Abstract
Lake Xolotlán (Nicaragua) is an important industrial area including heavy industries such as a fuel refinery and numerous industries that discharge their effluents to the lake. Mercury distribution was studied in soil samples from six different sites close to an old chlor-alkali plant (CAP) which has historically released mercury wastes to the lake and its surroundings. A Hg-specific sequential extraction procedure was used to assess Hg partitioning. Hg content was subdivided in operationally defined fractions named as labile mercury species, humic and fulvic complexes, elemental Hg and bound to crystalline oxides and bound to sulphide Hg and refractory species. The total mercury concentrations ranged between 1 and 123 mg kg−1. Sequential extractions revealed that both humic and fulvic complexes and elemental Hg constituted the major forms of mercury in the most samples. Both fractions are related with the accumulation of mercury from both atmospheric deposition and sewage outflow. Moreover, accumulation of the elemental Hg in these soils decreased with the increasing distance from the CAP. In addition, the study of the distribution of other elements revealed a remarkable availability of Al, Ba, Ca, Fe, Pb, Sr, V and Zn that are commonly related to petroleum treatment and combustion. This suggests that these soils are also affected by the releasing of other pollutants from a nearby refinery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mercury cell chlor-alkali products are used to produce thousands of other products including paper, soap, detergents, textiles, aluminium, petrochemical refining and even food ingredients such as citric acid or sodium benzoate. In the mercury cell process, chlorine and sodium hydroxide are produced simultaneously by electrolyzing seawater (sodium saltwater or brine) in a mercury cell where liquid mercury acts as a cathode. It attracts sodium cation forming an amalgam. Chlorine gas is collected at the anode (graphite) and then removed from the anode, cooled, dried and compressed. When the amalgam is added to water, sodium reacts with water to form sodium hydroxide and hydrogen, leaving mercury, which can then be reused. The chlor-alkali industry based on mercury cell technology has historically been a significant source of atmospheric mercury emissions and direct releases in surrounding environments (Ebinghaus et al. 1999; Hintelmann and Wilken 1995). The main mercury contamination streams from chlor-alkali plants (CAPs) process are wastewater, air emissions and solid waste (Garron et al. 2005).
Lake Xolotlán (also known as Lake Managua) is a lake located in Western-Nicaragua, in the North of the city of Managua. More than 12,000 micro-industries, 1850 small and medium-sized industries and 25 large-scale chemical factories (including a fuel refinery) are located along the lake’s southern shore. The lake has been severely polluted by decades, mostly by dumping of Hg-containing sewage into the lake. During the period from 1967 to 1992 a chlor-alkali plant (CAP) (Pennwalt/HERCASA-ELPESA Company) dumped about 40 tons of mercury into the Lake Xolotlán (Wyrick 1981). Despite the pollution, some of the people of Managua still live along the lake’s shores. In addition, this lake is a fish source not only for feeding population in Nicaragua, but also other Central American countries. Previous studies evidenced the impact of Hg emissions to local population and environment (Hassan et al. 1981; Lacayo et al. 1991; McCrary et al. 2006). In the literature the characterisation of Hg contamination in lake Xolotlán mainly consists either of Hg spatial distribution studies in sediments and water (Lacayo et al. 1991) or Hg concentration in fish or hair samples (McCrary et al. 2006) or airborne Hg levels and even health effects of Hg exposure on workers (Hassan et al. 1981). However, no studies about Hg availability in the surrounding soils of the CAP have been previously reported.
It is widely accepted that the use of total Hg concentration is not a suitable criterion to assess the potential effects of soil contamination. Sequential extraction procedures (SEP) can be used to determine metal contents associated to relevant solid phases in solid environmental samples. In this process, the sample is submitted to specific reagents of successively stronger dissolving power that, under controlled conditions, will remove metals from the particular phases of concern. SEPs constitute a common and feasible approach to understand and establish interactions between Hg or other metals with soil and sediments components, as well as to evaluate their availability to plants (Bloom et al. 2003; Digiulio and Ryan 1987; Fernandez-Martinez and Rucandio 2013; Han et al. 2003; Revis et al. 1989; Sakamoto et al. 1992).
The aim of this study was to investigate the impact of Hg emissions and other metals in soils from the vicinity of the Pennwalt CAP. For this purpose a recent SEP specifically developed for the evaluation of the binding fractions of Hg was applied to the studied soils combined with a complete characterisation to evaluate the chemical factors controlling Hg in the affected area. In addition, the distribution of other potentially-polluting elements was evaluated to assess the impact of the other local industries in the studied soils.
Experimental
Sampling area
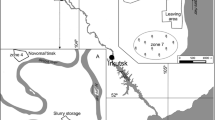
The former chlor-alkali plant PENWALT is located at the 12,15,244 N and 86,32,210 W geographic coordinates, in Managua City 8 km Westside from the Political Centre of Nicaragua. An oil refinery is neighboring behind of the CAP (Fig. 1).
As a tropical country there are two well defined seasons: dry and rainy seasons. The rainy season runs from May to November and the rainfall in Managua where the study area is located is about 1150 mm per year with temperatures ranging between 23 and 32 °C (Climate-Nicaragua 2015).
The study area is situated next to the CAP covering an area of 37 500 m2 (220 m × 150 m rectangular shape), facing north to the Miraflores Bay in the Lake Xolotlan (Fig. 1). Despite the distance between the study area and the shore of the lake, which in the year 2003 was more than 2000 m, several flooding episodes have affected this area since then, and remaining covered with a tiny water mirror until January or February each year (dry season).
Several soil samples were systematically collected by mixing samples obtained at five spots per square in a grid of 100 m × 100 m. With this five spot mixing method, individual samples were collected from a total of five spots: the centre point of each grid and four subpoints set around it. These five samples were combined to obtain one final composite sample for each site. This enhances the representativeness of the soil samples obtained from each grid. To get samples from the four subpoints, they were collected at points north, south, east and west 10 m equidistant of the centre point of the grid (Fig. 2).
At each sampling point, the soil samples were collected with a soil column cylinder auger (inner diameter 5 cm). The core corresponding at 10–20 cm depth was taken, labelled and stored in special PET bags with special zip.
Each sample was put in a tray to remove most coarser objects (pebbles, roots, little stones, etc.) and each sample was homogenised by mixing with the quarter method. After homogenisation, 5 g of each point and subpoint were mixed to obtain a final composite sample and re-homogenised using the quarter method to obtain one composite sample for the mercury sequential extraction.
A selection of six sampling sites was considered, located at different distances from the Pennwalt CAP, to determine the mercury partitioning and to study the pollution spread in the area.
Characterisation techniques
The Hg determinations in solid samples as well as in liquid extracts were performed by using a Direct Mercury Analyser DMA-80 (Milestone, Sorisole, Italy). This is a specific mercury analyser, capable to determine Hg concentrations in solid and liquid samples without any pretreatment. Samples are weighed into a quartz boat and placed on the autosampler tray. Then, the sample boat is inserted into an oxygenated decomposition furnace to liberate Hg. Samples are dried and decomposed and the combustion products carried out by oxygen flow through a heated catalyst to reduce Hg species to elemental Hg and to trap halogens, nitrogen and sulphur oxides. Then mercury vapour is preconcentrated in a gold amalgamator and rapidly desorbed releasing mercury vapours to the absorbance cells where absorbance is measured at 253.7 nm.
The determination of major and minor elements (Al, As, B, Ba, Be, Bi, Ca, Cd, Co, Cr, Cu, Fe, Mg, Mn, Mo, Ni, Pb, Sr, Ti, V and Zn) in the different extracted solutions obtained from the selected soil samples, as well as the total fractions were determined by simultaneous inductively coupled plasma optical emission spectrometer (ICP-OES), model Varian (now Agilent) 735-ES in a radially viewed configuration and a VistaChip image-mapped charged coupled device (CCD) detector. Na was analysed by flame atomic emission spectroscopy (FAES) by using a Perkin–Elmer spectrophotometer 2280 model.
For wavelength dispersive X-ray fluorescence analysis (WDXRF), an aliquot of approximately 8 g of each sample was pulverised, at least fine enough to pass through a 200 mesh screen (74 µm opening size). The ground powder was mixed with 0.07 g of Elvacite dissolved in acetone, and after acetone evaporation, the sample was compressed using a hydraulic press (HTP 40, Herzog) into a 40 mm pellet. 30 trace elements were determined in the pellets by wavelength dispersive X-ray fluorescence spectrometry using a PANalytical AXIOS automated spectrometer employing a method (PRO-TRACE) developed by PANalytical. 11 major elements were analysed using fused beads. For fused bead preparation, 0.5 g of finely ground powder was mixed with approximately 6 g of flux (67 % lithium tetraborate +33 % lithium metaborate) and 0.02 g of lithium iodide as bead-releasing agent. The mixture was subjected to high-temperature treatment in a fusion bead machine (Perl’ X3, PANAlytical). The fused beads were analysed with a quantitative (MAYORES) method developed by PANalytical.
The structural characterisation of crystalline phases present in the samples was performed by X-ray diffraction (XRD) technique. For XRD analyses, data were registered from 20 to 120° 2θ employing a PANalytical X´Pert PRO diffractometer operating in θ–θ configuration, with Cu Kα radiation.
Scanning electron microscopy (SEM) was employed for surface morphology studies. These studies were carried out by using Zeiss (Model EVO LS 15) and semi-quantitative chemical microanalysis was performed by an energy dispersive X-ray spectrometer (EDX) from Oxford (Model INCA-Energy 350).
The total content of C was determined by combustion employing Leco CS 244 instrument.
Accuracy and precision of the analysis procedures for total element concentration by ICP-OES, FAES, WDXRF and DMA-80 were verified using several certified reference materials (CRM): Montana Soil NIST 2710, Estuarine Sediment IAEA-405 and Marine Sediment IAEA-433. The obtained results are consistent with the certified values with errors lower than 5 %. Element concentrations in procedure blanks and in all reagents were always below the detection limit.
Hg and concomitant elements fractionation
An overview of the sequential scheme for the fractionation of Hg used in this research is given in Table 1. This is a recent SEP specifically developed to evaluate Hg fractionation in solid environmental samples according to the unique physical and chemical properties of the numerous Hg species (Fernandez-Martinez and Rucandio 2013). Extractions were conducted in centrifuge tubes with 0.5 g of dried soil. Continuous agitation was maintained during appropriate time by an end-over-end shaker. Samples were centrifuged at 4000 rpm for 10 min after each extraction step. The supernatants were removed using a Pasteur pipette and transferred to a vial. All samples were washed with 5 mL of ultrapure water between the different extractions. Washed solutions were combined with their corresponding extracts, filtered through 0.45 μm cellulose paper, diluted with water to a 50 ml-final volume and then analysed by electrothermal atomic absorption spectrometry using the DMA-80 instrument. To dissolve the present silicates a final aqua regia and hydrofluoric acid digestion of the residue was added (residue digestion). Hg from the residual phase is not determined as stated for other metals, because it is seldom associated with silicate minerals (Martian-Doimeadios et al. 2000). Validation of the proposed extraction method was carried out by its application to the certified reference material (CRM) NIST 2710 (Montana Soil).
Results and discussion
Total element characterisation
The pressed pellets were analysed with two different methods, an application for major elements and another for trace elements. The elements were classified in three groups (Table 2) as a function of their concentrations: (1) the major elements with concentrations in all cases were expressed as a percentage. (2) The minor elements, with concentrations ranging between tens and thousands of mg kg−1. (3) The elements in lower concentrations or trace elements with concentrations between units and tens of mg kg−1 or even below the quantification limit of WDXRF.
The samples were collected in a volcanic region. Momotombo volcano is located on the shores of Lake Xolotlan (Fig. 1). Therefore, WDXRF analysis showed that part of the major elements (Si, Fe, Al, Ca and Na) were structural components of the most common minerals to be found in these soils such as silicates (usually orthoclase, plagioclase), quartz, biotite, mica, olivine, amphibole and pyroxene coming mainly from volcanic ashes (Ruggieri et al. 2012).
The total concentrations of metals are shown in Table 2. The total metal concentrations varied significantly with sampled sites. As a general tendency, the minimum concentration values were recorded at the point N4 with the exception of As and Ni. Si was the most abundant major element, which ranged from 30 to 24 %. It was followed by Al and C with concentration ranging from 6.4 to 2.3 and 8.1 to 1.8 %, respectively. Fe showed concentrations between 7.0 and 1.8 %. The other major elements (Ca, Mg, S, Ti and V) showed concentrations below 3 %. It is remarkable that the concentrations of V, which ranged from 0.72 to 0.077 %, were higher than those found in other volcanic soils (Beccaluva et al. 1991; Musta et al. 2008; Ruggieri et al. 2012).
Ba and Mn showed the highest concentrations of minor elements. The concentrations of other minor elements, such as As, Cu, Sr and Hg, h were less than 225 mg kg−1. Hg concentrations ranged between 1.0 and 122.5 mg kg−1. Hg concentration range, measured by DMA-80, was similar to those found in soils impacted by CAPs in Portugal (1.0–90.8 mg kg−1) or Romania (0.074–114 mg kg−1) (Frentiu et al. 2013; Reis et al. 2010) but were higher than Hg concentrations found in other impacted sites as a mountainous catchment in France (0.016–0.399 mg Kg−1) or Germany (0.15–4.2 mg kg−1) (Biester et al. 2002; Hissler and Probst 2006). It is remarkable that, although the point N2 is sited immediately close to the drain ditch, great Hg enrichment occurred in the site N6, which means that the sinking of the area in N2 occurred in a larger extent than in the other points.
The results of the crystalline characterisation by XRD technique showed a very similar composition in the six samples, as it is shown in Table 3, where the four main phases are summarised. Also, it is shown evidence of amorphous content in the samples, particularly in the samples N3 and N4.
The results obtained by XRD analysis were corroborated by using a scanning electron microscope with an energy dispersive X-ray (SEM/EDX).
The crystalline phases found were clearly identified with the volcanic type of soil. The main mineral phase was the feldspar labradorite [Ca0.65Na0.32 (Al1.62Si2.38O8)], an intermediate to calcic member of the plagioclase series, a common mineral in volcanic ash soils (Shoji et al. 1994). Maghemite (Fe2O3) has the same structure as magnetite, that is, it is spinel ferrite and is also ferromagnetic. It was associated with ilmenite (FeTiO3) which also appeared in SEM analysis. Ilmenite is a common accessory mineral found in igneous rocks.
The analysis by SEM/EDX concluded that the sample N4 had a high content of diatoms (Fig. 3a). This is consistent with the results obtained by XRD technique that showed the highest amorphous content in the sample N4. Diatoms are unicellular algae, distinguished from other structures by having a siliceous algae shell, formed by the accumulation of the amorphous silica (opal, SiO2·nH2O), accompanied by other metals mainly as oxides. The sample N2 did not present diatoms in its composition, however a labradorite structure was present. Iron oxides were also observed in this sample, combined with Ti forms (ilmenite), again confirming the XRD results.
The structure of iron oxide, in our case the spinel type structure Fe2O3, was also observed in the sample N4. SEM analyses, performed in the samples N4 and N6, also showed the adsorption of V and other metals onto iron oxides, which are, in some cases, combined with C, that suggests an anthropogenic source for all of them (Fig. 3b, c). SEM also indicated the presence of Hg in the sample N6 (Fig. 3d), that is the closest site to the Pennwalt CAP, and also the point with the highest concentration of Hg.
Hg fractionation
The Hg-specific sequential extraction scheme was applied to the studied samples and CRM NIST 2710. The results are summarised in Table 4. Hg recovery from CRM as the comparison of the sum of Hg concentration in each fraction with the certified value was found acceptable with a value of 95.6 %. Hg recoveries above 90 % were reached in all the samples studied, which can be considered satisfactory. In general, comparison among replicates of the same fractions was in agreement and the relative standard deviations were below 10 % in most cases. The percentages of each fraction referred to the total Hg contents are represented in the Fig. 4. The distribution of mercury among the different fractions was globally similar in all samples with the exception of sample N6. In samples N1–N5 mercury was mainly distributed in the elemental mercury fraction followed by the humic and fulvic bound mercury fraction. On the contrary, the Hg distribution pattern was very different in the sample N6, where the Hg sulphide and refractory species fraction clearly prevailed representing about 65 % of the total Hg content. Hg Hum/Ful was the second most abundant fraction while Hg Elem/CrystOx only represented 11 % of total Hg content.
The first fraction (F1) represents the most labile mercury species and consequently the most available mercury present in soils. The Hg extracted in the first step was very low, remaining below 0.5 mg kg−1 in the N1–N5 samples and only 4.4 mg kg−1 in the sample N6. Moreover, this fraction accounted for very low percentages and only in the N5 soil, the farthest sampled point from the plant, and whose location in the lowest topographic level (56.7 m above sea level), represents approximately 7 % of the total mercury. As a priori assumption, part of the mercury released by the CAP must have been deposited as Hg(II) since it has been hypothesised that 30 % of all the Hg emissions from chlor-alkali plants correspond to Hg(II) species (USEPA 1997). Hence, it would be predictable a significant content of mobile mercury, especially in the nearest soils (Biester et al. 2002). However, the poor results obtained may indicate that the available mercury could be leached by run-off since the area is affected by abundant precipitations or it could be complexed by strong ligands such as humic and fulvic acids or sulphides present in soils.
In the second step (F2), the Hg complexed with humic and fulvic acids present in soil organic matter was extracted. In terms of Hg concentration Hg Hum/Ful was the second most important fraction for all the samples. The Hg concentrations ranged between 0.1 mg kg−1 in the sample N5 and 23.6 mg kg−1 in the sample N6. Regarding total Hg, the Hg Hum/Ful fraction accounts for above 30 % in most samples with a maximum percentage of approximately 40 % in the sample N1. The obtained results indicated that Hg(II) complexation by humic and fulvic acids occurred in a large extent as it can be observed in other soils polluted by CAP emissions (Biester et al. 2002). The accumulation of Hg in this fraction might have occurred in two possible ways: (1) dry deposition of Hg(II) emitted by the CAP and a subsequent complexation by soil organic matter and (2) oxidation of a portion of deposited Hg(0) to Hg(II) (Panyametheekul 2004). In both cases the accumulation is expected to be increased with the deposition of Hg from the CAP. Then, the complexation of Hg by organic matter seems to depend on the total amount of deposited Hg in the studied soils (Biester et al. 2002). The strong correlation (r 2 = 0.9969 at P < 0.05) found between Hg in the Hum/Ful fraction and the total Hg supports this hypothesis.
The Hg extracted in the third fraction (F3) may be interpreted as an estimation of elemental Hg and Hg associated to Fe crystalline oxides present in the samples. Since deposition of elemental Hg vapour is a major source of Hg in areas affected by CAP emissions, it is predictable that most of Hg leached in this fraction corresponds to elemental Hg. The lack of correlation between Hg and Fe contents extracted in the Elem Hg/CrystOx. fraction seems to support this hypothesis (r 2 = 0.1007 at P < 0.05). In most samples (N1–N5), the F3 was the most abundant fraction ranging from 50 to 73 % of the total Hg content. The exception was the sample N6, where the elemental Hg concentration was only the third most abundant fraction. Hence, distribution of Hg clearly indicates an accumulation of the elemental Hg in the studied soils. This assumption is also confirmed by the very high correlation between the relative amount of Hg in the third fraction and the total Hg (r 2 = 0.9472 at P < 0.05). The obtained results were close to values reported by Frentiu et al. (2013) for soils in the surroundings of a former CAP who attributed 38–98 % of the total Hg to the elemental Hg fraction. Neculita et al. (2005) also reported contents of elemental Hg as high as 88–98 % of the total Hg in soils from CAPs in The Netherlands, Belgium and France. The presence of elemental mercury in soils is important because it could be re-emitted to the atmosphere by volatilisation, suffer oxidation to more reactive Hg(II) and even be transformed by bacteria into the more toxic methylated mercury compounds (Biester et al. 1999; Colombo et al. 2013; Gray et al. 2004; Hu et al. 2013).
The measurement of Hg after the fourth extraction (F4, Hg sulph/ref) interpreted as an estimation of the Hg present as cinnabar or other sulphide Hg forms, accounted for only 0.1–4 % at the N1–N5 sampling points indicating that the Hg sulphide complexes are not significant in these points. However the Hg concentration in this fraction was 77.4 mg kg−1 in the sample N6, which represents 63.2 % of the total Hg content. On the contrary to what occurred in the other studied sampling points, highly stable Hg species prevailed in the point N6, indicating that Hg stabilisation with naturally present sulphide may have occurred. Hg stabilisation in natural soils through the formation of sulphide complex is well established. It has been reported that Hg is preferentially bound to the sulphur-containing groups rather than other scavengers present in soils as organic matter or Fe and Mn oxides (Feyte et al. 2010; Fuhrmann et al. 2002).
Distribution of other concomitant elements
Figure 5 shows the distribution of the main elements in the samples among the operationally defined fractions of the applied SEP (F1–F4) and the subsequent residue digestion. The percentages were calculated with respect to the total concentrations obtained by XRF (Table 2).
Al and Ca were present mostly in the residual fraction. This behaviour was expected since these elements were present in labradorite (Table 3), which is a highly insoluble silicate. However, the samples N4, N5 and N6 showed high percentages of Ca (45–68 %) in the more labile fraction (F1) indicating a great availability of calcium in these points. Traditionally, Ca is extracted in large extent in the labile fraction in SEP associated with carbonates (Fernández-Martínez 2006; Fernandez-Martinez and Rucandio 2014).
The sum of the Ba recoveries in the F1–F4 steps from the SEP ranged from 57 to 91 % (Fig. 5). By SEM analysis, barite (BaSO4) has been observed in the sample N2. This is a very insoluble compound that must be present in the residual fraction, which can justify the variability in recoveries.
As occurred with Ca, the major proportions of extracted Ba and Mg were obtained in the fraction F1. F1 extracts the most labile compounds.
After submitting the samples to the SEP method, it can be seen that the Fe percentages were significant in all fractions. In the last two fractions the obtained recoveries ranged from 3 to 42.2 %. In these two fractions it is possible to recover elements of structural composites, in this case spinel type structure Fe2O3, crystalline compounds previously reported in the XRD analysis. The first and second extractants (F1 and F2) are able to recover metals that have high mobility and are also available for plants and organisms. The amounts of Fe recovered in these two fractions were also important (maximum of 26 %) and slightly higher than those found in the labile fractions in other works (Adaikpoh 2011). This behaviour suggests that Fe also has an anthropogenic influence.
High percentages of V (40–100 % of the total content) were extracted by the SEP in all sampling points with the exception of N2 in which the most V content remains as an unextracted residue. Moreover, the percentages extracted in the labile fraction showed a high availability of V in the samples N1, N3, N4, N5 and N6. Also Mn presented high percentages extracted in F1 (labile fraction) in the samples N3, N4 and N6. Such high extracted values also suggest an anthropogenic source of these elements.
Figure 1 shows that near CAP, an oil refinery is located, which can release these elements (Al, Ca, Fe, Mg, Mn and V) in a readily available form. The samples presented high percentages of these elements in the fraction 1, indicating an important availability of them, and suggesting that there were certain elements (Al, Ca, Cr, Fe, K, Mg, Mn, Na, Ni, S, Si, Ti and V) commonly released from petroleum combustion: concentrations of these elements in petroleum ashes ranged from units of mg kg−1 to percentages (Groen and Craig 1994).
Trace elements
The distribution data showed that the percentage of the residual fraction, of As changes within the wide range of 38.9–100 % of its total concentration. In the points N3 and N5, As was released in important amounts during the step 2 (sodium pyrophosphate at pH 10). This behaviour is expected since As is usually present in soils and sediments as anionic species such as arsenate and arsenite that are predominantly extracted under alkaline conditions (Jang et al. 2005; Muller et al. 2007).
Co was predominantly extracted in the residual fraction at all the sampling sites suggesting that it is mainly from lithogenic origin. However, high Co percentages in fraction 1 were found in the samples N3, N4 and N5 (21–48 % of the total Co content) indicating a certain availability of cobalt in these points. Since several studies have established that Co can be effectively adsorbed onto Fe and Mn oxyhidroxides (Stockdale et al. 2010; Tongtavee et al. 2005), the Co content in F1 could be related with that associated to Fe and Mn oxyhydroxides which were extracted mainly in the first step according to the applied SEP (Fernandez-Martinez and Rucandio 2013).
Cr appeared mainly bound to organic compounds (25–45 %) and sulphides (49–59 %) in most samples being the exception the samples N2 and N6. Binding of Cr(III) to humic and fulvic acids and sulphides is well established, and even reduction of Cr(VI) by organic matter in soils and groundwater has been described in the literature as a mechanism of natural attenuation of Cr(VI) content (Di Palma et al. 2012; Xiao et al. 2012). In the case of the samples N2 and N6, Cr was extracted in residual fraction, which means that is present associated to silicate minerals as occurs in other natural soils (Erzen and Stupar 2003).
Cu was predominantly associated with the non-residual fractions (78.1–89.7 % of total Cu content). High percentages were found in the F1 fraction indicating a high availability of Cu in these soils which again suggests the anthropogenic influence from the nearby refinery (Groen and Craig 1994). The same behaviour has been reported for soils surrounding copper smelters in Finland (Kabala and Singh 2001). Significant percentages found in the second fraction for Cu were expected since this metal shows high affinity for humic substances (Fytianos and Lourantou 2004; Kabata-Pendías 1992; Stevenson 1982).
More than 65 % of the total Ni was present in the residual fraction indicating that it was mainly from natural origin, which is in agreement with other studies on soils (Mester et al. 1998; Yuan et al. 2004). Substantial amounts of Ni were also found in F3 in the samples N3–N6. This could be related to the association of Ni with Fe oxides as occurred in other studies on soils (Sowder et al. 2003).
The concentration of Pb in the residual fraction at all sampling points accounted for <5 % of its total concentrations, being N4 the exception. Among the non-residual fractions, F3 was predominant (40–58 %). It has been stated that Pb can form stable complexes with low and highly crystalline Fe oxides which would be presumably leached during the third step of the SEP (Ramos et al. 1994). The high percentages found in the labile fraction also suggest the influence of the oil refinery in the studied area (Mahanta and Bhattacharyya 2011).
Sr was notably extracted in the first fraction accounting for 26–47 %. As occurred with Ca, the high Sr amounts extracted in the labile fraction cannot be attributed to the solubilisation of Ca due to the low content of inorganic C. The main fraction was the residual one, which means that Sr was associated with primary minerals as silicates. Sr chemical diffusion in plagioclase is well established being dominated by Sr + 2 → Ca + 2 exchange in calcic plagioclase (Cherniak and Watson 1994). However these phases were not detectable by XRD due to the low concentration of Sr.
Zn was uniformly distributed among the four operationally defined fractions with the highest percentages found in the most available fraction. This was in accordance with other fractionation studies in soils affected by oil refinery where Zn was found as the most available trace element as occurred with soils from two industrialised areas in Korea (Chon et al. 1998). Similar Zn mobility was observed in soils from urban areas affected by oil refineries (Adaikpoh 2011; Imperato et al. 2003). This high amount of Zn associated with the non-residual phases indicated that it can be easily mobilised through pH or ionic composition changes in the environment (Li et al. 2010).
Conclusions
The results of this study show that 20 years after CAP closure, the studied area is clearly affected by the historic dumping of mercury wastes and atmospheric mercury emissions from the CAP. Sequential extractions showed that mercury is present mainly as the elemental form and bound to organic complexes.
From these results it is reasonable to suggest that enrichment of elemental mercury from CAP emissions in nearby soils occurred, and the rate of deposition decreases as the distance to the plant increases. An important part of the Hg emitted by the CAP appeared fixed by the organic matter through the formation of complexes with humic and fulvic acids. This makes the soils from abandoned CAP a long term Hg reservoir for local environment which it could be released by weathering or drastic environmental changes.
Besides the influence of the volcanic emissions and flooding, the results also indicate the potential environmental impact of other contaminants from a different anthropogenic source. Our results suggest that the emissions from the oil refinery next to the CAP may be responsible for the impact caused by other elements such as Mg, Mn, Cu, Pb, V and Zn. Contrary to what happens with the CAP, the oil refinery is still working. Then, oil refinery probably constitutes a continuous input of contaminants to the surrounding area. Further studies should include an assessment of pollutants impacting the environment at Lake Xolotlán, as well as its source.
References
Adaikpoh EO (2011) Metal fractionation in soil profiles in Umutu oil field, Northwest Niger Delta Nigeria. Int J Chem 3:57–67. doi:10.5539/ijc.v3n1p57
Beccaluva L, Digirolamo P, Serri G (1991) Petrogenesis and tectonic setting of the Roman volcanic province, Italy. Lithos 26:191–221. doi:10.1016/0024-4937(91)90029-k
Biester H, Gosar M, Muller G (1999) Mercury speciation in tailings of the Idrija mercury mine. J Geochem Explor 65:195–204. doi:10.1016/S0375-6742(99)00027-8
Biester H, Muller G, Scholer HF (2002) Binding and mobility of mercury in soils contaminated by emissions from chlor-alkali plants. Sci Total Environ 284:191–203. doi:10.1016/S0048-9697(01)00885-3
Bloom NS, Preus E, Katon J, Hiltner M (2003) Selective extractions to assess the biogeochemically relevant fractionation of inorganic mercury in sediments and soils. Anal Chim Acta 479:233–248. doi:10.1016/s0003-2670(02)01550-7
Cherniak DJ, Watson EB (1994) A study of strontium difussion in plagioclase using a Rutherford backscattering spectroscopy. Geochim Cosmochim Acta 58:5179–5190. doi:10.1016/0016-7037(94)90303-4
Chon HT, Ahn JS, Jung MC (1998) Seasonal variations and chemical forms of heavy metals in soils and dusts from the satellite cities of Seoul, Korea. Environ Geochem Health 20:77–86. doi:10.1023/a:1006593708464
Climate-Nicaragua (2015). http://www.climatestotravel.com/Climate/Nicaragua. Accessed 15 Nov 2015
Colombo MJ, Ha JY, Reinfelder JR, Barkay T, Yee N (2013) Anaerobic oxidation of Hg(0) and methylmercury formation by Desulfovibrio desulfuricans ND132. Geochim Cosmochim Acta 112:166–177. doi:10.1016/j.gca.2013.03.001
Di Palma L, Mancini D, Petrucci E (2012) Experimental assessment of chromium mobilization from polluted soil by washing. In: Bosicon 2012: 3rd international conference on contaminated sites remediation, vol 28. Chemical Engineering Transactions, pp 145–150. doi:10.3303/cet1228025
Digiulio RT, Ryan EA (1987) Mercury in soils, sediments, and clams from a North-Carolina peatland. Water Air Soil Pollut 33:205–219. doi:10.1007/BF00191389
Ebinghaus R et al (1999) International field intercomparison measurements of atmospheric mercury species at Mace Head, Ireland. Atmos Environ 33:3063–3073. doi:10.1016/s1352-2310(98)00119-8
Erzen NK, Stupar J (2003) Fractionation of chromium in soils treated with aqueous solutions of Cr(VI) and Cr(III). Acta Chim Slov 50:67–81. doi:10.1039/A909597G
Fernández-Martínez R (2006) Desarrollo y aplicación de nuevas metodologías para el estudio del fraccionamiento y movilidad del mercurio en muestras medioambientales. Thesis Dissertation, Universidad Autónoma de Madrid
Fernandez-Martinez R, Rucandio I (2013) Assessment of a sequential extraction method to evaluate mercury mobility and geochemistry in solid environmental samples. Ecotoxicol Environ Saf 97(196–203):2013. doi:10.1016/j.ecoenv.07.013
Fernandez-Martinez R, Rucandio I (2014) Total mercury, organic mercury and mercury fractionation in soil profiles from the Almaden mercury mine area. Environ Sci Processes Impacts 16:333–340. doi:10.1039/c3em00445g
Feyte S, Tessier A, Gobeil C, Cossa D (2010) In situ adsorption of mercury, methylmercury and other elements by iron oxyhydroxides and organic matter in lake sediments. Appl Geochem 25:984–995. doi:10.1016/j.apgeochem.2010.04.005
Frentiu T, Pintican BP, Butaciu S, Mihaltan AI, Ponta M, Frentiu M (2013) Determination, speciation and distribution of mercury in soil in the surroundings of a former chlor-alkali plant: assessment of sequential extraction procedure and analytical technique. Chem Cent J 7:178. doi:10.1186/1752-153x-7-178
Fuhrmann M, Melamed D, Kalb PD, Adams JW, Milian LW (2002) Sulfur polymer solidification/stabilization of elemental mercury waste. Waste Manag 22:327–333. doi:10.1016/s0956-053x(01)00057-5
Fytianos K, Lourantou A (2004) Speciation of elements in sediment samples collected at lakes Volvi and Koronia, N. Greece. Environ Int 30:11–17. doi:10.1016/s0160-4120(03)00143-0
Garron C, Gagne F, Ernst W, Julien G, Bernier M, Caldwell C (2005) Mercury contamination of marine sediments and blue mussels (Mytilus edulis) in the vicinity of a mercury cell chlor-alkali plant in Dalhousie, New Brunswick, Canada. Water Qual Res J Can 40:1–15
Gray JE, Hines ME, Higueras PL, Adatto I, Lasorsa BK (2004) Mercury speciation and microbial transformations in mine wastes, stream sediments, and surface waters at the Almaden Mining District, Spain. Environ Sci Technol 38:4285–4292. doi:10.1021/es040359d
Groen JC, Craig JR (1994) The inorganic geochemistry of coal, petroleum, and their gasification/combustion products. Fuel Process Technol 40:15–48. doi:10.1016/0378-3820(94)90033-7
Han Y et al (2003) Speciation of mercury in soil and sediment by selective solvent and acid extraction. Anal Bioanal Chem 375:428–436. doi:10.1007/s00216-002-1701-4
Hassan A et al (1981) Mercury-poisoning in Nicaragua—a case study of the export of environmental and occupational-health hazards by a Multinational-Corporation. Int J Health Serv 11:221–226. doi:10.2190/n9y9-ymu2-d6r1-lpyp
Hintelmann H, Wilken RD (1995) Levels of total and methylmercury compounds in sediments of the pôlluted Elbe River—influence of seasonally and spatially varying environmental-factors. Sci Total Environ 166:1–10. doi:10.1016/0048-9697(95)04506-v
Hissler C, Probst JL (2006) Impact of mercury atmospheric deposition on soils and streams in a mountainous catchment (Vosges, France) polluted by chlor-alkali industrial activity: the important trapping role of the organic matter. Sci Total Environ 361:163–178. doi:10.1016/j.scitotenv.2005.05.023
Hu HY et al (2013) Oxidation and methylation of dissolved elemental mercury by anaerobic bacteria. Nat Geosci 6:751–754. doi:10.1038/ngeo1894
Imperato M, Adamo P, Naimo D, Arienzo M, Stanzione D, Violante P (2003) Spatial distribution of heavy metals in urban soils of Naples city (Italy). Environ Pollut 124:247–256. doi:10.1016/s0269-7491(02)00478-5
Jang M, Hwang JS, Choi SI, Park JK (2005) Remediation of arsenic-contaminated soils and washing effluents. Chemosphere 60:344–354. doi:10.1016/j.chemosphere.2004.12.018
Kabala C, Singh RR (2001) Fractionation and mobility of copper, lead, and zinc in soil profiles in the vicinity of a copper smelter. J Environ Qual 30:485–492. doi:10.2134/jeq2001.302485x
Kabata-Pendías APH (ed) (1992) Trace elements in soils and plants, 3rd edn. CRC Press, Boca Raton
Lacayo M, Cruz A, Lacayo J, Fomsgaard I (1991) Mercury contamination in Lake Xolotlan (Nicaragua) In: International association of theoretical and applied limnology—proceedings, Vol 24, Pt 2, vol 24. International Association of Theoretical and Applied Limnology—Proceedings, pp 1174–1177
Li JH, Lu Y, Shim HJ, Deng XL, Lian J, Jia ZL, Li JH (2010) Use of the BCR sequential extraction procedure for the study of metal availability to plants. J Environ Monit 12:466–471. doi:10.1039/b916389a
Mahanta MJ, Bhattacharyya KG (2011) Total concentrations, fractionation and mobility of heavy metals in soils of urban area of Guwahati, India. Environ Monit Assess 173:221–240. doi:10.1007/s10661-010-1383-x
Martian-Doimeadios RCR, Wasserman JC, Bermejo LFG, Amouroux D, Nevado JJB, Donard OFX (2000) Chemical availability of mercury in stream sediments from the Almaden area, Spain. J Environ Monit 2:360–366
McCrary JK, Castro M, McKaye KR (2006) Mercury in fish from two Nicaraguan lakes: a recommendation for increased monitoring of fish for international commerce. Environ Pollut 141:513–518. doi:10.1016/j.envpol.2005.08.062
Mester Z, Cremisini C, Ghiara E, Morabito R (1998) Comparison of two sequential extraction procedures for metal fractionation in sediment samples. Anal Chim Acta 359:133–142. doi:10.1016/S0003-2670(97)00687-9
Muller K, Daus B, Morgenstern P, Wennrich R (2007) Mobilization of antimony and arsenic in soil and sediment samples—evaluation of different leaching procedures. Water Air Soil Pollut 183:427–436. doi:10.1007/s11270-007-9391-3
Musta B, Fitria H, Soehady W, Tahir S (2008) Geochemical characterization of volcanic soils from Tawau, Sabah. Bull Geol Soc Malaisya 54:33–36. doi:10.7186/bgsm2008006
Neculita CM, Zagury GJ, Deschenes L (2005) Mercury speciation in highly contaminated soils from chlor-alkali plants using chemical extractions. J Environ Qual 34:255–262. doi:10.2134/jeq2005.0255
Panyametheekul S (2004) An operationally defined method to determine the speciation of mercury. Environ Geochem Health 26:51–57. doi:10.1023/B:EGAH.0000020967.03217.5f
Ramos L, Hernandez LM, Gonzalez MJ (1994) Sequential fractionation of copper, lead, cadmium and zinc in soils from or near Donana-National-Park. J Environ Qual 23:50–57. doi:10.2134/jeq1994.00472425002300010009x
Reis AT, Rodrigues SM, Davidson CM, Pereira E, Duarte AC (2010) Extractability and mobility of mercury from agricultural soils surrounding industrial and mining contaminated areas. Chemosphere 81(1369–1377):2010. doi:10.1016/j.chemosphere.09.030
Revis NW, Osborne TR, Sedgley D, King A (1989) Quantitative method for determining the concentration of mercury(II) sulfide in soils and sediments. Analyst 114:823–825. doi:10.1039/AN9891400823
Ruggieri F et al (2012) Contribution of volcanic ashes to the regional geochemical balance: the 2008 eruption of Chaiten volcano, Southern Chile. Sci Total Environ 425:75–88. doi:10.1016/j.scitotenv.2012.03.011
Sakamoto H, Tomiyasu T, Yonehara N (1992) Differential determination of organic mercury, mercury(II) oxide and mercury(II) sulfide in sediments by cold vapor atomic-absorption spectrometry. Anal Sci 8:35–39
Shoji S, Nanzyo M, Dahlgren RA (1994) Volcanic ash soils: genesis, properties and utilization. Elsevier, Amsterdam
Sowder AG, Bertsch PM, Morris PJ (2003) Partitioning and availability of uranium and nickel in contaminated riparian sediments. J Environ Qual 32:885–898. doi:10.2134/jeq2003.8850
Stevenson JWL (ed) (1982) Humus chemistry: genesis, composition, reactions. Wiley, New York
Stockdale A, Davison W, Zhang H, Hamilton-Taylor J (2010) The association of cobalt with iron and manganese (oxyhydr)oxides in marine sediment. Aquat Geochem 16:575–585. doi:10.1007/s10498-010-9092-1
Tongtavee N, Shiowatana J, McLaren RG, Buanuam J (2005) Evaluation of distribution and chemical associations between cobalt and manganese in soils by continuous-flow sequential extraction. Commun Soil Sci Plant Anal 36:2839–2855. doi:10.1080/00103620500306023
USEPA (1997) Mercury study report to congress. Volume III: Fate and transport of mercury in the Environment Office of Air Quality Planning and Standards. US Environmental Protection Agency (USEPA), Washington, DC
Wyrick B (1981) Chemical plant’s poison inflames a nation, part VIII: hazards for export. Newsday, USA
Xiao WD, Zhang YB, Li TQ, Chen B, Wang H, He ZL, Yang XE (2012) Reduction kinetics of hexavalent chromium in soils and its correlation with soil properties. J Environ Qual 41:1452–1458. doi:10.2134/jeq2012.0061
Yuan CG, Shi JB, He B, Liu JF, Liang LN, Jiang GB (2004) Speciation of heavy metals in marine sediments from the East China Sea by ICP-MS with sequential extraction. Environ Int 30:769–783. doi:10.1016/j.envint.2004.01.001
Acknowledgments
We acknowledge Miguel Ángel Labajo for SEM-EDX measurements and the staff of the Chemistry Division at CIEMAT.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fernández-Martínez, R., Gómez-Mancebo, B., Peña, E.J. et al. Monitoring of mercury and other metals mobility by sequential fractionation in soils nearby an abandoned chlor-alkali plant in Managua (Nicaragua). Environ Earth Sci 75, 538 (2016). https://doi.org/10.1007/s12665-015-5171-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-015-5171-3