Abstract
Objective
The purpose of this study was to evaluate the safety and efficacy of CT-guided percutaneous cryoablation for osteoid osteoma in children.
Materials and methods
This study was approved by the institutional ethics committee. From January 2007 to July 2008, six children (four boys, two girls, mean age 12.6 years old) with osteoid osteoma were treated with CT-guided percutaneous cryoablation. The procedures were carried out under conscious sedation and local anesthesia. CT guidance was used for procedural planning, instrument guidance, and monitoring. An argon-based cryoablation system was used. Each cryoablation included two freezing-thawing cycles. Follow-up was performed to assess technical and clinical outcome for a minimum of 12 months. A visual analog scale (VAS) was used to assess severity of pain pre- and post-procedure, and mean VAS for the group was compared pre- and post-procedure with a t-test. The mean clinical follow-up period was 28.7 months (ranging from 18 to 36 months).
Results
Cryoablation was technically and clinically successful for all patients. No major immediate or delayed complications were observed. Significant pain relief (P <0.05) was observed in all patients after operation. Mean VAS were 6.57 ± 0.55 pre-procedure and 0.57 ± 0.10 1 month post-procedure. Patients were allowed to fully bear their weight and function without limitation within 3 days after the procedure. Pain recurrence was not observed in any patient.
Conclusion
Percutaneous cryoablation is safe and effective for the treatment of osteoid osteomas in children. Notably, this procedure can be accomplished without general anesthesia.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Osteoid osteoma represents 10–12% of primary benign bone tumors (third most common) and usually occurs in children or young adults, with a male predominance [1]. Pain is the most common presenting symptom, and those who do not respond or tolerate medical therapy with analgesics usually require surgical intervention for pain relief and possibly to prevent growth disturbances in the immature skeleton. Percutaneous image-guided interventional techniques, using trephination or tumor ablation of the nidus, have emerged to replace surgical excision [2–4]. These techniques are also described specifically in the pediatric population with osteoid osteoma [5–7]. Tumor thermal ablation is popular because of precise lesion targeting, minimal invasiveness, minimal complications or collateral damage, quick patient recovery, effectiveness for pain management, and reduced use of healthcare resources. Radiofrequency ablation has become the main technique applied for osteoid osteoma thermal ablation with an established durable effect [8–10]. However, the technical and clinical success rates of radiofrequency ablation are slightly lower in the pediatric population. In one study of 23 children, the lower clinical success of 78.2% was attributed to greater technical difficulty during ablation of osteoid osteoma in children due to the small body mass, difficulty in positioning and fixation of the needle, and shorter ablation time (range 2–6 min) for each procedure [11]. This observation was substantiated by another study of 95 subjects which found that older subjects (mean age 24 years in treatment success group versus 20 years in treatment failure group) had a lower risk for treatment failure of RFA for osteoid osteoma [12].
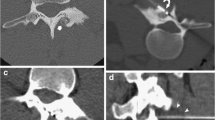
Cryoablation is another thermal ablation technology with particular biochemical and biophysical mechanisms of tissue destruction [13]. Room temperature argon gas is delivered through a sealed insulated probe. Rapid expansion of the gas results in cooling via the Joule-Thompson effect, with temperatures reaching just over −100°C at the exposed probe tip, and exceeding within a few seconds the effective temperature for cryoablation of a tumor (<−40°C). An ice ball is formed (Fig. 1) leading to the development of intercellular ice crystals. The change in electrolyte and osmotic pressure causes cell dehydration and damage to the cellular membrane with the development of intracellular ice crystals. Cytoclasis ensues with fragmentation, and the cell structure is completely destroyed [14]. During the cryoablation period, microvasculature membranes swell and break. When the temperature is recovered, a wide area of thrombus has formed in the local microcirculation. This leads to further lack of oxygen and necrosis of tissues [15]. Active thawing is achieved by the infusion of helium gas into the cryoprobes, instead of argon gas. Percutaneous cryoablation is widely used in the treatment of solid soft tissue tumors and more recently for musculoskeletal lesions including tumors involving bone [16, 17]. However, there are only limited data on image-guided percutaneous cryoablation in the treatment of osteoid osteoma [18, 19]. The rationale for why cryoablation of osteoid osteoma may be beneficial and potentially superior to radiofrequency ablation includes the following: patient tolerance without general anesthesia, intraprocedural ablation zone monitoring and control, preservation of critical structures/tissues, and improved pain management efficacy. The purpose of this study was to evaluate the safety and efficacy of CT-guided percutaneous cryoablation for osteoid osteoma in children.
Materials and methods
No commercial support was received for this study. Ethics committee approval was obtained and allowed review of records and images with informed consent from the parents/guardians.
Patients
The study design was a retrospective consecutive case series. During a 19-month period from January 2007 through July 2008, there were 13 pediatric patients who underwent a CT-guided cryoablation for substantial pain from presumed osteoid osteoma who were considered for review. While both radiofrequency ablation and cryoablation are performed at our institution, cryoablation is preferred for the rationale previously elaborated.
Inclusion criteria were clinical presentation suggesting osteoid osteoma, presence of pain ≥4 on a visual analog scale (VAS) of 0–10 that was worse at night and relieved by administration of oral anti-inflammatory medications, symptom duration for at least 6 months, CT scan showing a characteristic appearance for osteoid osteoma, age less than 21 years, histological confirmation of osteoid osteoma, and follow-up of at least 12 months with post-procedure MRI obtained at about 6 months after ablation. Exclusion criteria were adult age patients, incomplete imaging or clinical data, and clinical follow-up less than 12 months. In this study, seven patients were excluded: two because the diagnosis was not confirmed by pathology, two due to incomplete imaging or clinical data, and three lost to follow up. The remaining six children (four boys, two girls; aged 10–15 years; mean age 12.6 years) were included for review. Lesions were located in the proximal femur (n = 4) and patella (n = 2).
Technique
Pre-procedure preparation
Medical evaluation was performed on each patient, including coagulation profile of prothrombin time, bleeding time, and coagulation time. An intravenous infusion route was established. Electrocardiogram, respiratory rate, and oxygen saturation of patients were monitored during the procedure. All patients received conscious sedation using diazepam administered intravenously. Warming mats with water circulation (Hico-Aquatherm660, Hirtz, Germany) were prepared to keep patients warm during cryoablation. Procedures were performed on an inpatient basis as is the standard of care for the institution.
Procedure
The procedures utilized CT imaging guidance (Philips Brilliance Big Bore 16, slice thickness 1–2 mm, kVp 120, mAs 250). Radiopaque markers on the skin surface were used to plan the skin entry point. After precise localization of the lesion by thin section CT, the skin entry point was marked, and the best needle trajectory was chosen avoiding neurovascular and other critical structures. Once the puncture point was marked on the skin, the area was disinfected and covered by a drape. Local anesthesia was administered with skin and subcutaneous injection of 2% lidocaine. An 8-G (outer diameter 4.2 mm) T-Lok bone marrow biopsy needle system (Angiotech Medical Device Technologies, USA) was used in all patients to penetrate the cortex and target the lesion. During insertion, the trajectory and position were verified with CT imaging. We performed two insertions using the biopsy needle during the procedure. We obtained a pathology sample during the first insertion (noncoaxial technique), the tissue sample was placed in formalin and sent for histology. The biopsy needle was then reinserted through the channel formed in the first puncture. The stylet was then removed.
Percutaneous cryotherapy was performed using a cryotherapy delivery system (Cryohit; Galil Medical, Yokneam, Israel), equipped with cryoprobes of 2.0 mm diameter (approximately equal to 14 gauge). A single cryoprobe was inserted coaxially through the access needle (Fig. 2). The access needle was then withdrawn slightly so that the distal 1 cm of the cryoprobe was exposed. A scan was obtained to confirm that the position of the cryoprobe tip was within 5 mm of the inner edge of the nidus (Fig. 3). To prevent skin injury, a small amount of filtered sterile gas (5–10 ml) was injected into the subcutaneous region of the puncture site and a heated sterile water bag was put on the skin near the puncture area. A warming mat was placed under the body of the patient to prevent a substantial decrease in body temperature. Two freeze-thaw cycles were performed for each lesion with a time frame of 8-min freeze, 5-min passive thaw intended for each cycle. During freezing, the cryoneedle tip temperatures reached −175°C (based on a thermocouple measurement).
The size of the ablation zone (ice ball) generated could be adjusted by the length of the noninsulated tip, amount of freezing time, and rate of gas delivered to the probe. The length of available noninsulated tips was 40 mm. Using the whole length of the cryoprobe tip (40 mm), a freezing time of 10 min, and 3,200 psi of argon, a 4 × 6 cm ice -ball is generated. If one-third of the tip was covered with the cannula of the biopsy needle, the volume of the ice ball would be decreased correspondingly by approximately one-third. As an example, for a 1 cm lesion, the ice-ball coverage should be about 2 cm, so the settings used would be as follows: cryoprobe tip length of 2 cm (that is the amount extending beyond the cannula), argon gas pressure of 3,200 psi, freezing time of 8 min, and thawing time of 5 min.
CT scanning was performed periodically to monitor for changes in surrounding tissues (Fig. 4). CT scanning was performed for lesion localization, needle targeting, and therapy monitoring. Cryoablation may be observed on CT imaging as a hypoattenuating area in the soft tissues and is less conspicuous within bone. Monitoring allows control of the cryoablation zone. There were no direct signs of inadequate coverage. Signs of complications were if the cryoablation zone was extending into adjacent critical structures such as neurovascular bundle, skin, or articular cartilage. Upon completion of the cryoablation, a 3-min active thawing was performed (instilling helium gas into the cryoprobes instead of argon gas), and the probes were removed. As an additional measure to prevent bleeding, a small amount of fibrin glue was inserted into the puncture hole. After the probe was removed, a final CT scan (2 mm thickness) was obtained to check for possible complications such as fracture or hematoma.
Post-operative care
Immediately after needle removal, the puncture site was compressed for 5–10 min to achieve hemostasis, then disinfected and dressed. Patients were observed for 30 min in the radiology department and then returned to the ward if there was no discomfort. After the procedure, an electrocardiogram was monitored for 6 h, an antibiotic (oral ciprofloxacin 500 mg twice a day) was taken for 3 days, and urine alkalization (with sodium bicarbonate) was performed for 2–3 days.
Follow-up
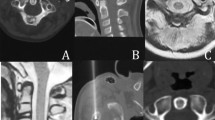
Patients were evaluated in the orthopedic clinic at 1 week, 1 month, and 3 months after the procedure for assessment of clinical success and adverse events. We also contacted patients by telephone at 6, 9, 12, 18, and 24 months for follow-up regarding complications or recurrence of pain. MRI scans were performed at approximately 6 months after the procedure. This examination was performed to evaluate the change in appearance of the ablated region and to capture possible late post-cryoablation complications. The cryoablation complications that might be expected and could be detected with MRI are osteonecrosis, stress reaction/fracture, articular cartilage damage, joint effusion/synovitis, and adjacent soft tissue collections or injury.
Outcome evaluation
Technical success was defined as the ability to localize and target the radiolucent nidus with accurate placement of the cryoprobe within the nidus under CT guidance with ablation performed for the desired period and desired coverage. Clinical success was defined as significant relief of symptoms without the use of pain medication and return to normal activities within 1 month after the procedure on the assumption that the effect of ablation would be observed within 4 weeks of the procedure. The degree of pain relief was evaluated using a visual analogue scale (VAS) from 1 (no pain) to 10 (extreme pain). Patients were evaluated for pain severity with a VAS at 3 days, 7 days, and 1 month post-procedure in addition to a pre-procedure VAS. The end point was defined as the 1 month VAS score. The intermediate time point VAS scores were used to provide information on the more immediate post-procedural time period. Major complications were defined as complications that resulted in an unplanned increase in the level of care, prolonged hospitalization, permanent adverse sequelae, or death. Minor complications were defined as complications that resulted in no sequelae.
Statistical analysis
Analysis of the pain severity end point was done with paired comparison of VAS values involving paired t tests comparing baseline with subsequent post-procedure time points (3 days, 7 days, and 1 month). All significance tests were two-sided, and P values less than 0.05 were considered statistically significant. Data processing and analysis were performed with Chinese Health Information Standardization Society (CHISS) statistical software (version 3.0).
Results
Results of the cryoablation are summarized in the Table 1. Percutaneous cryoablation was technically successful in all six patients. The mean time of cryoablation was 18 min (range 12–25 min) for each procedure. There were three cases where the cryoablation zone (ice ball) could be partially visualized within the soft tissues because of a superficial location of the osteoid osteoma and a portion of the active cryoprobe tip was situated within the adjacent soft tissues. The entire procedure duration ranged from 70 to 110 min (mean 90 min). The mean length of in-hospital stay for each admission was 3.5 days (range 3–5 days). All patients tolerated full weight-bearing and could function without limitation within 3 days after the procedure without need for casts or crutches. There were no fractures, hematomas, myoglobinuria, or neurovascular injuries during or immediately after the procedure. One minor complication was observed in a patient who demonstrated moderate fever, discomfort, and had body core temperature of 38°C for 1 day after the operation. These symptoms were relieved after 2 days of physical cooling and administration of acetylsalicylic acid. Significant pain relief was observed in all patients after the ablation. The VAS score averages were 6.57 ± 0.55 pre-procedure, 3.24 ± 0.23 on day 3 post-procedure, 1.54 ± 0.14 on day 7 post-procedure, and 0.57 ± 0.10 on day 30 post-procedure. The results of the t-test showed a statistically significant difference (P < 0.05) between the VAS of patients pre-procedure, and those at 3 days, 1 week, and 1 month post-procedure. The clinical success rate was also 100%, and for all six patients there was no evidence of recurrent symptoms at latest follow-up. No delayed complications (e.g., infection or myoglobinuria) were observed during the follow-up period. No skin changes were identified in the subjects who had superficial lesions treated. No major complications were encountered. For each patient, MRI after the procedure showed an area of signal alteration corresponding to the cryoablation zone (Fig. 5). These post-ablation MRIs depicted no complications that resulted from the procedure. No stress fractures were observed during the 18–36 month follow-up.
Discussion
We report a case series of six children with osteoid osteoma who were treated successfully, technically and clinically, using percutaneous image-guided cryoablation with intermediate term relief of pain symptoms and no major complications. Prior communications of cryoablation for osteoid osteoma consist of case reports [18, 19]. In one case report there was a pediatric patient with a femoral lesion who was treated with cryoablation under general anesthesia using CT guidance and remained symptom free at 19 months of follow-up [19]. Our series supports these results and extends this work by demonstrating that these procedures can be done without general anesthesia while producing a sustained effect. The post-procedure MRI appearance of cryoablation-treated osteoid osteomas corresponds to that described in the literature for radiofrequency-treated ones [20] with the lesion showing a target-like appearance of the central ablated zone surrounded by a well demarcated band. In our patients, since the scans were done 6 months after the procedure we did not observe a peripheral area of edema, consistent with the time course of edema resolution described post-radiofrequency ablation.
Due to improvements in imaging guidance and focal ablative therapies, the surgical treatment of osteoid osteomas has been replaced by percutaneous treatments. CT-guided percutaneous radiofrequency ablation of osteoid osteoma has high technical and clinical success rates [2, 6, 8–10]. Cryoablation, however, may be advantageous for treating osteoid osteomas. Cryoablation is well tolerated by patients under conscious sedation, and there is anecdotal evidence that they do not experience increased intra/periprocedural pain as radiofrequency ablation patients do [21–23]. The soft tissue extent may be monitored during the procedure with intermittent noncontrast CT, while the ablation zone for radiofrequency is not directly visible under CT monitoring. This capability is even better using MRI guidance where the entire ice ball is seen as a signal void on all pulse sequences. An advantage of cryoablation is the ability to actively control the extent of the ice ball to minimize collateral damage to critical structures. There are some disadvantages of cryotherapy compared to radiofrequency ablation. Cryotherapy takes longer because of the duration of freeze-thaw cycles required. The ablation zones tend to be larger with cryotherapy given the ice-ball configuration.
Cryoablation does require some protective measures but does not use a grounding pad as in radiofrequency ablation. Warming of the skin is needed, especially to manage lesions near the subcutaneous tissues. The potential hazard of skin frostbite is avoided by using an aseptic warm water/saline bag/glove, which is placed on the skin close to the puncture area during the procedure, and a small amount of filtered sterile gas injected into the subcutaneous tissues. The approach and targeting of lesions are similar between cryoablation and radiofrequency ablation. Cryoprobes, initially large gauge needles, are now available with smaller 17 gauge (1.47 mm) diameters and may be placed co-axially through a cannulated bone biopsy needle. A single cryoprobe can generate an “ice ball” with about 2.0 cm diameter and up to 3.5 cm length, large enough to result in complete ablation of an osteoid osteoma nidus (typically < 1.5 cm); the effective “kill zone” is up to 3 mm from the ice-ball outer margin. Also, as opposed to the inductive nature of radiofrequency heat generation, the conductive nature of heat dissipation from the probe itself with cryoablation does not necessitate direct placement of the needle within the nidus to destroy the lesion. A theoretical advantage of cryoablation is the preservation of extracellular collagen matrix, which may allow treatment of subarticular lesions near the chondral surface [24].
Further studies of cryoablation using MRI guidance may be useful to distinguish the relative benefit for difficult to treat lesions near critical structures as demonstrated for other musculoskeletal lesions by Tuncali et al. [17]. With clear demonstration of the ice ball margin on MRI, osteoid osteomas may be treated confidently and with greater safety in relative proximity to and while sparing adjacent critical structures, such as large nerves or vessels. MRI guidance uses nonionizing radiation and is favorable for the pediatric population with regards to biosafety. With the availability of smaller probes, preclusion of general anesthesia, the inherent risks of radiofrequency ablation, and the ability to monitor and control the ablative zone, MRI-guided cryoablation for osteoid osteomas warrants further consideration, especially in children and for lesions at risk of adverse sequelae because of precarious location.
Our study was limited because of a small number of patients and lack of direct comparison to radiofrequency ablation. The follow-up period was relatively short although within the time course for typical osteoid osteoma recurrences [9, 10]. We used a retrospective study design and therefore did not gather all detailed information to include all the osteoid osteoma patients treated with cryoablation. Our ablations were done as inpatient procedures, but many ablation procedures are performed as outpatient procedures or as same day admissions helping to control costs. It is possible that cryoablation may also be performed in these settings; however, the common practice in the hospital where this research was done is to perform ablations as an inpatient procedure, which is related to logistical and cultural issues and not inherent to the cryoablation therapy. We chose to use histology as a reference standard of this study, however in clinical practice a decision may be made to treat osteoid osteoma on the basis of characteristic clinical presentation and imaging appearance given that these lesions may be painful to biopsy and the histology may be indeterminate.
Conclusions
Our findings support that percutaneous cryoablation is a safe and effective treatment for osteoid osteomas in children that are refractory to medical therapy. Notably, this procedure can be accomplished without general anesthesia.
References
Kransdorf MJ, Stull MA, Gilkey FW, Moser Jr RP. Osteoid osteoma. Radiographics. 1991;11(4):671–96.
Rosenthal DI, Hornicek FJ, Wolfe MW, Jennings LC, Gebhardt MC, Mankin HJ. Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg Am. 1998;80(6):815–21.
Gangi A, Alizadeh H, Wong L, Buy X, Dietemann JL, Roy C. Osteoid osteoma: percutaneous laser ablation and follow-up in 114 patients. Radiology. 2007;242(1):293–301.
Towbin R, Kaye R, Meza MP, Pollock AN, Yaw K, Moreland M. Osteoid osteoma: percutaneous excision using a CT-guided coaxial technique. AJR Am J Roentgenol. 1995;164(4):945–9.
Sierre S, Innocenti S, Lipsich J, Lanfranchi L, Questa H, Moguillansky S. Percutaneous treatment of osteoid osteoma by CT-guided drilling resection in pediatric patients. Pediatr Radiol. 2006;36(2):115–8.
Peyser A, Applbaum Y, Simanovsky N, Safran O, Lamdan R. CT-guided radiofrequency ablation of pediatric osteoid osteoma utilizing a water-cooled tip. Ann Surg Oncol. 2009;16(10):2856–61.
Moser T, Giacomelli MC, Clavert JM, Buy X, Dietemann JL, Gangi A. Image-guided laser ablation of osteoid osteoma in pediatric patients. J Pediatr Orthop. 2008;28:265–70.
Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology. 2003;229(1):171–5.
Cioni R, Armillotta N, Bargellini I, Zampa V, Cappelli C, Vagli P, et al. CT-guided radiofrequency ablation of osteoid osteoma: long-term results. Eur Radiol. 2004;14(7):1203–8.
Sung KS, Seo JG, Shim JS, Lee YS. Computed-tomography-guided percutaneous radiofrequency thermoablation for the treatment of osteoid osteoma—2 to 5 years follow-up. Int Orthop. 2009;33(1):215–8.
Donkol RH, Al-Nammi A, Moghazi K. Efficacy of percutaneous radiofrequency ablation of osteoid osteoma in children. Pediatr Radiol. 2008;38(2):180–5.
Vanderschueren GM, Taminiau AH, Obermann WR, van den Berg-Huysmans AA, Bloem JL. Osteoid osteoma: factors for increased risk of unsuccessful thermal coagulation. Radiology. 2004;233(3):757–62.
Rubinsky B. Cryosurgery. Annu Rev Biomed Eng. 2000;2:157–87.
Baust JG, Gage AA, Clarke D, et al. Cryosurgery—a putative approach to molecular-based optimization. Cryobiology. 2004;48(2):190–204.
Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology. 1998;37(3):171–86.
Callstrom MR, Atwell TD, Charboneau JW, Farrell MA, Goetz MP, Rubin J, et al. Painful metastases involving bone: percutaneous image-guided cryoablation—prospective trial interim analysis. Radiology. 2006;241(2):572–80.
Tuncali K, Morrison PR, Winalski CS, Carrino JA, Shankar S, Ready JE, et al. MRI-guided percutaneous cryotherapy for soft-tissue and bone metastases: initial experience. AJR Am J Roentgenol. 2007;189(1):232–9.
Skjeldal S, Lilleås F, Follerås G, Stenwig AE, Samset E, Tillung T, et al. Real time MRI-guided excision and cryo-treatment of osteoid osteoma in os ischii—a case report. Acta Orthop Scand. 2000;71(6):637–8.
Liu DM, Kee ST, Loh CT, McWilliams J, Ho SG, Brower JS, et al. Cryoablation of osteoid osteoma: two case reports. J Vasc Interv Radiol. 2010;21(4):586–9.
Lee MH, Ahn JM, Chung HW, Lim HK, Suh JG, Kwag HJ, et al. Osteoid osteoma treated with percutaneous radiofrequency ablation: MR imaging follow-up. Eur J Radiol. 2007;64(2):309–14.
Callstrom MR, Kurup AN. Percutaneous ablation for bone and soft tissue metastases—why cryoablation? Skeletal Radiol. 2009;38(9):835–9.
Moser T, Buy X, Goyault G, Tok CH, Irani F, Gangi A. Image-guided ablation of bone tumors: review of current techniques. J Radiol. 2008;89(4):461–71.
Ullrick SR, Hebert JJ, Davis KW. Cryoablation in the musculoskeletal system. Curr Probl Diagn Radiol. 2008;37(1):39–48.
Bischof JC. Quantitative measurement and prediction of biophysical response during freezing in tissues. Annu Rev Biomed Eng. 2000;2:257–88.
Conflict of interest
The authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, B., Xiao, YY., Zhang, X. et al. CT-guided percutaneous cryoablation of osteoid osteoma in children: an initial study. Skeletal Radiol 40, 1303–1310 (2011). https://doi.org/10.1007/s00256-011-1119-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-011-1119-1