers are necessary for the DTI studies.
Our study has some limitations. First, the number of patients was limited; a larger series should be imaged in the future. Second, clinical severity was measured by JOA scores only; however, this is the most common method in the CCM literature. Third, only symptomatic patients who were scheduled for surgery were included. However, as shown in Tables 1 and 3, the patients’ symptoms and severity of myelopathy were variable. Fourth, the heterogeneity of the patient group is another limitation of the study. Because the age, cause of cord compression, compression level, and symptoms were different, it was more difficult to find significant differences in this small group of patients.
In conclusion, DTI parameters in CCM patients were significantly different from those of normal volunteers but were not significantly related with myelopathy severity. The tractography patterns appear to correlate with postoperative neurologic improvement.
References
Ducreux D, Fillard P, Facon D, Ozanne A, Lepeintre JF, Renoux J, et al. Diffusion tensor magnetic resonance imaging and fiber tracking in spinal cord lesions: current and future indications. Neuroimaging Clin N Am. 2007;17(1):137–47.
Clark CA, Werring DJ. Diffusion tensor imaging in spinal cord: methods and applications—a review. NMR Biomed. 2002;15(7–8):578–86.
Thurnher MM, Law M. Diffusion-weighted imaging, diffusion-tensor imaging, and fiber tractography of the spinal cord. Magn Reson Imaging Clin N Am. 2009;17(2):225–44.
Lee JW, Kim JH, Kang HS, Lee JS, Choi JY, Yeom JS, et al. Optimization of acquisition parameters of diffusion-tensor magnetic resonance imaging in the spinal cord. Invest Radiol. 2006;41(7):553–9.
Facon D, Ozanne A, Fillard P, Lepeintre JF, Tournoux-Facon C, Ducreux D. MR diffusion tensor imaging and fiber tracking in spinal cord compression. AJNR Am J Neuroradiol. 2005;26(6):1587–94.
Demir A, Ries M, Moonen CT, Vital JM, Dehais J, Arne P, et al. Diffusion-weighted MR imaging with apparent diffusion coefficient and apparent diffusion tensor maps in cervical spondylotic myelopathy. Radiology. 2003;229(1):37–43.
Mamata H, Jolesz FA, Maier SE. Apparent diffusion coefficient and fractional anisotropy in spinal cord: age and cervical spondylosis-related changes. J Magn Reson Imaging. 2005;22(1):38–43.
Hesseltine SM, Law M, Babb J, Rad M, Lopez S, Ge Y, et al. Diffusion tensor imaging in multiple sclerosis: assessment of regional differences in the axial plane within normal-appearing cervical spinal cord. AJNR Am J Neuroradiol. 2006;27(6):1189–93.
Renoux J, Facon D, Fillard P, Huynh I, Lasjaunias P, Ducreux D. MR diffusion tensor imaging and fiber tracking in inflammatory diseases of the spinal cord. AJNR Am J Neuroradiol. 2006;27(9):1947–51.
Lee JW, Park KS, Kim JH, Choi JY, Hong SH, Park SH, et al. Diffusion tensor imaging in idiopathic acute transverse myelitis. AJR Am J Roentgenol. 2008;191(2):W52–7.
Yu C, Lin F, Li K, Jiang T, Qin W, Sun H, et al. Pathogenesis of normal-appearing white matter damage in neuromyelitis optica: diffusion-tensor MR imaging. Radiology. 2008;246(1):222–8.
Ozanne A, Krings T, Facon D, Fillard P, Dumas JL, Alvarez H, et al. MR diffusion tensor imaging and fiber tracking in spinal cord arteriovenous malformations: a preliminary study. AJNR Am J Neuroradiol. 2007;28(7):1271–9.
Ellingson BM, Ulmer JL, Kurpad SN, Schmit BD. Diffusion tensor MR imaging in chronic spinal cord injury. AJNR Am J Neuroradiol. 2008;29(10):1976–82.
Ducreux D, Lepeintre JF, Fillard P, Loureiro C, Tadie M, Lasjaunias P. MR diffusion tensor imaging and fiber tracking in 5 spinal cord astrocytomas. AJNR Am J Neuroradiol. 2006;27(1):214–6.
Werring DJ, Clark CA, Barker GJ, Thompson AJ, Miller DH. Diffusion tensor imaging of lesions and normal-appearing white matter in multiple sclerosis. Neurology. 1999;52(8):1626–32.
van Hecke W, Nagels G, Emonds G, Leemans A, Sijbers J, van Goethem J, et al. A diffusion tensor imaging group study of the spinal cord in multiple sclerosis patients with and without T2 spinal cord lesions. J Magn Reson Imaging. 2009;30(1):25–34.
Xiangshui M, Xiangjun C, Xiaoming Z, Qingshi Z, Yi C, Chuanqiang Q, et al. 3 T magnetic resonance diffusion tensor imaging and fibre tracking in cervical myelopathy. Clin Radiol. 2010;65(6):465–73.
Song T, Chen WJ, Yang B, Zhao HP, Huang JW, Cai MJ, et al. Diffusion tensor imaging in the cervical spinal cord. Eur Spine J. 2011;20(3):422–8.
Kerkovsky M, Bednarik JAP, Dusek L, Sprlakova-Pukova A, Urbanek IAP, Mechl MP, et al. Magnetic resonance diffusion tensor imaging in patients with cervical spondylotic spinal cord compression: correlations between clinical and electrophysiological findings. Spine (Phila Pa 1976). 2011; doi:10.1097/BRS.0b013e31820e6c35
Kara B, Celik A, Karadereler S, Ulusoy L, Ganiyusufoglu K, Onat L, et al. The role of DTI in early detection of cervical spondylotic myelopathy: a preliminary study with 3-T MRI. Neuroradiology. 2011; doi:10.1007/s00234-011-0844-4
Budzik JF, Balbi V, Le Thuc V, Duhamel A, Assaker R, Cotten A. Diffusion tensor imaging and fibre tracking in cervical spondylotic myelopathy. Eur Radiol. 2011;21(2):426–33.
Aota Y, Niwa T, Uesugi M, Yamashita T, Inoue T, Saito T. The correlation of diffusion-weighted magnetic resonance imaging in cervical compression myelopathy with neurologic and radiologic severity. Spine (Phila Pa 1976). 2008;33(7):814–20.
Bammer R, Auer M, Keeling SL, Augustin M, Stables LA, Prokesch RW, et al. Diffusion tensor imaging using single-shot SENSE-EPI. Magn Reson Med. 2002;48(1):128–36.
Mori S, van Zijl PC. Fiber tracking: principles and strategies—a technical review. NMR Biomed. 2002;15(7–8):468–80.
Naderi S, Ozgen S, Pamir MN, Ozek MM, Erzen C. Cervical spondylotic myelopathy: surgical results and factors affecting prognosis. Neurosurgery. 1998;43(1):43–9. discussion 49–50.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, J.W., Kim, J.H., Park, J.B. et al. Diffusion tensor imaging and fiber tractography in cervical compressive myelopathy: preliminary results. Skeletal Radiol 40, 1543–1551 (2011). https://doi.org/10.1007/s00256-011-1161-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-011-1161-z