Abstract
The mobility and bioavailability of mercury in the soil from the area near a plant using elemental mercury for manufacturing thermometers, areometers, glass energy switches and other articles made of technical glass has been evaluated. Mercury has been determined by sequential extraction method and with additional thermo desorption stage to determine elemental mercury. The procedure of sequential extraction involves five subsequent stages performed with the solutions of chloroform, deionized water, 0.5 M HCl, 0.2 M NaOH and aqua regia. The mean concentration of total mercury in soil was 147 ± 107 μg g−1 dry mass (range 62–393), and the fractionation revealed that mercury was mainly bound to sulfides 56 ± 8% (range 45–66), one of the most biounavailable and immobile species of mercury in the environment. The fractions that brought lower contribution to the total mercury content were semi-mobile humic matter 22 ± 9% (range 11–34) and elemental mercury 17 ± 5% (range 8–23). The contributions brought by the highly mobile and toxic organomercury compounds were still lower 2.3 ± 2.7% (range 0.01–6.5). The lowest contributions brought the acid-soluble mercury 1.5 ± 1.3% (range 0.1–3.5) and water-soluble mercury 1.0 ± 0.3% (range 0.6–1.7). The surface layer of soil (0–20 cm) was characterized by higher mercury concentrations than that of the subsurface soil (60–80 cm), but the fractional contributions were comparable. The comparison of mercury fractionation results obtained in this study for highly polluted soils with results of fractionation of uncontaminated or moderately contaminated samples of soil and sediments had not shown significant statistical differences; however, in the last samples elemental mercury is usually present at very low concentrations. On the basis of obtained correlation coefficients it seems that elemental mercury soils from “Areometer” plant are contaminated; the main transformation is its vaporization to atmosphere and oxidation to divalent mercury, probably mainly mediated by organic matter, and next bound to humic matter and sulfides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The “Areometer” plant was established in 1941 located in a densely populated area, southern part of Warsaw suburbs. During its operation time the plant has produced mainly glass thermometers, areometers, glass energy switches and other articles made of technical glass. Since 1990 all installations in the production line have been hermetically sealed, and gaseous effluents from the process have been directed to a wet scrubber, where mercury is washed out and filtered off. Hazardous waste resulting from this process has probably been buried all around the site in the subsurface volume of the surrounding soil. In 1986 it was revealed that the whole plant site and the surrounding area were heavily contaminated with mercury. Then, according to the measurements conducted, the mean level of mercury concentrations found in the soil taken from the vicinity of the junction building was over 200 μg g−1 (Bobrowicz 1999). In 1992 the factory was obliged, by the decision of local administration, to clean up the surrounding areas, especially those owned by other parties. The cleaning and remediation of the plant site started in 1992 using two methods: including removal of contaminated soil and replacing it by standard soil, and the introduction of zeolite bed agent in the form of small cubes into the soil. After the remediation, measurements of mercury contamination were made by the Institute of Environmental Protection in Warsaw, in the years 2001–2003. The results obtained showed that the mean value of mercury concentration over the whole site was 41 μg g−1 in the surface soil layer and 12 μg g−1 in the subsurface layer (Polubiec et al. 2003).
The aim of the studies was to determine the degree of pollution of the soil in the area surrounding the plant, known to have been exposed to emission of mercury, with different mercury species. Another aim of this study was statistical comparison of results obtained from this study with results of fractionation of mercury in uncontaminated or moderately contaminated soils and sediments.
Materials and methods
Sample collection and preparation for analysis
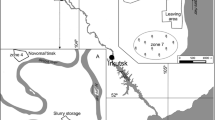
Soil samples for this study were taken in August 2005 from the most contaminated sites within the plant area using a hand soil borer made of stainless steel (Fig. 1). The soil samples were taken from two layers: top layer (0–20 cm) and subsurface (60–80 cm). The samples from a given layer were collected at 6–10 sites, to ensure that the result would be a representative average. The samples were placed in plastic boxes of 1,000 mL capacity and were transported to the laboratory, where they were dried at room temperature to constant weight. For mercury analysis, the samples were refined in an agate mortar but not to damage the structure of the grains. From the samples prepared the subsamples of uniform mass were collected and sieved through a copper sieve of mesh size 0.150 mm.
Reagents and apparatus
The analytical agents used for mercury determination were of the highest available purity, made by Merck (Darmstadt, Germany). All dilutions were made with deionized water from Milli-Q system (Millipore, France). Laboratory vessels used were made of boron-silica glass of the highest quality (Boszke et al. 2007).
Sequential extraction procedure
After a review of the literature covering the determination of various species of mercury in solid samples, a new sequential extraction procedure (Fig. 2) permitting determination of six fractions has been proposed and developed (based partially on the works of Wallschläger et al. 1996; Lechler et al. 1997, Renneberg and Dudas 2001, Bloom et al. 2003).
Five grams of a sediment sample were treated with four subsequent extractions using chloroform, deionized water, 0.5 M HCl and 0.2 M NaOH, followed by oxidative digestion with aqua regia (Fig. 2). The sample was shaken, centrifuged and filtered after each stage of sequential extraction. The chloroform phase was reextracted with sodium thiosulfate, and the solution obtained was treated with concentrated nitric acid. The extracts obtained at separate stages were treated with 37% HCl and 0.2 M KBr/KBrO3 to oxidize all mercury species to Hg(II). Next, 12% NH2OH × HCl was added to the extract to remove free bromine. The sample obtained at the fourth stage was treated with aqua regia and was heated in a glass apparatus consisting of a water cooler and a partial condenser. The same procedure as at the fifth stage was applied to determine the content of the total mercury in bulk sediment. Details of sequential extraction procedure are presented in Boszke et al. (2007). In this study, an additional stage was proposed, i.e. determination of elemental mercury. Concentration of this form of mercury was calculated as a difference between the mercury concentrations in the subsamples at the fourth stage and those at the same stage but heated at 150°C for 6 h (Fig. 2). A similar fractionation method, without elemental mercury determination but with the same extraction method, was used for analysis of freshwater sediment samples from Warta river (Boszke et al. 2007) and floodplain soils of this river (Boszke and Kowalski 2007b), post-tsunami sediments from Thailand (Boszke et al. 2006), sediments of the Vistula river (Boszke and Kowalski 2007a) and one sample of certified estuarine sediment (Boszke et al. 2007). The results obtained during this research and presented in the “Discussion” part will be partially related to the samples mentioned above.
Mercury determination
Mercury was determined by cold-vapor atomic fluorescence spectroscopy (CV-AFS) on Millenium Merlin (PS Analytical, England). The calibration was performed with the mercury standard Hg(NO3)2 (Merck) with the nominal mercury content of 980 ± 020 ng L−1. The limit of detection and quantification of the method depend on the purity of the reagents used. The limit of quantification (LOQ) of the method reached 0.8 ng Hg L−1 and was calculated according to the formulas presented by Konieczka et al. (2004).
Other procedures
The grain size analysis of soils was made by the areometric Bouyuocosa-Casagrande method in the modification of Prószyński (Lityński et al. 1976; Ostrowska et al. 1991). The total nitrogen was determined by the Kjeldahl method, and the total organic carbon was determined by the dry burns using the Shimadzu dry TOC-10A, according to the norm PN-ISO 10694 (2002). The acidity was determined in water solution and 1 M KCl solution (1:2.5 m/m) by the potentiometric method.
Reference samples
Along with determination of the total mercury, analyses were routinely performed on the certified materials, including: SRM 2711 (Montana Soil), SRM 2709 (San Joaquin Soil) and LGC 6137 (Estuarine Sediment). The total mercury concentrations obtained in our study, taking into regard the values of expanded uncertainty (Konieczka et al. 2004), 0.606 ± 0.351 μg g−1 (SRM 2711, n = 5), 0.144 ± 0.098 μg g−1 (SRM 2709, n = 6), 0.370 ± 0.021 μg g−1 (LGC 6137, n = 7) well correspond with those obtained for the certified materials of 0.6250 ± 0.190, 0.140 ± 0.080 and 0.340 ± 0.050 μg g−1, respectively (Kowalski 2006).
For comparison with the results of sequential extraction, one certified sample LGC 6137 was used. The sum of mercury concentrations obtained from particular fractions in the certified sample studied was 0.373 μg g−1 dry mass, whereas the corresponding sum obtained for the certified material was 0.340 μg g−1 dry mass. The method of sequential extraction is thus characterized by the recovery of about 110% (range108–112%) and by the reproducibility (Relative Standard Deviation) from 1 to 7% (Boszke et al. 2007).
Statistical testing
To statistically assess differences in percentage contribution of mercury between samples collected at the area of “Areometer” plant and uncontaminated or moderately contaminated samples of soils/sediments, non-parametric Kołmogorov–Smirnov test was applied. Similar procedure was applied to comparison of fractionation of mercury in coastal and freshwater sediments (Boszke and Astel 2007). To assess correlations, non-parametric Spearman r was calculated. All calculations in this study were performed by applying Statistica 6.0 software (StatSoft, Inc 2001) running on MS Windows 2000 platform.
Results
Acidity of the soil studied ranged from 4.9 to 7.4 in water and from 4.4 to 7.2 in KCl. The pH values do not show much variation in the soil profile (Table 1). The content of organic carbon and nitrogen in the surface layer of the soil studied (0–20 cm) ranged from 0.76 to 2.20% and from 0.07 to 0.18%, respectively. The deeper layer (60–80 cm) was characterized by lower organic carbon and nitrogen content, and the enrichment factor in the soil studied was 1.7 (range 0.8–2.7) for organic carbon and 1.4 (range 0.6–3.6) for nitrogen. There were no significant differences (Kołmogorov–Smirnov test: p > 0.05 in the grain size distribution in the surface (0–20 cm) and the subsurface (60–80 cm) layers of the soil (Table 2). The soil studied was characterized by very low sorption capacity; it was built of loose sands in which the content of the clay minerals did not reach 3–4% (Table 2).
The concentrations of total mercury in the soil samples studied are shown in Tables 3 and 4. The average concentration of total mercury is 147 ± 107 μg g−1 dry mass (range 62–393) calculated as a sum of mercury concentrations in individual fractions, and 169 ± 116 μg g−1 dry mass (range 62–390) in bulk soils. The surface layer of the soil (0–20 cm) is characterized by higher total mercury concentrations than that of subsurface soil (60–80 cm) (K–S: p < 0.05). The enrichment factor defined as a ratio of the total mercury concentration in the surface soil to that in the subsurface soil is 2.7 (Table 3). When the total mercury concentration is normalized to organic carbon and nitrogen, the enrichment factor is 1.7 and 2.0, respectively (Table 4).
Division to surface and subsurface layers appears not to be statistically important (K–S: p > 0.1) for mercury contribution in all fractions. The fractionation revealed that mercury was mainly bound to sulfides 56 ± 8% (range 45–66). The fractions that brought low contribution to the total mercury content were the humic matter fraction 22 ± 9% (range 11–34) and elemental mercury 17 ± 5% (range 8–23). The lowest contributions brought the organomercury compounds 2.3 ± 2.7% (range 0.01–6.5), acid-soluble mercury 1.5 ± 1.3% (range 0.1–3.5) and water-soluble mercury 1.0 ± 0.3% (range 0.6–1.7). The surface layer of the soil (0–20 cm) was characterized by higher mercury concentrations, in particular fractions than that of the subsurface layer (60–80 cm) (Table 5). For fractions F1, F2, F3, F4, F5 and F6 the average enrichment factors were: 2.2, 2.7, 1.4, 2.7, 2.6 and 2.8, respectively. In comparison to mercury concentrations, the contributions of mercury in the surface and subsurface layers were comparable. The average enrichment factors characterizing the contributions of particular fractions are 0.9, 1.0, 0.6, 0.9, 1.2 and 1.0 (Table 5).
Discussion
Total mercury distribution
The mercury concentration calculated as a sum of mercury concentrations in individual fractions obtained in this study (147 ± 107 μg g−1, range 62–393) is higher than 41 and 12 μg Hg g−1 determined in surface and subsurface layer of soil from “Areometer” plant (Polubiec et al. 2003). The mercury concentration obtained in this study is much higher than the total mercury concentrations established for uncontaminated and moderately contaminated soils in Poland (Umińska 1987; Falandysz 2002; Falandysz et al. 2002, 2004, 1996; Boszke and Kowalski 2006). The average concentration of mercury in the soil samples from farmlands under direct effect of anthropopressure in Poland was 0.130 μg g−1 dry mass (range 0.0023–0.450) (Falandysz et al. 1996). In the soil samples from typical villages near industrial centers in Poland much higher mercury concentrations were found: between 0.150 and 3.70 μg g−1 (Umińska 1987).
Much higher mercury concentrations were determined in the soil and sediment samples collected from area where mercury was used in production or mining, e.g. chloroalkali plants, gold mines, cinnabar mines. In the soil samples collected from the area of the chloroalkali plant, the total mercury was detected at concentrations up to 73,300 μg g−1 (Bloom et al. 2003) or 1497 ± 302 μg g−1 (Biester and Scholz 1997). In the soil samples collected from the area of the cinnabar mine, the total mercury was detected at concentrations up to 78,400 μg g−1 (Bloom et al., 2003), up to 699 μg g−1 (Miller et al. 1995) or 29 ± 3.5–612 ± 29.2 μg g−1 (Biester and Scholz 1997). In the soil samples collected from the area of the gold mine, the total mercury was detected at concentrations up to 635 μg g−1 (Bloom et al. 2003), up to 3,126 μg g−1 (Miller et al. 1995). In the soil samples collected from the mercury switch disposal area, the total mercury was detected at concentrations up to 229 μg g−1 (Miller et al. 1995) and in the soil samples from the timber conservation plant 144 ± 5.8 μg g−1 (Biester and Scholz 1997).
Fractionation
The contribution of mercury in particular fractions in the soil samples studied can be only semiquantitatively compared with the analogous data reported by other authors because of the slight differences in the methods of fractionation. The total mercury concentrations in “Areometer” plant samples were very high comparing with various uncontaminated or moderately contaminated soils or sediment samples. The total mercury concentrations, calculated as a sum of mercury concentrations in individual fractions were: 0.134 ± 0.074 μg g−1 (range 0.051–0.307) in sediments of the Warta river (Boszke et al. 2007), 0.248 ± 0.187 μg g−1 (range 0.058–0.829) in floodplain soils of the Warta river (Boszke and Kowalski 2007b), 0.119 ± 0.050 μg g−1 (range 0.066–0.230) in post-tsunami sediments (Boszke et al. 2006), 0.065 ± 0.014 μg g−1 (range 0.054–0.092) in sediments of the Vistula river (Boszke and Kowalski 2007a) and 0.373 μg g−1 in estuarine sediment, respectively (Boszke et al. 2007).
Organomercury compounds
Organomercury compounds are those in which mercury is bonded directly to the carbon atom [e.g. CH3Hg(I)]. They are at least an order of magnitude more mobile than inorganic mercury species, and thus are more toxic and more readily bioaccumulated (Stein et al. 1996; Ullrich et al. 2001; Boszke et al. 2002, 2003). Usually, concentration of methylmercury in sediments varies between 1 and 1.5% of total mercury, while it makes less than 0.5% in marine and estuarine sediments (Ullrich et al. 2001).
The contribution of organomercury species in the total mercury concentration obtained in this study (2.3 ± 2.7%) was in general similar to that obtained in the samples of floodplain soils of the Warta river 1.6 ± 0.8% (K–S: p > 0.05) and the Vistula river sediments 6.4 ± 5.3% (K–S: p > 0.5). Higher contributions of organomercury species comparing to those obtained in this study were determined in relatively uncontaminated sediments of the Warta river 12 ± 9% (K–S: p < 0.01) and post-tsunami sediments 14 ± 7% (K–S: p < 0.005), respectively. Average value, range and SD of percentage contribution of mercury of the various forms related to sequential extraction procedures for all kinds of soils or sediments are shown in Fig. 3.
In general, the contribution of organomercury compounds to the total content of mercury in soils and sediments is low and greater in uncontaminated and not much in contaminated soil and sediment samples. This pattern was observed in other studies (Sakamoto et al. 1995; Eguchi and Tomiyasu 2002). In the samples of sediments from the Minamata Bay (Japan) contaminated with mercury, the contribution of organomercury compounds varied from 1 to 4%, while in the relatively less-contaminated sediments in the Kagoshima Bay (Japan) it was from 7 to 37% (Sakamoto et al. 1995; Eguchi and Tomiyasu 2002). In the samples of soil and sediments from the regions highly polluted with mercury, the contribution of methyl mercury to the total mercury concentration was very low, and the maximum contributions (from 0.01 to 0.05%) were found in the sediment samples from the area of the abandoned mercury mine in Alaska (Bloom et al. 2003). In these sediments concentrations of methylmercury ranged from 0.65 to 9.85 μg g−1 (Bloom et al. 2003). In other studies, in soils from the area of the cinnibar refinery and storage contribution was lower than 0.0001%, and concentration of mercury in this fraction was relatively low <0.012 μg g−1 (Miller et al. 1995). In the soils from the area of the gold mine, where the total mercury concentration ranged from 41.7 to 635 μg g−1 methylmercury was not detected (Bloom et al. 2003). However, in other studies where stream sediments from former gold mine area were studied, organomercury species were detected (<1 μg g−1), and the contribution this fraction was significant (<0.03%) (Miller et al. 1995). In contaminated soils from the mercury switch disposal area, the contribution of organomercury species was much higher (2.2%), and the absolute concentration of organomercury species was 5 μg g−1 (Miller et al. 1995).
Water-soluble mercury
The fraction called water-soluble mercury includes mercury species present in pore water. Usually mercury is not present in the form of water-soluble ionic species in the water phase but as species bound to organic matter (without a Hg–carbon bond) or suspended mineral particles (Biester and Scholz 1997; Wallschläger et al. 1998; Wasay et al. 1998; Renneberg and Dudas 2001). Mercury species extracted by water may be easily transported by natural processes and serve as the substrate for mercury methylation process (Stein et al. 1996; Ullrich et al. 2001; Boszke et al. 2003).
The contribution of water-soluble species in the total concentration of mercury in the soil samples examined was low (1.0 ± 0.3%, range 0.6–1.7), but their absolute concentration was much higher than in the samples uncontaminated with mercury. Relative to the results of this study, higher contributions of water-soluble mercury were found in the sediments of the Vistula river (6.9 ± 2.9%; K–S: p < 0.005), while comparable contributions were observed in the post-tsunami sediments 0.8 ± 1.0% (K–S: p > 0.05), in the floodplain soils of the Warta river 2.2 ± 1.8% (K–S: p > 0.05), in the sediments from this river 2.1 ± 0.9% (K–S: p > 0.10) and in estuarine sediment 0.3%.
The contribution of water-soluble mercury species is very small, and often the concentrations determined in the soil samples uncontaminated with mercury are below the limit of detection or limit of determination of the method applied (Kot et al. 2002; Kot and Matyushkina 2002). A low contribution of the water-soluble mercury (<0.001%) was found in the soil samples from the cinnibar refinery and storage area (Miller et al. 1995). Contributions lower than in the samples studied by us were also found in the stream sediments from the former gold mine 0.03% (Miller et al. 1995), in the soil from the cinnibar mine: 0.04% (Miller et al. 1995), 0.002–0.12% (Biester and Scholz 1997), in the soil from the mercury switch disposal area 0.4% (Miller et al. 1995), in the soil from the chloralkali plant area 0.15% (Biester and Scholz 1997). A low contribution of water-soluble mercury (0.03%) was found in the soils from the vicinity of a former wood preservation area, where HgCl2 solution was used as preservative (Biester and Scholz 1997).
Acid-soluble mercury
The fraction called the acid-soluble mercury species include strongly bound Hg species extracted by solution of acids, e.g. HCl (Ching and Hongxiao 1985; Lechler et al. 1997). Acid solution extracts are operationally defined as “reactive mercury species” or “bioavailable inorganic mercury” bonded to iron monosulfides (i.e., AVS), iron and manganese hydroxides and carbonates. They can also include the species bound to organic matter and adsorbed on surface of minerals (Lechler et al. 1997; Bloom et al. 2003; Shi et al. 2005).
The contributions of acid-soluble species in the total concentration of mercury in the soil samples examined were low (1.5 ± 1.3%, range 0.1–3.5) and statistically comparable to those in relatively uncontaminated floodplain soils of the Warta river 2.0 ± 1.7% (K–S: p > 0.05), in the sediments of the Warta river 0.4 ± 0.1% (K–S: p > 0.05), post-tsunami sediments 0.9 ± 0.5% (K–S: p > 0.05), the sediments of the Vistula river 0.3 ± 0.2% (K–S: p > 0.05) and estuarine sediment 0.2%.
In the soil samples the contribution of the acid-soluble species in the total concentration of mercury is relatively low (Inacio et al. 1998; Bloom and Katon 2000; Barnett and Turner 2001; Bloom et al. 2003). For example, the contribution of the acid-soluble mercury fraction in the soil samples collected near the chlorine-alkali plant varied from 0.3 to 2.3% (Inacio et al. 1998) or 5.3% (Barnett and Turner 2001). Low contributions were also in the stream sediments from the former gold mine area (0.2%) (Miller et al. 1995). Higher contributions were observed in the floodplain soil contaminated with Hg(NO3)2 (7.7%) (Bloom et al. 2003), in the soil from the cinnabar refinery and storage area (8.6%) (Miller et al. 1995) and in the soils from cinnabar mine area (7.8%) (Miller et al. 1995).
Mercury bound to humic matter
Organic matter is an important component of sediments and soils. It is also to a significant degree, responsible for binding metals. Organic mercury includes mercury Hg(II) complexes with organic ligands e.g. humic and fulvic acids, amino acids (but without a Hg–carbon bond). Binding of mercury in organic matter is mainly due to the reduced sulfur species in such functional groups as thiol (R-SH), disulphide (R-SS-R) or disulphane (R-SSH) (Xia et al. 1999). Besides sulfur, some mercury in organic matter is also bound by oxygen and nitrogen atoms but in much lower amounts (Hesterberg et al. 2001).
Contributions of mercury bound to humic matter in total concentration of mercury in examined soil (22 ± 9%, range 11–34), besides post-tsunami sediments 9 ± 7% (K–S: p < 0.01) and estuarine sediment (4.6%) are similar to the contributions obtained in other studies for uncontaminated samples of soils and freshwater sediments (floodplain soils of the Warta river: 28 ± 6% (range 17–42; K–S: p > 0.05); sediments of the Warta river: 23 ± 9%, (range 4–36; K–S: p > 0.05); sediments of the Vistula river 19 ± 10% (range 10–35; K–S: p > 0.05).
Greater contributions of mercury bound to organic matter are found in bottom sediments from not very polluted rivers and smaller in those from highly polluted ones. For example, in the sediments uncontaminated with mercury sampled from the river Carson (USA) the contribution of mercury bound to humic matter was <40% but in the region highly polluted with mercury it decreased to a few percent (Lechler et al. 1997). The contribution of mercury species bound to humic acids in the bottom sediments of the rivers Ji Yun and Zijan (China) ranged from 2.7 to 77.4% (Peng and Wang 1985). The lowest contribution of the mercury species bound to organic matter was found in a sample from the region of waste release from a plant producing chlorine alkaline compounds. It amounted to 54,000 ng g−1, while the concentration of mercury in the sample with the greatest contribution of the organic matter bound mercury was 4,400 ng g−1 (Peng and Wang 1985). Other authors (Ching and Hongxiao 1985) reported the contribution of humic acid bound mercury in the bottom sediments from the same river Ji Yun varying from 6.52 to 23.7% (800–46,650 ng g−1).
Elemental mercury
The contribution of elemental mercury in the total concentration of mercury in the soil samples examined was 17 ± 5% (range 8–23), and the absolute concentration was 25.2 ± 22.2 μg g−1 (range 10.0–76.5). It shows that the soil studied was contaminated with mercury because elemental mercury is not detected in the soil and sediments uncontaminated with mercury soils (Lechler et al. 1997; Pestana et al. 2000; Renneberg and Dudas 2001). For example, the contribution of elemental mercury was 30% in the soil sample with the total mercury concentration 16,000 μg g−1, but in another one with the total mercury concentration ranging from 30 to 540 μg g−1, elemental mercury was not found (Renneberg and Dudas 2001). However, in the floodplain soils highly contaminated with Hg(NO3)2, in which the total mercury concentration was 549 μg g−1, elemental mercury was not detected (Bloom et al. 2003). Higher elemental mercury contributions were noted in the soils highly contaminated with this form of mercury (Lechler et al. 1997; Pestana et al. 2000). In the area of a gold mine where elemental mercury was used to recover gold (Lavras so Sul, Brazil), the contribution of elemental mercury was very high and ranged from 82.0 to 83.1% (0.2–91.4 μg g−1) (Pestana et al. 2000). In the uncontaminated mercury sediments of the Carson river (USA) the contribution of elemental mercury was nearly naught but in the contaminated sediments flowing by mill tailing it was nearly 90% (Lechler et al. 1997).
Mercury bound to sulfides
In the presence of sulfides, the mercuric ion becomes tightly bound to them as insoluble HgS, and is not available for methylation. Sulfhide activity may be the main factor influencing the availability of Hg(II) and the concentration of methylmercury in the sediment. If conditions become aerobic due to a decrease in the organic load or seasonal turnover, sulphide can be oxidized to sulfate, releasing the mercury in the ionic form Hg(II), which is available for methylation (Stein et al. 1996; Ullrich et al. 2001; Boszke et al. 2003).
The contributions of mercury bound to sulfide in the total concentration of mercury in the soil samples examined (56 ± 8%, range 45–66) were similar to those in the floodplain soils of the Warta river (66 ± 7%, K–S: p > 0.05), in the sediments of the Warta river (61 ± 13%, K–S: p > 0.10) and the sediments of the Vistula river (68 ± 11%, K–S: p > 0.05). Higher contributions comparing to soil samples from “Areometer” plant area were found in the post-tsunami sediments 75.5 ± 6.2% (K–S: p < 0.001) and the estuarine sediment 93%.
The contribution of mercury bound to sulfides is greater in the sediments and soil samples characterized by reducing conditions (Lechler et al. 1997; Bełdowski and Pempkowiak 2003). For instance, the contribution of mercury bound to sulfides in the bottom sediments of the Gdańsk Bay (Poland) was close to 40%, with a maximum value of 96% in the bottom sediments characterized by reducing conditions (Bełdowski and Pempkowiak 2003). In the mercury-polluted sediments from the river Ji Yun (China), the contribution of mercury bound to sulphides varied from 5.8 to 17.1% (800–76,520 ng g−1) (Ching and Hongxiao 1985). These values can be contrasted with the contribution of mercury bound to sulfides in the mercury-polluted soil being of <99.1% in the vicinity of the plant-producing chlorine alkaline compounds (Bloom and Katon 2000), <93.1% in the vicinity of the gold mine (Bloom and Katon 2000; Miller et al. 1995) and 74.4% (406,000 ng g−1) in the floodplain soil contaminated with Hg(NO3)2 (Bloom et al. 2003). Lower contributions of mercury sulfides were in the soils from HgS mine area: 1.9–20.1% (Bloom and Katon 2000) or 7% (Miller et al. 1995).
Distribution of mercury in soil profiles
Mercury has a high ability to get bound to sulfur in organic matter and to form mercury sulfides. In the surface layers where the content of organic matter is higher, the concentration of mercury is also higher. In the soil samples studied in this work, the organic matter content and concentrations of mercury were significantly higher in the upper layer than in the deeper subsurface layer (Tables 1, 5). This pattern was observed in other studies of soil from the site highly contaminated with mercury. For example, in the soil samples from the gold mine area, the total mercury concentration was higher in the surface layer (635 μg g−1) than in the deeper layer (41.7 μg g−1) (Bloom et al. 2003). However, for other highly contaminated soil samples, the reversed pattern was observed. For example, in the soil profiles from the cinnabar mine, from the retort area, the total mercury concentration was 511 μg g−1 in the surface layer, while 860 μg g−1 in a layer 10 cm below the surface and 7,180 μg g−1 in a layer 210 cm below the surface (Bloom et al. 2003).
Although the concentrations of the total mercury and mercury in particular fractions were higher in the upper layer, the contributions of particular fractions were similar (Table 5; Fig. 4). Other authors studying soils contaminated with mercury reported different patterns of mercury fractions distribution in the vertical profiles, which can result from different specific forms of mercury released to the soils and from the properties of the soils. The presence of specific mercury species along with physical and chemical properties of these soils can influence mercury transformations in the soil profiles. For example, in the soil profile from the cinnabar mine in the surface layer, the layers 10 and 210 cm below the surface, the contributions of methylmercury (0.001, 0.0001 and 0.0004%) water-soluble mercury (0.09, 0.01 and 0.19%) and acid-soluble mercury (0.01, 0.00 and 6.0%) were very low, and the lowest in the layer 10 cm below the surface (Bloom et al. 2003). In the soil sample from the vicinity of the gold mine, methylmercury was not detected, but the contributions of water-soluble mercury and acid-soluble mercury were relatively higher than in the soil from the vicinity of the cinnabar mine (Bloom and Katon 2000). In the gold mine soil samples, the contributions of water- and acid-soluble mercury in the surface layer (1.3 and 1.2%) were lower than in a deeper layer (5.3 and 36%) (Bloom and Katon 2000).
In the soil from the vicinity of mill tailing highly contaminated with mercury, the contribution of mercury bound to humic matter was relatively low (<0.1–6.9%) but higher in the upper layer at the sites where the content of organic matter was the highest (Lechler et al. 1997). In the soil profile in the vicinity of the cinnabar mine, the contribution of mercury bound to humic matter was 0.26% (1.33 μg g−1) in the surface layer, while 1.3% (11.6 μg g−1) and 0.68% (49.3 μg g−1) in the layers 10 and 210 cm below the surface, respectively. In the soil sample from the vicinity of the gold mine, the contribution of the acid-soluble mercury species was 0.26% (1.33 μg g−1) on the surface and 1.0% (6.39 μg g−1) in a deeper layer (Bloom and Katon 2000; Bloom et al. 2003).
In the soil profile from the vicinity of mill tailing, the contribution of elemental mercury shows no clear pattern with increasing depth of the profile; its maximum contribution was 98% (Lechler et al. 1997). In the soil samples from the gold mine the concentration of elemental mercury vapor was higher in samples collected from deeper layer (72.7 μg m−3) than from the surface layer (5.6 μg m−3). However, the total mercury concentrations were higher in the surface layer (635 μg g−1) than in the deeper layer (41.7 μg g−1) (Bloom et al. 2003). Also in the soil profiles from the area of the HgS mine, the highest concentration of elemental mercury vapor (18,000 μg m−3) was detected in the deepest layer, where the total mercury was the highest (Bloom et al. 2003). However, in relatively uncontaminated soils elemental mercury is detected in the upper layer where the total mercury is the highest (Lechler et al. 1997).
In deeper layers characterized by anoxic conditions, the contributions of mercury sulfide are higher (Lechler et al. 1997; Bełdowski and Pempkowiak 2003). For example in the soil profile from mill tailing the contribution of residual mercury increased with increasing depth of the profile, from 8.1% in the surface layer (0–30 cm) to 69% in the layer at 50–80 cm (Lechler et al. 1997). In other studies the highest contribution of HgS was in the surface layer (97.8%), lower in the layer 20 cm below the surface (96.6%) and the lowest in the layer 210 cm below the surface (73.0%) although the highest HgS concentrations were in deeper layers: 500, 831 and 5,236 μg g−1, respectively (Bloom and Katon 2000; Bloom et al. 2003). In the soil sample from the vicinity of the gold mine, the contribution of the acid-soluble mercury species was 93.1% (591 μg g−1) on the surface and 9.8% (4.07 μg g−1) in a deeper layer (Bloom and Katon 2000; Bloom et al. 2003).
Correlations
There are some correlations between the parameters of the soils and the mercury concentrations and contributions (Table 6). Among them an interesting one is that between the concentration of the water-soluble mercury species and that of mercury bound to humic matter (r = 0.79) and between contribution of the water-soluble mercury and contribution of organic carbon (r = 0.76). There is also significant correlation between concentration of the mercury bound to humic matter and mercury sulfide concentration (r = 0.76) and between the concentration of elemental mercury and that of organomercury species (r = 0.79).
These correlations show that in the water phase, mercury may not be present in the form of water-soluble ionic species but rather as the species bound to organic matter, which agrees with the results of other authors (Biester et al. 2002; Renneberg and Dudas 2001; Wallschläger et al. 1996). These correlations also suggest that part of elemental mercury may be oxidized to divalent mercury—species soluble in water, and next converted to organomercury species, e.g. by biotic transformations. Some portion of divalent mercury (e.g. water-soluble mercury) may be bound to sulfides or humic matter.
Wang et al. (2003) concluded that transformations of atmospheric Hg(0) take place as soon as this element is deposited on the soils. Approximately 25% of the deposited from atmosphere mercury remained in the Hg(0), and a part will likely volatilize and enter the gaseous phase but some will likely be adsorbed onto the surface of soils particles. Approximately 75% of the deposited mercury remained into the so-called active forms, soluble HCl, organically bound and residual mercury (Wang et al. 2003). It seems that contaminated with elemental soils from “Areometer” plant the main transformation of mercury is vaporization of elemental mercury to atmosphere and oxidation to divalent mercury and that organic matter is the main factor in this process as was observed in other studies (Ravichandran, 2004). It is also known that organic matter may also dissolve some mercury bound to sulphides (Ravichandran 2004; Waples et al. 2005).
Conclusion
In the soil from the vicinity of the thermometer and “Areometer” plant, contaminated with mercury, it occurs mainly in the form of mercury sulfides (hardly soluble and hardly bioavailable), elemental mercury and mercury bound to humic matter. Although a large majority of mercury is bound to mercury sulfides, if conditions become more aerobic due to a decrease in the organic load or seasonal turnover, sulfides can be oxidized to sulfates, releasing mercury in the ionic form Hg(II), which is available for methylation. It seems that one of the most important factor influencing the mercury mobility and bioavailability in soil samples studied is the organic matter content. It is known that not only divalent mercury is strongly bound to humic matter but also elemental mercury. Thus, divalent and elemental mercury bound to humic matter/organic mater may fall into the “semi-mobile” category. Contribution of organomercury species in the samples studied is relatively low but the concentration of organomercuries is relatively high and it may pose a real threat to man and other living organisms because of their extreme toxicity and capability of bioaccumulation and biomagnification in trophic chains. The concentration and contribution of the water-soluble mercury species is relatively low, but these species can be easily transported by natural processes and serve as substrates for mercury methylation process and thus accumulate in organism.
References
Barnett MO, Turner RR (2001) Bioaccessibility of mercury in soils. Soil Sediment Contam 10:301–316
Bełdowski J, Pempkowiak J (2003) Horizontal and vertical variabilities of mercury concentration and speciation in sediments of Gdańsk Basin, Southern Baltic Sea. Chemosphere 52:645–654
Biester H, Scholz C (1997) Determination of mercury phase in contaminated soils. Mercury pyrolysis versus sequential extractions. Environ Sci Technol 31:233–239
Biester H, Müller G, Schöler HF (2002) Binding and mobility of mercury in soils contaminated by emissions from chlor-alkali plants. Sci Total Environ 284:191–203
Bloom NS, Katon J (2000) Application of selective extractions to the determination of mercury speciation in mine tailings and adjacent soils. In: Proceeding of assessing and managing mercury from historic and current mining activities conference, San Francisco, 28–30 November 2000
Bloom NS, Preus E, Katon J, Hilter M (2003) Selective extractions to assess the biogeochemically relevant fractionation of inorganic mercury in sediment and soils. Anal Chim Acta 479:233–248
Bobrowicz M (1999) Measurement of mercury concentration in soil samples within the area of “Areometer” Plant. PPH Biomed, Warsaw (in Polish)
Boszke L, Astel A (2007) Fractionation of mercury in sediments from coastal zone inundated by tsunami and in freshwater sediments from rivers. J Environ Sci Health A42:847–858
Boszke L, Kowalski A (2006) Spatial distribution of mercury in bottom sediments and soils from Poznań City, Poland. Polish J Environ Stud 15:211–218
Boszke L, Kowalski A (2007a) Mercury fractionation in sediments of the lower Vistula river (Poland). Oceanol Hydrobiol Stud 36:79–99
Boszke L, Kowalski L (2007b) Mercury fractionation in floodplain soils of the Warta river, Poland. Oceanol Hydrobiol Stud (in press)
Boszke L, Głosińska G, Siepak J (2002) Some aspects of speciation of mercury in a water environment. Polish J Environ Stud 11:285–298
Boszke L, Kowalski A, Głosińska G, Szarek R, Siepak J (2003) Selected factors affecting the speciation of mercury in the bottom sediments: an overview. Polish J Environ Stud 12:5–13
Boszke L, Kowalski A, Szczuciński W, Rachlewicz G, Lorenc J, Siepak J (2006) Assessment of mercury availability by fractionation method in sediments from coastal zone inundated by the 26 December 2004 tsunami in Thailand. Environ Geol 51:527–536
Boszke L, Kowalski A, Siepak J (2007) Fractionation of mercury in sediments of the Warta river (Poland). In: Pawłowski L, Dudzińska M, Pawłowski A (eds) Environmental engineering science. Taylor & Francis, London, pp 403–413
Ching IL, Hongxiao T (1985) Chemical studies of aquatic pollution by heavy metals in China. In: Irgolic KJ, Martel AE (eds) Environmental inorganic chemistry. VCH, Deerfield Beach, pp 359–371
Eguchi T, Tomiyasu T (2002) The speciation of mercury in sediments from Kagoshima Bay and Minamata Bay, southern Kyusyu, Japan, by fractional extraction/cold-vapor AAS. Bunseki Kagaku 51:859–864 (in Japanese)
Falandysz J (2002) Mercury in mushrooms and soil of the Tarnobrzeska Plain, South-Eastern Poland. J Environ Sci Health A37:343–352
Falandysz J, Kawano M, Danisiewicz D, Chwir A, Boszke L, Gołębiowski M, Boryło A (1996) Investigation on the occurrence of mercury in soils in Poland. Bromat Chem Toksykol 29:177–181 (in Polish with English abstract)
Falandysz J, Bielawski L, Kannan K, Gucia M, Lipka K, Brzostowski A (2002) Mercury in wild mushrooms and underlying soil substrate from the great lakes land in Poland. J Environ Monit 4:473–476
Falandysz J, Jędrusiak A, Lipka K, Kannan K, Kawano M, Gucia M, Brzostowski A, Dadej M (2004) Mercury in wild mushrooms and underlying soil substrate from Koszalin, North-central Poland. Chemosphere 54:461–466
Hesterberg D, Chou JW, Hutchison KJ, Sayers DE (2001) Bonding of Hg(II) to reduced organic sulfur in humic acid as affected by S/Hg ratio. Environ Sci Technol 35:2741–2745
Inacio MM, Pereira V, Pinto MS (1998) Mercury contamination in sandy soils surrounding an industrial emission source (Estarreja, Portugal). Geoderma 85:325–339
Konieczka P, Namieśnik J, Zygmunt B, Bulska E, Świtaj-Zawadka A, Naganowska A, Kremer E, Rompa M (2004) Quality assessment and quality control of analytical results—QA/QC. Centre of Excellence in Environment Analysis and Monitoring, Gdańsk (in Polish)
Kot FS, Matyushkina LA (2002) Distribution of mercury in chemical fraction of contaminated urban soils of Middle Amur, Russia. J Environ Monit 4:803–908
Kot FS, Matyushkina LA, Nikorych VN, Polivichenko VG (2002) Mercury in chemical and thermal fraction of soils of Eastern Ukrainian Polissya. Soil Sci 3:94–101
Kowalski A (2006) Determination of the total mercury and its species in the environmental samples. Doctoral Thesis, Adam Mickiewicz University, Poznań (in Polish)
Lechler PJ, Miller JR, Hsu LC, Desilets MO (1997) Mercury mobility at the Carson River superfund site, west-central Nevada, USA-interpretation of mercury speciation data in mill tailing, soils, and sediments. J Geochem Explor 58:259–267
Lityński T, Jakubowska H, Gorlach E (1976) The chemical–agricultural analysis. PWN, Warszawa (in Polish)
Miller EL, Dobb DE, Heithmar EM (1995) Speciation of mercury in soils by sequential extraction. Presented at the USEPA Metal Speciation and Contamination of Surface Water Workshop, Jekyll Island
Ostrowska A, Gawliński S, Szczubiałka Z (1991) Methods of analysis and evaluation of soil properties, catalogue. Wydawnictwo Instytutu Ochrony Środowiska, Warszawa (in Polish)
Peng A, Wang Z (1985) Mercury in river sediment. In: Irgolic KJ, Martel AE (eds) Environmental inorganic chemistry. VCH, Deerfield Beach, pp 393–400
Pestana MHD, Formoso MLL, Lechler PJ (2000) Mercury and cupper contamination from historic and recent mining activities in the Camaqua River Basin, South Brazil. In: Nriagu JO (ed) 11th Annual international conference on heavy metals in the environment. University of Michigan, School of Public Health, Ann Arbor (CD-ROM, Contribution Number:1115)
PN-ISO 10694 (2002) Soil quality—determination of organic and total carbon after dry combustion (elementary analysis) (in Polish)
Polubiec E, Bruszewski H, Kostrzewa-Szulc J, Sienkiewicz J (2003) The study on the incorporation of mercury to the biological cycle. Institute of Environmental Protection, Warsaw (in Polish)
Ravichandran M (2004) Interactions between mercury and dissolved organic matter––a review. Chemosphere 55:319–331
Renneberg AJ, Dudas MJ (2001) Transformations of elemental mercury to inorganic and organic forms in mercury and hydrocarbon co-contaminated soils. Chemosphere 45:1103–1109
Sakamoto H, Tomiyasu T, Yonehara N (1995) The content and chemical forms of mercury in sediments from Kagoshima Bay, in comparison with Minamata Bay and Yatsushiro Sea, Southwestern Japan. Geochem J 29:97–105
Shi JB, Liang LN, Jiang GB, Jin XL (2005) The speciation and bioavailability of mercury in sediments of Haihe River, China. Environ Int 31:357–365
StatSoft, Inc (2001) STATISTICA—data analysis software system, version 6. http://www.statsoft.com
Stein ED, Cohen Y, Winer AM (1996) Environmental distribution and transformation of mercury compounds. Crit Rev Environ Sci Technol 26:1–43
Ullrich SM, Tanton TW, Abdrashitowa SA (2001) Mercury in the aquatic environment: a review of factors affecting methylation. Crit Rev Environ Sci Technol 31:241–293
Umińska R (1987) Evaluation of mercury level in waters, bottom sediments and soils in selected rural areas. J Hyg Epidemiol Microbiol Immunol (Prague) 31:9–22
Wallschläger D, Desai MVM, Wilken R-D (1996) The role of humic substances in the aqueous mobilization of mercury from contaminated floodplain soils. Water Air Soil Pollut 90:507–520
Wallschläger D, Desai MVM, Spengler M, Windmöler CC, Wilken R-D (1998) Mercury speciation in floodplain soils and sediments along a contaminated river transect. J Environ Qual 27:1034–1044
Wang D, Shi X, Wie S (2003) Accumulation and transformation of atmospheric mercury in soil. Sci Total Environ 304:209–214
Waples JS, Nagy KL, Aiken GR, Ryan JN (2005) Dissolution of cinnabar (HgS) in the presence of natural organic matter. Geochim Cosmochim Acta 69:1575–1588
Wasay SA, Barrington S, Tokunaga S (1998) Retention form of heavy metals in three polluted soils. Soil Sediment Contam 7:103–119
Xia K, Skyllberg UL, Bleam WF, Bloom RP, Nater EA, Helmke PA (1999) X-ray absorption spectroscopic evidence for the complexation of Hg(II) by reduced sulfur in soil humic substances. Environ Sci Technol 33:257–261
Acknowledgment
Kind assistance and helpful critical remarks of prof. dr hab. Jerzy Falandysz are gratefully acknowledged. This work was partly supported by the Polish Committee for Scientific Research (KBN) under grant No 3 T09D 079 26.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boszke, L., Kowalski, A., Astel, A. et al. Mercury mobility and bioavailability in soil from contaminated area. Environ Geol 55, 1075–1087 (2008). https://doi.org/10.1007/s00254-007-1056-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-007-1056-4