Abstract
Objective
To determine the functional relevance of diffusion tensor imaging (DTI) metrics and conventional MRI (signal intensity change in T2, compression ratio) by measuring the correlation of these parameters with clinical outcome measured by the modified Japanese Orthopedic Association (mJOA) score.
Materials and methods
A total of 20 cervical myelopathy (CM) patients participated in this prospective cohort study. The severities of CM were assessed using the mJOA score. Conventional MRIs (T2-weighted images) measuring the signal changes of spinal cords and the degree of compression at the lesion level and DTI metrics [fractional anisotropy (FA), apparent diffusion coefficient (ADC)] at each lesion and below each lesion (C7/T1) level were acquired using a 3-T Achieva MRI. These parameters were correlated with the mJOA scores to determine the functional relevance.
Results
Ninety percent of CM patients showed signal changes and 30 % of patients noted a more than 40% canal compression ratio in conventional MRIs at the lesion level; however, these findings were not correlated with the mJOA score (p < 0.05). In contrast, FA values on DTI showed high sensitivity to CM (100%), which was well correlated with the mJOA score (p = 0.034, r = 0.475) below the lesion level (C7/T1).
Conclusions
This study showed a meaningful symptomatic correlation between mJOA scores and FA values below the lesion levels in CM patients. It could give us more understanding of the pathological changes in spinal cords matched with various clinical findings in CM patients than the results from conventional MRI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cervical myelopathy (CM) is an insidiously progressive condition usually showing a chronic course of clinical symptoms. Most of the cases are easily diagnosed by conventional T2-weighted magnetic resonance imaging (MRI) together with a history of specific symptoms and neurological examinations demonstrating myelopathic signs such as ankle clonus, increased deep tendon reflex, and spasticity [1,2,3]. Although MRI has played an important role in the diagnosis and follow-up of spinal cord lesions at present, it still lacks relevance to the functional outcome and prognosis [1, 4,5,6,7]. In addition, it has been reported that up to 21% of patients identified as having as CM did not show any clinical findings [8]. This controversy may stem from the methodological limitations to estimating and quantifying the spinal cord condition, which could possibly explain the change in white matter integrity linking with the clinical symptoms of CM [3, 6, 7, 9].
A recently introduced technique, diffusion tensor imaging (DTI), has been investigated for estimating the neural tissue integrity in the brain and spinal cord [2, 3, 10,11,12,13]. Limited evidence has recently been published regarding the possibility of using DTI in CM patients, showing a correlation with the modified Japanese Orthopedic Association (mJOA) score for CM [14, 15].
As the first step toward using this technique in the clinical field, it would be important to validate the advantage of DTI in terms of functional relevance compared to conventional MRI. Thus, the underlying hypothesis in this study was that the DTI technique would be better at estimating the clinical correlation in terms of functional recovery (recovery after surgery) compared to conventional MRI. Thus, the aim of this study was to determine the functional relevance of DTI metrics [fractional anisotropy (FA), apparent diffusion coefficient (ADC)], and conventional MRI (intensity change in T2, compression ratio) and whether these parameters correlated with the clinical outcome measured by mJOA scores.
Materials and methods
Participants
This is a prospective cohort study. A total of 20 CM patients participated in this study. The eligibility criteria were as follows: patients with a history of clinical symptoms such as neck pain with motor dysfunction or sensory deficits with compressive lesions and/or signal changes (cervical spondylotic myelopathy, ossification of the posterior longitudinal ligament, ossification of the ligament flavum, cervical disc herniation, and cervical spinal stenosis) as visualized on MRI for whom conservative treatment had had no effect for more than 6 weeks and with an acute worsening of neurological conditions. The exclusion criteria were as follows: any previous types of spine surgery, rheumatoid arthritis, spinal infection, trauma, instability, tumor, neurological disorders other than those of spinal origin, congenital anomaly of the spine, and contraindications to laminoplasty. Informed consent was obtained from all individual participants included in the study. Along with compressive lesions on MRI, signal changes, meaning increased signal intensity of the spinal cords on T2 images, have been regarded as one of the important radiological markers in CM patients [3, 6, 14, 15]. Besides, because of the vague symptoms of CM, not directly relevant to the MR findings, we focused on CM patients who had not responded to conservative treatment for more than 6 weeks, had not undergone a previous surgery, and had no other clinical conditions that might affect the final outcomes, just like the requirements for patient eligibility for surgery.
Conventional T2-weighted MRI acquisition and analysis
MRIs were obtained using a 3-T Achieva MRI scanner (Achieva, Philips, Best, The Netherlands) with the following parameters: same orientation plane as for diffusion-weighted data (axial), slice thickness 3 mm, no gap, time repetition/echo time 3600/120 ms, and field of view 250 mm. The signal changes in T2-weighted images were measured automatically by the computer and qualitatively assessed.
The compression ratio of the most compressed region for the cord diameter (di) on MRI was measured as a percentage of the non-compressed areas above (da) and below (db) the lesion level using the following equations according to the method described by Arvin et al. [14]. Compression ratio (%) = 1 - [di/{(da + db)/2}] × 100. The sensitivity of the compression ratio was considered a positive condition when the compression ratio was over 40% [15]. The sensitivity of signal change was considered a positive condition when a signal change existed. At least two investigators confirmed the signal change and compression ratio on MRI.
Diffusion tensor imaging (DTI) acquisition and analysis
DTI data were obtained using the same scanner. Single-shot echo-planar imaging was used for the transverse DTI of the cervical spinal cord with the following parameters: b value 500 s/mm2, number of diffusion gradient directions 15, slice thickness 3 mm, no gap, time repetition/echo time 6300/63 ms, field of view 145 mm, and acquisition matrix 128 × 128.
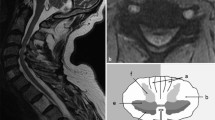
For the DTI analysis, the raw image files were converted to NIfTI files using MATLAB 2009b software (MathWorks, Natick, MA, USA). The NIfTI files were split using FSL software (University of Oxford, Oxford, UK) and then co-registered according to their gradient directions using SPM8 software (University College London, London, UK). DTIFIT implemented in FSL software was used to obtain DTI parameters including fractional anisotropy (FA) and the apparent diffusion coefficient (ADC). Regions of interest (ROIs) were marked at the anterior, lateral, and posterior white matter regions in each half of the spinal cord according to the method described by Thurnher et al. (Fig. 1) [16]. However, voxels of ROIs were difficult to distinguish with severe compression at the spinal cord lesion level. Therefore, the ROIs were defined as six ROIs at and below the lesion level (C7/T1) in the cervical vertebrae. FA and ADC values were measured in a given ROI (Fig. 2). Also, an average of FA and ADC values of six ROIs was used for statistical comparison of sensitivity to CM and correlation with the mJOA score of CM between MRI and DTI findings. FA and ADC values were measured in a given ROI and averaged for six ROIs for statistical comparison of sensitivity to CM and correlation with the mJOA score.
Sensitivity of the metrics on DTI was measured as variation of the lesion and below lesion level (C7/T1) from the non-compressed areas above the lesion level (C2) [5, 13, 17,18,19]. DTI findings were confirmed with two investigators using both MRI and color-coded FA maps.
Cervical myelopathy severity measurement
All cases of CM patients were carefully evaluated, and their neurological status including motor and sensory changes was recorded. Symptoms of CM were also evaluated according to their severity as measured by the mJOA score, a scoring system for evaluating functional outcome in cervical myelopathy patients. It assesses motor function in the upper and lower extremities, upper extremity sensory function, and bladder function. Each scale ranges from 0 to 7, 5, 3, and 3, respectively, with a total score of 18. Fehlings et al. defined the severity of myelopathy as mild if the mJOA score was 15 or larger, moderate if the mJOA score ranged from 12 to 14, and severe if the mJOA score was less than 12 [20]. To reduce errors, the two investigators were unaware of the intention of this research and the clinical scoring (mJOA) results of participants, which were measured twice at weekly intervals, and the average of the measured values was used as the measured value. Intra- and interobserver reliability of the measurements on ROIs was analyzed using the interclass correlation coefficient (ICC) and 95% confidence interval. The ICC values were interpreted according to the Landis-defined categories for the interpretation of κ [21]. All physical signs regarding the patients’ main discomfort were also recorded and videotaped using a digital camera (D-90; Nikon, Tokyo, Japan).
Statistical analysis
The Pearson correlation test was used to determine correlation among the signal change, compression ratio, and mJOA scores. It was also used to determine the correlation between all DTI metrics and mJOA scores on both the lesion level and the below lesion level as well as on conventional MRI. The SPSS 13.0 statistical package (SPSS, Chicago, IL, USA) was used. A value of p < 0.05 was accepted as significant.
Results
-
1.
Baseline characteristics, conventional T2-weighted MRI findings, and DTI findings.
A total of 20 patients were enrolled, and 17 patients were male. Average age of the patients was 52.79 ± 8.01 years. All patients had spinal stenosis; of these, 18 had signal changes on conventional MRI. The average compression ratio at the lesion level was 34.6% (11.0%–74.5%). The range of mJOA scores was 12 to 17. MRI and DTI findings of CM patients are shown in Table 1.
-
2.
Correlation of the MRI signal changes and compression ratio on conventional T2-weighted MRI with the mJOA score at the lesion level.
At the lesion level, signal changes of spinal cords on T2 image MRI showed high sensitivity to CM (90%). However, it was not correlated with the functional score (mJOA score) (p = 0.102). The compression ratio on MRI showed low sensitivity to CM (30%), which was also not correlated with the mJOA score (p = 0.489) (Fig. 3). The signal change on MRI was not correlated with the compression ratio (p = 0.271) (Table 2).
-
3.
Correlation of DTI metrics with the mJOA score at and below the lesion level.
At the lesion level, both FA and ADC values showed high sensitivity to CM (85%, 95%) on DTI. However, there was no statistically significant correlation with the mJOA score of CM (p = 0.456, 0.929) (Fig. 4). On the other hand, below the lesion level (C7/T1), the FA value on DTI indicated high sensitivity to CM (100%) and showed statistically significant positive correlation with the mJOA score of CM [Pearson coefficient = 0.475, p = 0.034, coefficient of determination (r2) = 0.23] (Tables 3 and 4, Fig. 5).
-
4.
Comparison of the DTI FA value below the lesion level and the mJOA score in the CM patient groups.
The mean FA value below the lesion level of the mild CM group (mJOA ≥15) was 0.539 (95% CI: 0.530–0.549) and that of the moderate-to-severe CM group (mJOA ≤14) was 0.485 (95% CI: 0.476–0.494). The mild CM group showed a relatively higher mean FA value below the lesion level [+0.0542 (95% CI: 0.00891–0.0994)] than the moderate-to-severe CM group, and the difference was statistically significant (p = 0.022) (Table 5).
-
5.
Intra- and interobserver reliability of measurements
Intraobserver reliabilities of two observers were categorized as perfect agreement with ICCs of 0.974 (95% CI: 0.935–0.990) and 0.970 (95% CI: 0.925–0.988), respectively. Interobserver reliability between two observers was also interpreted as perfect agreement with an intraclass correlation coefficient (ICC) of 0.933 (95% CI: 0.830–0.973) (Table 6).
Discussion
CM developing progressively for years is a common degenerative disorder presenting symptoms of unsteady gait, worsening balance, clumsy hands, spasticity, and voiding difficulty. Also, these functional disorders can result in motor paralysis and sensory disturbances [11].
Nowadays, MRI is essentially used for the diagnosis and evaluation of CM in the clinical field. Besides the neurological examination, MR findings such as signal changes and compression of the spinal cord are diagnostic markers for CM. The signal changes in the spinal cord caused by ossification of the posterior longitudinal ligament and ligament flavum, cervical disc herniation, and cervical spinal stenosis, however, have pitfalls when it comes to estimating functional recovery. Much evidence has been published about the uncertainty of MRI in diagnosing CM [1, 3, 7, 9, 13, 22, 23]. In this study, we confirmed that neither the signal changes nor the compression ratio had any significant correlation with the functional scores in CM patients. The reason it is not relevant to functional scores is that MRI lacks the sensitivity to assess the condition of CM [1, 3, 22, 23] because MRI cannot disclose the injured neuron of the spinal cord. By diagnosing an abnormal spinal cord at an early stage, CM patients are able to obtain better results with optimal treatment from spine surgeons. However, it is difficult to assess the condition of the injured neuron of the spinal cord with MRI at an early stage. Therefore, these problems lead to difficulty in deciding the optimal treatment for CM [7]. In contrast, some patients had normal anatomical cords on MRI despite having severe symptoms of CM. Therefore, it is very difficult to explain the difference between the MR imaging and clinical findings [7, 9, 24].
Several investigators recently assessed the feasibility of performing diffusion tensor imaging (DTI), which is an advanced MRI technique that can objectify and quantify neurological changes using several parameters. These parameters are obtained by measuring the diffusion of extracellular water molecules in ROIs, which is drawn to neuronal tracts. One parameter, the FA value (fractional anisotropy, whose value is from 0 to 1), is known to be related to the directionality of water diffusivity and generally considered to be related to function [3, 25]. A decrease in motor and sensory function happens with a reduced number of fibers or reduced density results in increased extracellular space [26]. The other parameter, the ADC value (apparent diffusion coefficient), is used to measure diffusive strength and thought to be useful for monitoring the pathological condition such as the progression of recovery from a spinal cord injury [27]. As the FA value moves closer to 0 and the ADC values move closer to 1, a CM subject has a greater possibility of being diagnosed with poorer function [3].
Some DTI studies used different parameters, such as the mean diffusivity (MD) and radial and axial diffusion (AD) coefficients, because they are believed to reflect the degree of axonal and myelin damage, respectively [28,29,30,31], and the standard deviation (SD) of the primary eigenvector orientation, std. (q), was evaluated as a measure of white matter disorganization and tract disruption [32]. Among these parameters, the FA and ADC values, most commonly used in DTI-related research, were measured and analyzed in our study. Previous studies tried to show the usefulness of DTI in spondylosis and myelopathy patients. Ying et al. described the positive correlation between the change of mean FA values and mJOA scores in multilevel compressive myelopathy; however, they did not explain the change in FA values below and above the lesion level, only focusing on the change in general mean FA values in white matter; we could not identify the actual symptomatic correlation with the change in FA values at the most compressed lesion sites [33]. Lindberg et al. explained that DTI parameters could detect any change in the spinal cords even in cervical spondylosis with no evidence of cord damage on T2-weighted MRI imaging. However, unfortunately, there is no practical way to prove the actual effect and symptomatic correlation of only the change of DTI parameters with clinical findings, especially in patients with no evidence of structural change of the spinal cord on conventional MRI at this time. To overcome these limitations of DTI studies, we measured the DTI parameters above and below the lesion levels as well as the lesion sites to determine any correlation of the change in DTI parameters with clinical findings. [34].
At the lesion level, both the FA and ADC values on DTI indicated high sensitivity to cervical myelopathy (85%, 95%). Although the DTI metrics in ROIs definitely showed significant changes of the spinal cord at the lesion level in this study, it did not correlate with the functional scores. Unfortunately, when there are many structural changes with severe compression, the DTI technique does not display the spinal cord tract’s neurological disruption at the compressed lesion level. Also, some secondary histopathological changes, such as gliosis, cystic degenerative changes, extracellular edema, extracellular space, inflammatory cell proliferation, and decreased perfusion of the cell membrane in the spinal cord, could influence problems not correlated with CM symptoms [6]. Besides, other problems such as artifacts of susceptibility, movement of cerebrospinal fluid (CSF), heart motion, respiration motion, a high signal-to-noise ratio, and low resolution of DTI images could influence the measurement of neurological changes of the spinal cord [4, 7, 9]. Therefore, DTI findings at the lesion level should be interpreted cautiously when analyzing sensitivity to CM and correlation with CM symptoms. Nevertheless, DTI can quantify the anatomical structure and neurological changes of the spinal cord with DTI metrics such as FA and ADC. DTI can be more objective and sensitive to CM than MRI.
CM develops progressively for years but is sometimes caused by acute events. In patients who have injured spinal cord neurons, Wallerian degeneration can develop toward the distal part of an injured neuron after spinal cord injury and be detected at sites distant from the lesion as well [17]. Harsan et al. also conducted a study showing water diffusion at great distances from the damaged axons was restricted because of accumulations of neurofilaments (NF) and other transported proteins [35]. That is, changes to the DTI metrics below the lesion level are likely to be caused by damage to the axonal integrity of the descending tract of the lesion level.
Therefore, this study for measuring sensitivity to CM and correlation with symptoms of CM was implemented at the lesion level as well as below the lesion level because of current DTI technical limitations at the lesion level and Wallerian degeneration. As a result, FA values below the lesion level (C7/T1) indicated high sensitivity to CM (100%) and also showed statistically significant positive correlation with CM symptoms.
In our previous study, DTI metrics sensitively reflected the pathological status and were correlated with behavioral outcome. DTI analysis could also give us more objective and reliable information about the spinal cord structure symptoms of CM than that MRI according to the results [3].
This study showed not only the diagnostic and predictive value of DTI but also the important clinical significance of neurological changes in spinal cords below the lesion level.
Because of technical limitations of current DTI imaging analysis, we could not go down to the thoracic spine level for measurement. The maximum level was C7/T1 as other studies suggested [3, 7, 14, 15]. This is why we chose the C7–T1 level as below the lesion level. As myelopathy symptoms are closely related to the severity of the damage in the spinal cord at the lesion level as well as the axonal integrity of the descending tract below the lesion level, one can easily expect to measure the exact extent of the damage according to the location as the lesion distortion and change in spinal morphometry due to compression; at the same time, the integrity of the spinal cord below the lesion level would give us valuable information regarding pathological changes without the problems of structural deformation.
According to this study, unfortunately, we could not suggest the exact lower or upper threshold for FA that is predictive of a mild (mJOA ≥15) and moderate to severe (mJOA ≤14). This is because each patient could express different degrees of symptoms or clinical findings at all different areas, even with the same amount of FA value change. It is the same in the mJOA scoring system. As the mJOA scoring system is just based on the patients’ subjective symptoms, and simply divided into four categories such as motor (upper and lower extremities), sensory, and sphincter dysfunction, almost all patients with the same mJOA score could have different clinical findings; for instance, some patients might only have motor dysfunction in their upper extremities or others might have symptoms in their lower extremities or only sensory dysfunctions. This discrepancy indicates the difficulty in deciding the exact upper or lower threshold for the FA value predicting the mild and moderate CM groups. Nevertheless, based on our observations, the mean FA value below the lesion level of the mild CM group was 0.539 (95% CI: 0.530–0.549) and that of moderate to severe CM group was 0.485 (95% CI: 0476–0.494); the difference was statistically significant (p = 0.022). Further investigation will be needed in regard to this matter.
Our research has several limitations. First, the small numbers of the participants could be a weakness of this research. Second, there could be a bias in studying mostly mild and moderate patients according to the mJOA score.
In conclusion, this DTI study showed a meaningful symptomatic correlation between mJOA scores and FA values below lesion levels in CM patients. It could give us more understandings of pathological changes in spinal cords, matched with various clinical findings in CM patients than conventional MRI.
References
Ducreux D, Fillard P, Facon D, et al. Diffusion tensor magnetic resonance imaging and fiber tracking in spinal cord lesions: Current and future indications. Neuroimaging Clin N Am. 2007;17(1):137–47.
Acharya S, Srivastava A, Virmani S, Tandon R. Resolution of physical signs and recovery in severe cervical spondylotic myelopathy after cervical laminoplasty. Spine (Phila Pa 1976). 2010;35(21):E1083–7.
Yoo WK, Kim TH, Hai DM, et al. Correlation of magnetic resonance diffusion tensor imaging and clinical findings of cervical myelopathy. Spine J. 2013;13(8):867–76.
Cercignani M, Horsfield MA, Agosta F, Filippi M. Sensitivity-encoded diffusion tensor MR imaging of the cervical cord. AJNR Am J Neuroradiol. 2003;24(6):1254–6.
Mamata H, Jolesz FA, Maier SE. Characterization of central nervous system structures by magnetic resonance diffusion anisotropy. Neurochem Int. 2004;45(4):553–60.
Rajasekaran S, Kanna RM, Shetty AP. Diffusion tensor imaging of the spinal cord and its clinical applications. J Bone Joint Surg Br. 2012;94(8):1024–31.
Song T, Chen WJ, Yang B, et al. Diffusion tensor imaging in the cervical spinal cord. Eur Spine J. 2011;20(3):422–8.
Rhee JM, Heflin JA, Hamasaki T, Freedman B. Prevalence of physical signs in cervical myelopathy: A prospective, controlled study. Spine (Phila Pa 1976). 2009;34(9):890–5.
Clark CA, Werring DJ. Diffusion tensor imaging in spinal cord: Methods and applications—a review. NMR Biomed. 2002;15(7–8):578–86.
Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006;6(6 Suppl):190S–7S.
Fehlings MG, Skaf G. A review of the pathophysiology of cervical spondylotic myelopathy with insights for potential novel mechanisms drawn from traumatic spinal cord injury. Spine (Phila Pa 1976). 1998;23(24):2730–7.
Uda T, Takami T, Tsuyuguchi N, et al. Assessment of cervical spondylotic myelopathy using diffusion tensor magnetic resonance imaging parameter at 3.0 tesla. Spine (Phila Pa 1976). 2013;38(5):407–14.
Xiangshui M, Xiangjun C, Xiaoming Z, et al. 3 T magnetic resonance diffusion tensor imaging and fibre tracking in cervical myelopathy. Clin Radiol. 2010;65(6):465–73.
Arvin B, Kalsi-Ryan S, Mercier D, Furlan JC, Massicotte EM, Fehlings MG. Preoperative magnetic resonance imaging is associated with baseline neurological status and can predict postoperative recovery in patients with cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2013;38(14):1170–6.
Ono K, OH TK, Yamamoto T. Cervical myelopathy secondary to multiple spondylotic protrusion: A clinico-pathologic study. Spine (Phila Pa 1976). 1977;2:109–25.
Mueller-Mang C, Law M, Mang T, Fruehwald-Pallamar J, Weber M, Thurnher MM. Diffusion tensor MR imaging (DTI) metrics in the cervical spinal cord in asymptomatic HIV-positive patients. Neuroradiology. 2011;53(8):585–92.
DeBoy CA, Zhang J, Dike S, et al. High resolution diffusion tensor imaging of axonal damage in focal inflammatory and demyelinating lesions in rat spinal cord. Brain. 2007;130(Pt 8):2199–210.
Gao SJ, Yuan X, Jiang XY, et al. Correlation study of 3T-MR-DTI measurements and clinical symptoms of cervical spondylotic myelopathy. Eur J Radiol. 2013;82(11):1940–5.
Budzik JF, Balbi V, Le Thuc V, Duhamel A, Assaker R, Cotten A. Diffusion tensor imaging and fibre tracking in cervical spondylotic myelopathy. Eur Radiol. 2011;21(2):426–33.
Fehlings MG, Wilson JR, Kopjar B, et al. Efficacy and safety of surgical decompression in patients with cervical spondylotic myelopathy: Results of the AOSpine North America prospective multi-center study. J Bone Joint Surg Am. 2013;95(18):1651–8.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74.
Dorenbeck U, Schreyer AG, Schlaier J, Held P, Feuerbach S, Seitz J. Degenerative diseases of the cervical spine: Comparison of a multiecho data image combination sequence with a magnetisation transfer saturation pulse and cervical myelography and CT. Neuroradiology. 2004;46(4):306–9.
Yousem DM, Atlas SW, Hackney DB. Cervical spine disk herniation: Comparison of CT and 3DFT gradient echo MR scans. J Comput Assist Tomogr. 1992;16(3):345–51.
Werring DJ, Clark CA, Barker GJ, Thompson AJ, Miller DH. Diffusion tensor imaging of lesions and normal-appearing white matter in multiple sclerosis. Neurology. 1999;52(8):1626–32.
Johansen-Berg H, Rushworth MF. Using diffusion imaging to study human connectional anatomy. Annu Rev Neurosci. 2009;32:75–94.
Facon D, Ozanne A, Fillard P, Lepeintre JF, Tournoux-Facon C, Ducreux D. MR diffusion tensor imaging and fiber tracking in spinal cord compression. AJNR Am J Neuroradiol. 2005;26(6):1587–94.
Ellingson BM, Ulmer JL, Kurpad SN, Schmit BD. Diffusion tensor MR imaging in chronic spinal cord injury. AJNR Am J Neuroradiol. 2008;29(10):1976–82.
Ellingson BM, Kurpad SN, Schmit BD. Functional correlates of diffusion tensor imaging in spinal cord injury. Biomed Sci Instrum. 2008;44:28–33.
Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. NeuroImage. 2003;20(3):1714–22.
Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage. 2002;17(3):1429–36.
Budde MD, Xie M, Cross AH, Song SK. Axial diffusivity is the primary correlate of axonal injury in the experimental autoimmune encephalomyelitis spinal cord: A quantitative pixelwise analysis. J Neurosci. 2009;29(9):2805–13.
Ellingson BM, Salamon N, Grinstead JW, Holly LT. Diffusion tensor imaging predicts functional impairment in mild-to-moderate cervical spondylotic myelopathy. Spine J. 2014;14(11):2589–97.
Ying J, Zhou X, Zhu M, et al. The contribution of diffusion tensor imaging to quantitative assessment on multilevel cervical Spondylotic myelopathy. Eur Neurol. 2016;75(1–2):67–74.
Lindberg PG, Sanchez K, Ozcan F, et al. Correlation of force control with regional spinal DTI in patients with cervical spondylosis without signs of spinal cord injury on conventional MRI. Eur Radiol. 2016;26(3):733–42.
Harsan LA, Poulet P, Guignard B, et al. Brain dysmyelination and recovery assessment by noninvasive in vivo diffusion tensor magnetic resonance imaging. J Neurosci Res. 2006;83(3):392–402.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study was supported by Hallym University Research Fund (HRF-01-2010-13) and the National Research Foundation of Korea (2015R1D1A1A01061562, 2013R1A1A2012562).
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Yang, YM., Yoo, WK., Yoo, J.H. et al. The functional relevance of diffusion tensor imaging in comparison to conventional MRI in patients with cervical compressive myelopathy. Skeletal Radiol 46, 1477–1486 (2017). https://doi.org/10.1007/s00256-017-2713-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-017-2713-7