Abstract
Fluoride contamination in India’s groundwater is nowadays widespread, with serious health consequences. We collected 205 water samples from both rural and urban areas of the Dhar district in Madhya Pradesh, India, to determine fluoride concentrations in drinking water supplies. According to the hydrochemical data, most values are over the acceptable limit of drinking water standards. Fluoride concentration in the Dhar district vary from 0.01 to 4.75 mg/L, including a mean of 0.50 mg/L. The health risk assessment by fluoride contamination was calculated using estimated daily intake (EDI) and the hazard quotient (HQ). Adults have a variation in EDI and HQ of 0.00–0.15 and 0.01–2.54 mg/kg/day, respectively. Teenagers have a variation of 0.00–0.19 and 0.01–2.17 mg/kg/day, children have a variation of 0.00–0.27 and 0.01–4.49 mg/kg/day, and infants have a variation of 0.00–0.04 mg/kg/day. Our studies revealed that among hydrochemical types in the Dhar district are mostly of Ca2+–HCO3¯ and Ca2+–Cl¯ types, whereas samples with HCO3¯–Na+ and SO42¯–Cl¯–Na+ have high fluoride content. The characteristics of groundwater in Dhar are dominated by weathering of rocks. Proper groundwater monitoring and mitigation are thus required in the research region to protect residents from various diseases associated with drinking water with different fluoride levels. Using appropriate treatment technologies to reduce or remediate fluoride from drinking water, such as adsorption and electrocoagulation, would lessen the health hazards associated with groundwater fluoride exposure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, the rapid industrialization and population growth are placing more strain on natural resources, making their preservation one of the most significant challenges facing humanity. Groundwater is the main source of water used for drinking, agriculture, manufacturing, and recreation. In dry and semi-arid regions with inadequate surface water resources, groundwater is a suitable substitute (Huan 2020). About 20% of the world’s freshwater resources are found in groundwater, which serves as the key drinking water source in many countries (Chen 2017). According to the fluoride concentration in drinking water, fluoride is a common inorganic pollutant and could be harmful to the general population (Golaki 2022). The occurrence of levels of fluoride is a global challenge, as exposure to fluoride-rich water adversely affects a large number of individuals worldwide (Jalili 2018). Natural waters primarily include fluoride in free ions, which are often created when minerals like (CaF2) dissolve in groundwater (Mukherjee & Singh 2018). In nations including India, Bangladesh, Pakistan, Kenya, South Africa, China, Thailand, Nigeria and Iran, fluoride-rich water has led to issues (Li 2013; Kaur 2020).
According to the World Health Organization (WHO 2011), fluoride in water is essential for maintaining good health and promoting the formation of teeth and bones, but if the concentration goes above a safe level of 1.5 mg/L, it can be hazardous (Chen 2017). One of the widely used methods for determining the potential dangers of both carcinogens and non-carcinogens to humans is HRA (Mohammadi et al. 2019). To ascertain the adverse effects of exposure to water contamination, Human Risk Assessment techniques can be used (Ghoochani 2017). Human health risk assessment is defined by the United States Environmental Protection Agency (US EPA) as a methodical way of evaluating the possible health impacts of exposure to certain dangerous compounds in contaminated ecological systems, such as water resources (EPA 2011; Shalyari 2019). To put it another way, this approach is a scientific paradigm for quantitatively or semi-quantitatively quantifying the damaging effects of exposure to dangerous substances. This technique can be used to evaluate the danger of pollutants in various age groups.
Fluoride contamination of groundwater is a significant issue. It requires an understanding of the geochemical processes as well as the spatial distribution pattern of fluoride. The key governing factor of fluoride content in groundwater is the mineralogical structure of the underlying bedrocks (Yadav 2019). The presence of fluoride-bearing minerals in the bed rocks, such as fluorite, mica (biotite and vermiculite), cryolite, topaz, and fluoro-apatite, is mainly responsible for the presence of fluoride levels in groundwater (Dehbandi 2018).
According to a new study, India has a high amount of groundwater fluoride, which has been linked to detrimental health impacts (Nizam et al. 2022). The maximum permissible limits for F were determined to be 1.5 mg/L by WHO and Indian standards, and it was discovered that states like Madhya Pradesh (5.98 mg/L), Punjab (4.67 mg/L), Chhattisgarh (3.80 mg/L), Rajasthan (3.30 mg/L), Karnataka (2.56 mg/L), Andhra Pradesh (2.03 mg/L), Maharashtra (1.70 mg/L), Haryana (1.65 mg/L), Telangana (1.64 mg/L), Uttar Pradesh (1.63 mg/L), and Bihar (1.54 mg/L). All of these high levels have a clear connection to the aquifers’ geology and to dry and semi-arid areas (Ali et al. 2019). Concerns about the level of fluoride in drinking water arose as a result of these findings, causing researchers to look into remediation methods. It is obvious that modelling the long-term health effects of fluoride as well as reporting its concentration in drinking water is necessary to develop strategies to prevent its adverse effects on health (Dehbandi 2018). The health risk assessment is an excellent approach for calculating and evaluating the probability of health impacts from fluoride and other pollutants. Various health risk evaluation cases have used the health risk assessment methodology.
Madhya Pradesh is one of Central India’s largest states. Dhar is one of the districts in Madhya Pradesh that has been affected by fluoride in groundwater. It is a district of southern Madhya Pradesh where about 82% of the population belong to the Scheduled Tribes. According to the 2011 census, 21,84,672 people live in the district, out of which 11,14,267 are men and 10,70,405 are women. Population growth is occurring at the rate of 25.53%. However, past studies of fluoride in groundwater in the region have mainly focused on a few small local region or cover only geological aspects of that particular area (Guha 1983; Khosla 2003; Kumar 2018; Umak 2022), and to date, no study has attempted to characterize the hydrogeochemistry, correlation with its competing ions and human health risk assessment of groundwater across the whole Dhar district.
In the Dhar district, groundwater is the primary source for their people to drink and other basic requirements. As a result, various studies must be conducted to evaluate the quantity and quality of underground water in different Indian regions. This means that groundwater’s hydrochemical properties can show whether or not the resources are chemically unfit for drinking (Srinivas 2015). Also, some spatial maps of fluoride contamination and health quotient for infants, children, teenagers and adults and hazard quotient are present which could determine the risk of contaminated water and subsequently health risk in various age groups all over the study area (Sheikhy Narany 2014).
Therefore, the current study’s objectives are to ascertain the spatial distribution of fluoride-contaminated groundwater, its geological aspects, understand the fluoride hydro geochemistry, correlation with its competing ions as well as the status and potential health risks assessment in Dhar district. The possible outcome of this study will be helpful for respective regional organizations, NGOs, and government bodies to provide effective measures to mitigate groundwater pollution definitely beneficial for the residents of the Dhar region. This study is carried out in the month of January 2022 in Dhar district, M.P, India.
Materials and methods
Study area
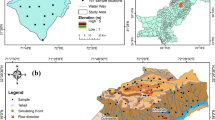
The Dhar district is located in Madhya Pradesh’s south-western region as shown in Fig. 1. It is situated between the parallels of East longitude 74°28ʹ15ʺ and 75°42ʹ43ʺ E and North latitude 22o01ʹ14ʺ and 23o08ʹ49ʺ. West Nimar (Khargone) in the south, Ratlam in the north, Jhabua in the west and Ujjain in the northeast define the district’s boundaries. The city of sword blades, Dhara Nagri, is where the name Dhar originated. The district is covered by topo-sheets nos. 46J, 46M, and 46N by the Survey of India.
Sample collection
In the Dhar district, 9 tehsils (Badnawar, Dahi, Dhar, Dharampuri, Gandhwani, Kukshi, Manawar, Pithampur, and Sardarpur), 205 groundwater samples were collected. Total hardness (TH) as CaCO3, sodium (Na+), potassium (K+), calcium (Ca2+), magnesium (Mg2+), chloride (Cl−), nitrate (NO3−), sulphate (SO42−), and fluoride (F−) are only a few of the hydrochemical parameters that were examined. Onsite physico-chemical parameters like pH, EC, and TDS, were measured using the Hanna multiparameter (model no. HI-98494). Major cations (Na+, K+, Ca2+, and Mg2+) and anions (F¯, Cl¯, SO42¯, NO3¯) were determined in the samples using ion chromatography, and for HCO3¯, a titration method is used. The table shows the various location along with the tested parameters can be viewed in ESI (Table S1†).
To represent the spatial data, we are using Piper trilinear diagram and Gibb’s plot by Rockware’s AqQa 1.5.0 and to produce geospatial maps, ArcGIS 10.4 were used respectively.
Geology and fluoride contamination
Geological mapping in the Dhar district has indicated that the rocks of the gneissic complex, Bagh Group, Aravali supergroup, and Deccan Trap are exposed, as illustrated in Fig. 2A. The current study is centred on the Bagh Group of sediments. The Bagh group rocks are found as discontinuous outcrops that lie unconformably on top of the Archean rocks. These are largely visible in the middle of Toposheet 46J/15, near Khojakuan and Ajantar (Khosla 2003). Bagh beds are divided into three units that are horizontally arranged: bottom Nimar Sandstone/red Shale, middle Nodular Limestone, and high Coralline Limestone. The Deola Marl deposit revealed at Sitapuri and Chirkhan is the bottom section of the Coralline limestone. The thickness of Bagh beds, on the other hand, ranges from a few metres to 20 m (Umak 2022).
The oldest litho-units are part of the Aravali supergroup, which is more than 2500 million years old and includes the Champaner, Lunavada, and Udaipur groups. The rocks of the Aravali super group are unconformably layered beneath the Bagh group, which is lower Cretaceous in age and 110 million years old. At the bottom, there is Nimar sandstone, followed by calcareous units made up of Coralline and nodular limestone (Kumar 2018).
The Malwa group of Deccan volcanic basalt flows, which date to the late Cretaceous Palaeogene (68–62 million years ago), cover a significant portion of the district. There are 38 basaltic flows in this, totalling 610 m in thickness. The rock is grained from fine to medium range, aphyric, and porphyritic moderately and having plagioclase phenocryst (Guha 1983). The Narmada lineament runs parallel to the dykes, which typically trend ENE-WSW to E-W.
The spatial distribution map of fluoride-contaminated groundwater in Dhar was obtained in the study using ArcGIS 10.4 and the inverse distance weighting (IDW) interpolation method, as shown in Fig. 2B. The majority of the time, the fluoride content of GW is less than 1.5 mg/L, and there is no detectable regularity in the distribution characteristics. Most of the district’s lower region’s groundwater sources have a comparatively high fluoride content.
Health risk assessment
Health risk assessment is the one of the most crucial risk indicator for humans is the fluoride in drinking water. The framework outlined in this study was based on the current WHO guideline (1.5 mg/L) of drinking water for assessing the health risk of fluoride contaminant in water (WHO 2011). As a result, the levels of fluoride in this study was included in the final health risk assessment following the guidelines of World Health Organisation (WHO 2017). GIS has primarily been used in environmental epidemiology. This software is a handy tool for identifying spatial relationships between environmental parameters and human health effects (Abbasnia 2018).
In recent research, fluoride concentration was used to assess health risks using GIS software. Non-carcinogenic health hazards can be estimated by comparing an exposure level over a defined time period to a reference dose acquired over a comparable time period. Various techniques have been employed to evaluate the potential non-carcinogenicity of contaminants for health risk assessment (Karim 2011; Chen 2017). These techniques are used to determine HQ for both individual and combined contaminants (Zabin 2008; Abouleish 2016).
In this research, the guidelines of US-EPA were followed when investigating the health issues of fluoride in drinking water (EPA 2011). Health risk evaluations for Infants, children, teens, and adults have been calculated using water consumption and body weight (Yousefi 2018; Asgari et al. 2019). As a consequence, the quantitative HRA of fluoride in drinking water intake in the Dhar district population was estimated. To accomplish this objective, we divided the population into categories of four age group depending on their physiological or behavioural characteristics, namely infants of age 2 years, children having age from 2 to 6 years, teens with age 6–16 years, and adults for age over 16 years (Ghoochani 2017). The expected daily consumption of fluoride in drinking water in different age groups was computed using Eq. (1):
Cf and Cd represent the daily average drinking water consumption and fluoride content in drinking water, respectively. The EDI unit is mg/kg/day, and Bw refers for body weight (Kg). Data on water consumption and body weight were collected using a questionnaire. Infants, children, teens, and adults consumed 0.08, 0.85, 2, and 2.5 L of water each day, respectively. Body weights of 10, 15, 50, and 78 kg were chosen for infants, children, teens, and adults, respectively. The non-carcinogenic HRA of fluoride concentration to human health can be represented as HQ using Eq. (2):
where RfD denotes the reference dose (mg/kg/day). The RfD is an estimate of the human population’s daily exposure. The HQ is the ratio of the EDI to the RfD. When HQ is less than 1, the health of human caused by contamination is acceptable, when HQ is greater than 1 exhibit that the HRA is unacceptable (USEPA 1991). Finally, the ArcMap 10.4 software-based project GIS was designed to incorporate various levels of information.
Quality control/quality assessment QC/QA
Every single sample has been examined for ions thrice, and the resulting mean was used to analyse the final outcomes. The parameters were evaluated using the ionic charge balance error (ICBE) method described in (Rehman et al. 2022). The ICBE findings were within ± 5%, indicating that the data is result-driven and accurate. According to the verified value of the standard reference material, the recovery percentage of the F¯ via ISE (HANNA) instrument was 97%.
Results and discussion
Chemical compositions of water
Groundwater typically contains a variety of chemical constituents and travels through the soils (via pore space) and by the fractures of rocks, depending on the geochemical circumstances and lithological basis (Adimalla 2019). It is critical to determine the properties and chemical conformations of groundwater exist in an aquifer. Table. 1. shows various parameters that were investigated in the water samples collected from the various regions in the study area. The various parameters from groundwater samples should be in ranges as suggested by (WHO 2017) and (BIS 2012). The collected samples had a pH range of 6.90–8.45, with an average value of 7.56; Manawar block had the lowest pH and Kukshi block had the highest. The geospatial distribution of various indices such as TDS, TH, Na+, K+, Ca2+, Mg2+, HCO3¯, CO32¯, SO42¯, Cl¯ and F¯ based on IDW interpolation shown in Fig. 3. TDS and electrical conductivity are both important factors for determining the quality of water; high TDS water, on the other hand, is unfit for human consumption owing to health dangers and is also unsuitable for cooking due to differences in flavour. In Umarban, the EC was reported to be 300 µS/cm at its lowest and 2615 µS/cm at its highest. As can be seen from Table 1, majority of the water samples that were collected, or 93.17 per cent, met the EC’s permissible standards, however calcium carbonate concentrations in water was found in range from 50 to 795 mg/L (mean: 286 mg/L). Additionally, the Dharmapuri area had the highest total hardness, while the sardarpur area had the lowest where the total hardness in water samples was primarily derived from the magnesium and calcium ions. All of the collected water samples (98.54 per cent) had calcium content below the established permissible limits, with the exception of the Dhar water sample (16.00 mg/L), which had a higher calcium ion concentration of 253.47 mg/L (mean is 67.08 mg/L). Concentration of magnesium contained from 1.24 to 142.33 mg/L (mean is 24.17 mg/L), with Tirla water having the lowest Mg2+ concentration and Sardarpur area water having the highest of all the samples collected, 97.56 per cent of water samples fell below the allowable limit for Mg2+ concentration. When water percolates through the soil profile and contacts calcite and dolomite, the Ca2+ and Mg2+ are transferred from the soil profile into the groundwater. The average concentrations of Na+ and K+ in the water sample were 69.71 and 184 mg/L, respectively, with standard deviations of 20.33 mg/L. The groundwater in Badnawar had the highest potassium content, while the Umarban surface water had the lowest sodium ion concentration, while potassium was absent from some water samples. According to the Bureau of Indian Standards, all collected samples had sodium levels below the allowable limit and nearly 96.4 per cent of the water samples had potassium levels below the allowable limit for drinking.
Anionic content such as HCO3¯, CO32¯, SO42¯, Cl¯ and F¯ were also determined to assess the purity of water for drinking reasons, and the anions dominance was reported in the following order.: HCO3¯ > Cl¯ > SO42¯ > CO32¯ but CO32¯ was not detected in water because it gets precipitated easily. With a mean value of 298.58 mg/L, the bicarbonate content of the water samples varied between Bagh (37 mg/L) and Sardarpura (755 mg/L). The primary silicate minerals underwent intense chemical weathering due to the high concentration of HCO3¯ in the water. High levels of bicarbonate in the water are caused by the weathering of minerals having carbonate and silicate as the breakdown of organic waste, which releases HCO3¯ (Sahu 2021). In water samples, the amounts of Cl¯ and SO42¯ ranged from 10 to 404 mg/L (average: 82.13 mg/L) and 2–260 mg/L (mean: 43.28 mg/L), respectively.
Groundwater samples from the Sardarpur and Kuksi areas had higher Cl¯ and SO42¯ contents, but all of the samples of water were found to be below the allowable limit for both contaminants. F¯ concentrations in the water samples varied from 0.01 to 4.75 mg/L, with a mean value of 0.50 mg/L. The surface water of Manawar had the lowest fluoride content, while the groundwater of Dharmapuri had the highest. The majority of the 87.32 per cent of water samples tested were safe to drink, with the exception of a few samples with fluoride levels that were higher than allowed by standards and could be harmful to health if consumed regularly.
Hydrogeochemical facies
This is characterized by the predominance of major ions in groundwater. Human health is compromised when some major ions exceed their permissible limits. Water consist with concentration of sodium more than 50 mg/L is unfit for drinking perspective as it can cause some major health problems such as nausea and vomiting, headache, loss of energy, drowsiness, and fatigue (Karunanidhi 2021). The HCO3¯ has no effects on human health but is beyond permissible limit in water as it can cause some stones in the kidney due to higher Ca2+ concentrations, particularly in dry climates (Patnaik 2002; S. A. Ali & Ali 2018). The Piper trilinear diagram has been widely used by numerous researchers to comprehend issues regarding the geochemical evolution of groundwater (Ravikumar & Somashekar 2015). A collection of ion analyses is graphically represented in a single plot.
The several groundwater facies aids in understanding the chemical processes that occur among minerals and their effects on groundwater. The concentrations of major dissolved ions have a clear relationship with aquifer properties (Lokhande 2016). Several variables influence groundwater quality, including the composition of recharge water, the geological structure and mineralogy of watersheds and aquifers, as well as the residence time and reactions that occur inside the aquifer. The combination of all components results in diverse water kinds. Improved understanding of the geochemical evolution of groundwater quality might lead to a better understanding of hydro-chemical processes in such places, resulting in more sustainable water resource development and effective groundwater resource management. The location of the samples on the figure indicates that the weathering of rocks, rather than evaporation or precipitation, has an enormous effect on water–rock interaction as a key source of dissolved ions (Hamzaoui-Azaza et al. 2011). A Piper trilinear diagram (Fig. 4) was created using hydrogeochemical analysis (Piper 1944). The Piper diagram categorizes water samples into six significant fields that are (i) Ca2+–HCO3¯ type, (ii) Ca2+–Cl¯ type, (iii) Na+–HCO3¯ type, (iv) Na+–Cl¯ type, and (v) Ca2+–Mg2+–Cl¯ type (Kumar 2013). Ca2+ is the dominant cation in all samples, and Cl¯ is the dominant anion, followed by bicarbonate. Ca2+–Cl¯ and Ca2+–HCO3¯ have significant interactions. The Ca2+–Cl¯ field contains the maximum of the samples of water. The remaining samples are Ca2+–Mg2+–Cl¯ and Ca2+–HCO3¯ in nature. In the piper plot, the diamond region shows that alkaline earth metal (Ca2+ + Mg2+) exceeds the number of alkalies (K+ + Na+) and strong acidic anion (Cl¯ + SO42¯) out numbers weak acidic anions (CO32¯ + HCO3¯). The Ca2+–Mg2+–Cl¯ and Ca2+, Mg2+ ion dominant type of water type represents most of the samples.
The Gibbs diagram is a tool for understanding groundwater geochemistry (Gibbs 1970). It can give insights into water quality, the behaviour of contaminants, and the geological processes that define the subsurface environment by analysing the chemical stability of minerals in groundwater (Wang 2021). In the Gibbs diagram, the ratio I (Na+/Na+ + Ca2+) for cations and ratio II (Cl¯/Cl¯ + HCO3¯) for anions were plotted against the relative values of TDS to determine the mechanism identifying the composition of the groundwater, with ion concentrations expressed in meq/L (Li 2018). As shown in Fig. 5, most samples fall within the dominating range of rock dominance, demonstrating that rock weathering is the primary source of hydrochemical composition. It shows that rock weathering is the primary factor controlling the hydrochemical properties of groundwater in Dhar. The figure illustrates that rock weathering is the most significant process impacting groundwater quality in the studied region, and that the mechanism governing groundwater chemistry in the Deccan trap is rock-water interaction (Surjeet Singh et al. 2021). Additionally, river water intrusion and evaporation impacted only a few water samples.
The distribution of several groundwater sample points in this figure’s Na+/(Na+ + Ca2+) > 0.2 regions suggests that cation exchange may affect these groundwater samples. Higher fluoride concentrations (F > 2 mg/L) are present in the groundwater samples, but they are scattered in the Na+/(Na+ + Ca2+) > 0.5 zone (Liu 2021). This suggests that cation exchange is probably related to the fluoride in water.
Fluoride presence can be determined by the fluoride-bearing rock’s mineral composition, which includes fluorite, cyrolite, and fluorapatite. Equations (3)–(7) illustrate the hydrogeochemical processes involved in leaching the fluoride from the source rock. The rising fluoride ion concentration trend adds to the evidence that high fluoride concentration is the result of extensive evaporation (Ravikumar & Somashekar 2015). High F concentrations may be influenced by processes other than water–rock interactions and evaporation.
A signal of possible fluoride concerns could be found in the chemical composition of the groundwater. Fluorine can be leached out and dissolved in groundwater and steam gases during weathering and water transport in rocks and soils. The geological conditions and types of rocks have a significant effect on the fluoride concentration of groundwater (Surjeet Singh et al. 2021). Therefore, areas with the highest concentrations of these minerals in the host rocks are where fluoride issues are most likely to arise.
Correlation and water type
A correlation matrix is a statistical analysis and it was conducted to examine the relationship between the evaluated parameters in various water samples for the hydrochemistry evaluation (Ana Célia Maia Meireles 2010). The correlation matrix was displayed for many parameters, including pH, EC, TH, Ca2+, Mg2+, Na+, K+, HCO3¯, Cl¯, SO42¯, NO3¯ and F¯ in several samples of water. Table 2. demonstrates the statistical analysis of the physicochemical characteristics and ion concentrations. The average value was used to report all the studied data. According to the matrix, the first group included EC with TH, Ca2+, Mg2+, Na+, HCO3¯, Cl¯ and SO42¯ (0.78, 0.64, 0.62, 0.68, 0.69, 0.86 and 0.66) respectively have a very strong correlation. The second group included TH with Ca2+, Mg2+, NO3¯ (0.83, 0.76 and 0.68), respectively and the third group included Ca2+ with NO3¯ (0.62), and finally, the fourth group had Na+ with Cl¯ and SO42¯ (0.70 and 0.61), respectively. These findings demonstrated that evaporation and seasonal effects could impact water quality. Salt concentrations like sodium, chloride, and sulphate could be high during the dry season while bicarbonate concentration was highest during the rainy seasons. Sulphate and chloride significantly interacted with other parameters, as shown by the high correlation coefficient between water quality parameters. As a result of anthropological activities and natural materials like rock and soil, these parameters had increased amount of values (Abbasnia 2018). Alkaline water was created from water with a high HCO3¯ level, and by replacing OH¯ in the groundwater, it was able to mobilise fluoride from the minerals that held it (Rao 2008; Somvir Singh 2017; Li 2018; Jia 2019; S. Singh 2019; Sahu 2021).
Health risk assessment
On the basis of the results given in Fig. 6, the mean, minimum and maximum EDI values for fluoride in children were 0.03 and 0.27 mg/kg bw/d, respectively and values of mean and maximum HQ of fluoride were 0.48 and 4.49, respectively. The calculation of HQ and EDI of all age groups can be viewed in ESI (Table S1†). Infants, children, teens, and adults were the four age groups utilised to calculate the HQ by ingestion, dermal exposure, and overall hazard. The HQ ranged in infants from 0.00 to 0.63 with a mean of 0.07, children from 0.01 to 4.49 with a mean of 0.48, Teenagers from 0.01 to 3.17 with a mean of 0.34 and adults from 0.01 to 2.54 with mean 0.27 respectively. Hence, this shows that the HQ is higher in children than in other age groups which are clearly shown in Fig. 7. Due to the consumption of high fluoridated groundwater, it can also be deduced that children in the study area are more susceptible to non-carcinogenic human health risks than adults (Golaki 2022).
The risk of fluoride contamination posing a health risk was significantly high for infants and children than for male and female adults. In this regard, a survey was conducted in areas with high fluoride levels, including Himmatgarh (1.90 ppm), Shikarpura (2.08 ppm), Petlawad (7.15 ppm) and Musapura (3.70 ppm), and some cases of dental fluorosis were noted. It was discovered that those villages with increased amount of fluoride concentration in their water are hazardous to human health (Fig. 8). Through constant consumption of high fluoride concentration in drinking water, fluoride can accumulate in children’s developing brains and affect their capacity for learning and memory (Keesari 2016; Yousefi 2018; Zhang 2020a; Rashid 2020; Zhang 2020b).
Study limitation and future scope
The current study gives a detailed analysis of fluoride distribution in Dhar district groundwater, as well as related fluorosis and possible health risk to various age groups. However, the study lacks information on the visual health effect. As a result, local surveys have to be conducted to document the effect of groundwater on local inhabitants and animals as a result of varied ground fluoride consumption. Furthermore, the average groundwater fluoride content in this study area is equivalent to or greater than in the majority of India’s fluoride-endemic areas. As a result, immediate mitigation measures must be performed in places of severe and extremely significant danger, particularly among children. The implementation of extensive rainwater harvesting in the basin to recharge the groundwater will reduce the fluoride concentration is one of the recommended methods to prevent the risks of fluorosis. The local administration will lessen the danger of fluorosis by treating fluoride-rich groundwater before offering drinking water supplies.
Conclusion
The hydrogeochemical characteristics of water in Dhar were investigated, as well as the health risks associated with groundwater. The following are the main conclusions:
-
1.
In Dhar, fluoride concentrations in the water samples were present in a range from 0.01 to 4.75 mg/L, with a mean of 0.50 mg/L. A total of 13% (205 samples) of the samples of groundwater showed fluoride concentrations over the 1.50 mg/L WHO-2017 and IS-10500 (2012) guidelines. The key influential factors for groundwater fluoride are the dissolution of fluorite, apatite, and hornblende.
-
2.
Based on the existing geological formation, mostly due bagh group of sediments, the contamination of fluoride in groundwater was found beyond the permissible limits in the Dhar district.
-
3.
In Dhar, the hydrochemical kinds of groundwater are mostly Ca2+–HCO3¯ and Ca2+–Cl¯ types. Groundwater containing HCO3¯–Na+ and SO42¯.Cl¯–Na+ water types, on the other hand, has a high fluoride level. Weathering of rocks plays a conclusive role in the hydrochemical composition features of groundwater in Dhar. On the other hand, river water intrusion has a considerable impact on groundwater in the northern section of Dhar.
-
4.
The health risk assessment of the contaminants studied revealed that when the HQ was calculated separately for each contaminant, it was not greater than one in any of the age groups studied. As a result, it did not appear to pose a specific risk to any of the study groups.
-
5.
This study further discovered that more than 57% of the groundwater samples in the study area could be harmful to children’s dental fluorosis. Children with high IQs are more vulnerable, indicating that they are more prone than adults to fluoride-related health risks. According to the findings, proper groundwater monitoring, particularly for fluoride, is extremely important.
References
Abbasnia A (2018) Evaluation of groundwater quality using water quality index and its suitability for assessing water for drinking and irrigation purposes: case study of Sistan and Baluchistan province (Iran). Hum Ecol Risk Assess Int J 25(4):988–1005. https://doi.org/10.1080/10807039.2018.1458596
Abouleish MY (2016) Evaluation of fluoride levels in bottled water and their contribution to health and teeth problems in the United Arab Emirates. Saudi Dent J 28(4):194–202. https://doi.org/10.1016/j.sdentj.2016.08.002
Adimalla N (2019) Groundwater quality evaluation using water quality index (WQI) for drinking purposes and human health risk (HHR) assessment in an agricultural region of Nanganur, south India. Ecotoxicol Environ Saf 176:153–161. https://doi.org/10.1016/j.ecoenv.2019.03.066
Ali SA, Ali U (2018) Hydrochemical characteristics and spatial analysis of groundwater quality in parts of Bundelkhand Massif, India. Appl Water Sci. https://doi.org/10.1007/s13201-018-0678-x
Ali S, Fakhri Y, Golbini M, Thakur SK, Alinejad A, Parseh I, Bhattacharya P (2019) Concentration of fluoride in groundwater of India: a systematic review, meta-analysis and risk assessment. Groundw Sustain Dev. https://doi.org/10.1016/j.gsd.2019.100224
Asgari G, Ghalehaskar S, Kazemi Moghaddam V, Radfard M, Heidarinejad Z, Mohammadi AA, Faraji H (2019) Monitoring and health risk assessment of fluoride in drinking water in Babol, Mazandaran Province, Iran. Desalin Water Treat 165:141–147. https://doi.org/10.5004/dwt.2019.24553
BIS (2012) 10500, Indian Standard drinking water specification, second revision, Bureau of Indian Standard
Chen L (2017) Ion sieving in graphene oxide membranes via cationic control of interlayer spacing. Nature 550(7676):380–383. https://doi.org/10.1038/nature24044
Dehbandi R (2018) Geochemical sources, hydrogeochemical behavior, and health risk assessment of fluoride in an endemic fluorosis area, central Iran. Chemosphere 193:763–776. https://doi.org/10.1016/j.chemosphere.2017.11.021
EPA (2011) U.S. Exposure Factors Handbook 2011 Edition (Final report), Washington, DC, EPA/600/R-09/052F
Ghoochani M (2017) Risk assessment of haloacetic acids in the water supply of Tehran, Iran. Water Supply 17(4):958–965. https://doi.org/10.2166/ws.2016.189
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170:1088–1090
Golaki M (2022) Health risk assessment and spatial distribution of nitrate, nitrite, fluoride, and coliform contaminants in drinking water resources of kazerun, Iran. Environ Res 203:111850. https://doi.org/10.1016/j.envres.2021.111850
Guha A (1983) A correlation between the evolution of mineralizing fluids and the geomechanical development of a shear zone as illustrated by the Henderson 2 mine, Quebec. Econ Geol 78(8):1605–1618. https://doi.org/10.2113/gsecongeo.78.8.1605
Hamzaoui-Azaza F, Ketata M, Bouhlila R, Gueddari M, Riberio L (2011) Hydrogeochemical characteristics and assessment of drinking water quality in Zeuss-Koutine aquifer, southeastern Tunisia. Environ Monit Assess 174(1–4):283–298. https://doi.org/10.1007/s10661-010-1457-9
Huan H (2020) Groundwater nitrate pollution risk assessment of the groundwater source field based on the integrated numerical simulations in the unsaturated zone and saturated aquifer. Environ Int 137:105532. https://doi.org/10.1016/j.envint.2020.105532
Jalili D (2018) Data on Nitrate-Nitrite pollution in the groundwater resources a Sonqor plain in Iran. Data Brief 20:394–401. https://doi.org/10.1016/j.dib.2018.08.023
Jia H (2019) Fluoride occurrence and human health risk in drinking water wells from southern edge of Chinese Loess Plateau. Int J Environ Res Public Health 16(10):1683. https://doi.org/10.3390/ijerph16101683
Karim Z (2011) Health risk assessment of trihalomethanes from tap water in Karachi, Pakistan. J Chem Soc Pak 33(2):215–219
Karunanidhi D (2021) Groundwater pollution and human health risks in an industrialized region of southern India: impacts of the COVID-19 lockdown and the monsoon seasonal cycles. Arch Environ Contam Toxicol 80(1):259–276. https://doi.org/10.1007/s00244-020-00797-w
Kaur L (2020) Deterministic and probabilistic health risk assessment techniques to evaluate non-carcinogenic human health risk (NHHR) due to fluoride and nitrate in groundwater of Panipat, Haryana, India. Environ Pollut 259:113711. https://doi.org/10.1016/j.envpol.2019.113711
Keesari T (2016) Understanding the hydrochemical behavior of groundwater and its suitability for drinking and agricultural purposes in Pondicherry area, South India—a step towards sustainable development. Groundw Sustain Dev 2–3:143–153. https://doi.org/10.1016/j.gsd.2016.08.001
Khosla A (2003) Biodiversity during the Deccan volcanic eruptive episode. J Asian Earth Sci 21:895–908
Kumar PJS (2013) Interpretation of groundwater chemistry using piper and chadha’s diagrams: a comparative study from Perambalur Taluk. Elixir Geosci 54:12208–12211
Kumar S (2018) The age of the Nodular limestone formation (Late Cretaceous), Narmada Basin, central India. J Earth Syst Sci 127(8):1–7. https://doi.org/10.1007/s12040-018-1017-1
Li P (2013) Occurrence and hydrogeochemistry of fluoride in alluvial aquifer of Weihe River, China. Environ Earth Sci 71(7):3133–3145. https://doi.org/10.1007/s12665-013-2691-6
Li P (2018) Occurrence and health implication of fluoride in groundwater of loess aquifer in the Chinese Loess Plateau: a case study of Tongchuan, Northwest China. Expo Health 11(2):95–107. https://doi.org/10.1007/s12403-018-0278-x
Liu J (2021) An investigation into the hydrochemistry, quality and risk to human health of groundwater in the central region of Shandong Province, North China. J Clean Prod 282:125416. https://doi.org/10.1016/j.jclepro.2020.125416
Lokhande PB, Mujawar HA (2016) Graphic interpretation and assessment of water quality in the Savitri River Basin. Int J Sci Eng Res 7(3):1113–1123
Meireles ACM (2010) A new proposal of the classification of irrigation water. Revista Ciência Agronômica 41:349–357
Mohammadi AA, Zarei A, Majidi S, Ghaderpoury A, Hashempour Y, Saghi MH, Alinejad A, Yousefi M, Hosseingholizadeh N, Ghaderpoori M (2019) Carcinogenic and non-carcinogenic health risk assessment of heavy metals in drinking water of Khorramabad, Iran. MethodsX 6:1642–1651. https://doi.org/10.1016/j.mex.2019.07.017
Mukherjee I, Singh UK (2018) Groundwater fluoride contamination, probable release, and containment mechanisms: a review on Indian context. Environ Geochem Health 40(6):2259–2301. https://doi.org/10.1007/s10653-018-0096-x
Nizam S, Virk HS, Sen IS (2022) High levels of fluoride in groundwater from Northern parts of Indo-Gangetic plains reveals detrimental fluorosis health risks. Environ Adv 8:100200. https://doi.org/10.1016/j.envadv.2022.100200
Patnaik KN, Satyanarayana SV, Rout SP (2002) Water pollution from major industries in Paradip area—a case study. Indian J Environ Health 44(3):203–211
Piper AM (1944) A graphic procedure in the geochemical interpretation of water- analyses. Eos Trans Am Geophys Union 25(6):914–928. https://doi.org/10.1029/TR025i006p00914
Rao NS (2008) Fluoride in groundwater, Varaha river basin, Visakhapatnam district, Andhra Pradesh. India Environ Monitor Assess 152(1–4):47–60. https://doi.org/10.1007/s10661-008-0295-5
Rashid A (2020) Geochemical modeling, source apportionment, health risk exposure and control of higher fluoride in groundwater of sub-district Dargai, Pakistan. Chemosphere 243:125409. https://doi.org/10.1016/j.chemosphere.2019.125409
Ravikumar P, Somashekar RK (2015) Principal component analysis and hydrochemical facies characterization to evaluate groundwater quality in Varahi river basin, Karnataka state. India Appl Water Sci 7(2):745–755. https://doi.org/10.1007/s13201-015-0287-x
Rehman F, Siddique J, Shahab A, Azeem T, Bangash AA, Naseem A, Rehman Q (2022) Hydrochemical appraisal of fluoride contamination in groundwater and human health risk assessment at Isa Khel, Punjab, Pakistan. EnvironTechnol Innov. https://doi.org/10.1016/j.eti.2022.102445
Sahu S (2021) Groundwater solute chemistry, hydrogeochemical processes and fluoride contamination in phreatic aquifer of Odisha, India. Geosci Front 12(3):101093. https://doi.org/10.1016/j.gsf.2020.10.001
Shalyari N (2019) Health risk assessment of nitrate in groundwater resources of Iranshahr using Monte Carlo simulation and geographic information system (GIS). MethodsX 6:1812–1821. https://doi.org/10.1016/j.mex.2019.07.024
Sheikhy Narany T (2014) Groundwater irrigation quality mapping using geostatistical techniques in Amol-Babol Plain, Iran. Arabian J Geosci 8(2):961–976. https://doi.org/10.1007/s12517-014-1271-8
Singh S (2019) Development of indices for surface and ground water quality assessment and characterization for Indian conditions. Environ Monit Assess 191(3):182. https://doi.org/10.1007/s10661-019-7276-8
Singh S, MondalSingh NCVS (2017) Groundwater quality in and around Tuticorin town, Southeast coast of India. J Indian Geophys Union 21(1):34–43
Singh S, Sharma P, Mudhulkar R, Chakravorty B, Singh A, Sharma SD (2021) Assessment of hydrogeochemistry and arsenic contamination in groundwater of Bahraich District, Uttar Pradesh, India. Arabian J Geosci 15(1):1–18. https://doi.org/10.1007/s12517-021-09222-5
Srinivas R (2015) Groundwater quality assessment in some selected area of Rajasthan, India using fuzzy multi-criteria decision making tool. Aquat Proc 4:1023–1030. https://doi.org/10.1016/j.aqpro.2015.02.129
Umak DK, Rastogi SK, Das IC (2022) Spatial data analysis for ground water quality assessment with special reference to fluoride—a case study of Dhar District, Madhya Pradesh, India. Int J Chem 14(1):8
USEPA (1991) United Nation Environmental Protection Agency: environmental risk, your guide to analysis and reducing risk rush, no. 905/9-91-017, pp. 905
Wang Z (2021) Hydrochemistry characters and hydrochemical processes under the impact of anthropogenic activity in the Yiyuan city, Northern China. Environ Earth Sci 80(2):1–19. https://doi.org/10.1007/s12665-020-09361-0
WHO (2011) Guidelines for drinking water quality, Geneva
WHO (2017) Guidelines for drinking-water quality: first addendum to the fourth edition
Yadav KK (2019) Human health risk assessment: study of a population exposed to fluoride through groundwater of Agra city, India. Regul Toxicol Pharmacol 106:68–80. https://doi.org/10.1016/j.yrtph.2019.04.013
Yousefi M (2018) Health risk assessment to fluoride in drinking water of rural residents living in the Poldasht city, Northwest of Iran. Ecotoxicol Environ Saf 148:426–430. https://doi.org/10.1016/j.ecoenv.2017.10.057
Zabin SA (2008) Non-carcinogenic risk assessment of heavy metals and fluoride in some water wells in the Al-Baha region, Saudi Arabia. Hum Ecol Risk Assess Int J 14(6):1306–1317. https://doi.org/10.1080/10807030802494667
Zhang Q (2020a) Hydrogeochemistry and fluoride contamination in Jiaokou irrigation district, central China: assessment based on multivariate statistical approach and human health risk. Sci Total Environ 741:140460. https://doi.org/10.1016/j.scitotenv.2020.140460
Zhang Z (2020) Source and mobilization mechanism of iron, manganese and arsenic in groundwater of Shuangliao city, Northeast China. Water 12(2):534. https://doi.org/10.3390/w12020534
Acknowledgements
The authors thankfully acknowledge the financial support provided by the Department of Science and Technology (DST, India) grant No. (DST/TMD/EWO/MTI/2K19/EWFH/2019/397).
Author information
Authors and Affiliations
Contributions
AS contributed to conceptualization, investigation, data interpretation, supervision, visualization, review and writing–review & editing. DC contributed to methodology and writing–review & editing. AG helped in data collection & formal analysis, writing–review & editing. DkG done formal analysis.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflicts of interest.
Additional information
Editorial responsibility: S.Mirkia.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, A., Choudhary, D., Giri, A. et al. Deterministic health risk assessment, spatial analysis and hydrochemical assessment to toxic fluoride in groundwater of Dhar District, M.P, India. Int. J. Environ. Sci. Technol. 21, 5529–5542 (2024). https://doi.org/10.1007/s13762-023-05359-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-023-05359-w