Abstract
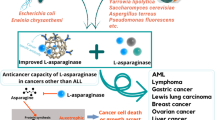
L-asparaginase, a cancer therapeutic enzyme, has attracted a great deal of attention from the scientific community. Although it was adopted in the treatment of leukaemia decades ago, its clinical formulations need to be improved. Current asparaginase formulations are derived from the terrestrial microorganisms, Escherichia coli and Erwinia chrysanthemi that elicit severe side effects to the patient. Moreover, these forms of asparaginase have been observed with a short half-life in the blood circulation, thereby requiring frequent dosage of the drug. Altogether, this results in increasing the cost of the treatment with lower clinical efficacy. Therefore, considerable research has been prompted in the acquisition of alternative sources of asparaginase with improved biochemical properties. Among the different sources, the marine environment is sparsely explored for biological products and enzymes. Marine resources that encompass vast diversity are a treasure trove of novel natural products. This chapter features the potential of marine-derived L-asparaginase and underlines the milestone chronicles of events in its discovery and adoption in the treatment of acute lymphoblastic leukaemia. Further, the nanoformulation of L-asparaginase has also been discussed, and the importance of bioinspired nanocarriers and nanozymes in the development of cancer therapeutics has been highlighted.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
4.1 Introduction
L-asparaginase is an established anti-leukemic drug in the treatment of various lymphoid system malignancies and Acute Lympphoblastic Leukaemia (ALL). Since then, it is used as an anti-leukemic drug by virtue of its catalytic activity that hydrolyses the amino acid L-asparagine into aspartate and ammonia, leading to depleted concentration in the bloodstream (Amylon et al. 1999; Pieters et al. 2011; Shrivastava et al. 2016). Due to the unavailability of exogenous L-asparagine, leukemic cells starve and are unable to proliferate, ultimately leading to cell death (Ueno et al. 1997; Balasubramanian et al. 2013; Lomelino et al. 2017). Due to its explicit toxicity against the leukemic cells, it was incorporated in the treatment regime in the 1960s, which showed a marked increase in the percentage of wholly recovered patients with a higher survival rate (Broome 1963, 1965; Mashburn and Wriston 1964).
However, the clinical use of L-asparaginase derived from Escherichia coli, Dickeya chrysanthemi, and the PEGylated form of L-asparaginase are associated with various side effects and allergic reactions that impose a risk to the patient’s health (Dellinger 1976; Evans 1982; Asselin et al. 1999; Duval et al. 2002; Fu and Sakamoto 2007). Moreover, L-asparaginase has low stability in the blood and rapid clearance from the body that requires a higher number of doses, which leads to the development of resistance against the drug and also increases the cost of the treatment (Pieters et al. 2011; Tong et al. 2013; Chien et al. 2015; Lee et al. 2019). Therefore, it is imperative to combat these therapeutic challenges by screening alternative sources of L-asparaginase and employing different strategies to develop a therapeutically efficacious drug with higher stability and less toxic side effects in the patient.
Lately, the marine environment has attracted the attention of researchers for exploring this vast ecosystem. A quote from the preeminent science fiction and nonfiction writer, Sir Arthur C. Clarke, distinctly advocates the abounding marine environment,
How inappropriate to call this planet “Earth,” when it is clearly “Ocean.”
Relatively, the marine environment that canvas two-thirds of the Earth’s surface with an expanded diversity of habitats has been mostly unexplored. It has an incredibly rich biological and chemical diversity, and the microorganisms in marine habitats possess a diverse range of potential enzymes (Das et al. 2006; Kennedy et al. 2008; Izadpanah Qeshmi et al. 2014; Prihanto and Wakayama 2016). These microorganisms that thrive under extreme conditions of the marine environment could prove to be a potential source for different enzymes with medicinal and industrial applications (Nguyen and Nguyen 2017; Izadpanah et al. 2018; Birolli et al. 2019; Cheng et al. 2020). Moreover, the nanotechnological advancements can be directed towards enhancing the stability of L-asparaginase in the blood plasma of patients. Several reports are available on the immobilization and encapsulation of L-asparaginase in nanocarriers, which have shown to increase the stability as compared to the native enzyme. Over the years, a plethora of nanomaterials have been employed for this purpose in different combinations, as well as by different surface modifications (He et al. 2014; Ulu and Ates 2017; Wahab et al. 2020). These formulations of the therapeutic enzyme have shown to acquire prolonged stability together with a slow and sustained release in the blood, which consequently aid in lowering the number of doses and improving the therapeutic efficacy of the drug (Vina et al. 2001; Zhang et al. 2004; Ghosh et al. 2012; Bahreini et al. 2014; Do et al. 2019).
Here, we have comprehensively reviewed the marine sources of L-asparaginase and the various improvements of this therapeutic drug by immobilization and nanoencapsulation techniques. Further, advents in the nanotechnology and their applications in therapeutics have been highlighted. The present chapter provides an essential insight into the potential role of the marine environment as a source for obtaining novel L-asparaginase and the role of nanotechnology in improving its therapeutic efficacy as an anti-leukemic drug.
4.2 Retrospective Analysis of L-asparaginase: Discovery and Background
The discovery of L-asparaginase was a fortuitous event when, in 1904, Lang observed L-asparaginase activity in the cow tissues (Lang 1904). From then on, several notable key events were registered in the history of L-asparaginase. Figure 4.1 illustrates the influential years that led to the development and characterization of L-asparaginase as an anti-leukemic drug. Years after Lang’s observation, Clementi reported that only herbivores exhibit L-asparaginase activity in all the organs, while in the case of omnivores, it is present in only specific organs, which disproved the Furth and Friedman’s statement (Clementi 1922). He unprecedently outlined the application of this enzyme in inhibition of cancer, which later got validated from the studies of Kidd (Kidd 1953) and Broome (Broome 1963, 1965), who found the inhibitory effect of guinea pig blood serum on murine lymphomas.
After gaining considerable attention as an anti-leukemic enzyme, researchers started exploring the microbial sources of L-asparaginase for clinical use. Mashburn and Wriston (Mashburn and Wriston 1964) demonstrated a highly purified L-asparaginase isolated from E. coli with comparable anti-lymphoma activity in guinea pig serum. Later, in 1966, two forms of L-asparaginase were determined in E. coli, out of which only one had the anti-leukemic property (Roberts et al. 1966). These findings encouraged several researchers in uncovering the potential of microorganisms in producing L-asparaginase that culminated in the production of the enzyme in quantities accompanied by several preclinical and clinical trials to study the therapeutic effects of the enzyme (Dolowy et al. 1966; Hill et al. 1967; Old et al. 1967; Ho et al. 1970). One such notable study was the finding of Erwinia carotovora (now called Erwinia chrysanthemi) as the L-asparaginase producer, which is now clinically used as an alternative formulation to E. coli asparaginase when patients are allergic to the latter (Wade et al. 1968; North et al. 1969). Although the bacterial source of L-asparaginase was being used for parenteral therapy, it was found to be associated with various issues like immunogenicity and hypersensitivity reactions when administered to the patient (Peterson et al. 1971; Killander et al. 1976). Consequently, studies were undertaken by several research laboratories directed towards reducing the immunogenicity. However, it only became possible in 1984, when a PEG-asparaginase adduct was prepared that considerably reduced the allergic reactions and immunogenicity (Abuchowski et al. 1984).
Meanwhile, when several researchers were indulged in screening alternative sources and clinical trials of L-asparaginase, some research groups were inclined towards investigating the physicochemical and biochemical properties of this enzyme (North et al. 1969; Irion and Voigt 1970; Cammack et al. 1972). It was 1970, when, for the first time, the molecular structure of L-asparaginase from E. coli was reported (Ho et al. 1970; Arens et al. 1970; Epp et al. 1971). These studies shed light on the subunit structure, molecular weight, and other physical and chemical properties of L-asparaginase. Soon after the Food and Drug Administration (FDA) approval of the first L-asparaginase formulation derived from E. coli (Elspar) in 1978, attempts were made towards investigating the primary structure together with the crystallization of the enzyme. Consequently, it was 1980 and 1988, when the amino acid sequence of L-asparaginase from E. coli and a preliminary crystal model of glutaminase-asparaginase (AGA) from Acinetobacter glutaminasificans, respectively, was established (Maita et al. 1974; Maita and Matsuda 1980; Ammon et al. 1988). By then, the amino acid sequence of L-asparaginase from different sources was available; therefore, Bronthon, in 1990, attempted to investigate the structure and location of the ansB gene encoding for L-asparaginase II in E. coli. He cloned, mapped, and sequenced the gene and reported differences in the amino acid sequence from those of the previously reported ones by direct amino acid sequencing (Bonthron 1990). Finally, in the same period, in 1993, Swain and his associates elucidated the true crystal structure of E. coli L-asparaginase at high resolution using a preliminary model of AGA as a template (Swain et al. 1993). A year later, in 1994, the PEGylated form of Asparaginase (Oncaspar) was granted FDA approval to treat ALL patients allergic to Elspar. However, in 2006, the FDA expanded Oncaspar as a first-line drug to treat ALL patients.
Presently, four formulations of Asparaginase are available in the market, namely, Asparaginase amidohydrolase, Erwinase (Erwinia chrysanthemi Asparaginase), Spectrila (recombinant E. coli Asparaginase), and Oncaspar, after the discontinuation of Elspar in 2013 by the manufacturer.
4.3 Efficacy of L-asparaginase Treatment: A Brief Overview
4.3.1 Asparagine Synthetase and L-asparaginase Therapy
The multi-drug treatment regimes for ALL and other malignant hematopoietic diseases incorporated asparaginase as an indispensable component for its capability to promote the tumour regression by diminishing the requirements of tumour cells.
To understand the distinct nutritional requirements of the tumour cells, Neuman and McCoy described a simplified growth medium for the Walker tumour-256 cells. Interestingly, they found that both asparagine and glutamine are needed by the tumour cells, and neither replacement with their corresponding carboxylic acids nor with each other restored their requirements (Neuman and Mccoy 1956). This finding was further confirmed by Haley and other studies (Haley et al. 1961), and henceforth, asparaginase was studied extensively as an anti-leukemic enzyme considering its catalytic property of hydrolysing asparagine. Nonetheless, some researchers found some contradicting results on the asparagine requirement of tumour cells. Lazarus et al. suggested asparagine synthetase activity as a more accurate determinant of the L-asparaginase sensitivity of tumour cells over their metabolic response to an exogenous source of asparagine (Lazarus et al. 1969). Asparagine synthetase catalyses the biosynthesis of asparagine and glutamate from aspartate and glutamine in an ATP (Adenosine Triphosphate) -dependent reaction. It has a critical role in treating ALL and is one of the aspects that decide the efficacy of L-asparaginase therapy. ALL is a type of malignancy of blood and lymphoid progenitor cells in the bone marrow, which is auxotrophic to the non-essential amino acid, asparagine. Due to the low expression level of asparagine synthetase, ALL cells stringently depend on the serum asparagine. L-asparaginase administration depletes the serum asparagine, and hence, tumour cells become sensitive to L-asparaginase therapy. Over the years, researchers have contemplated how the Asparagine synthetase activity of malignant cells is correlated with their acquired resistance to L-asparaginase (Aslanian and Kilberg 2001; Su et al. 2008; Liu et al. 2018). Some research groups concluded no relation between asparaginase synthetase expression and asparaginase sensitivity (Stams et al. 2003; Hermanova et al. 2012), while some explained the significance of thiamine, transcription factors like “Zinc Finger and BTB domain-containing protein 1” and metabolic profile of cancer cells in determining the asparaginase sensitivity of ALL cells (Guarecuco et al. 2020; Williams et al. 2020; Hlozkova et al. 2020).
In contrast to the asparaginase-sensitive cells, resistant cancer cells have been shown to overexpress asparagine synthetase that renders the asparaginase treatment ineffective (Horowitz et al. 1968; Kiriyama et al. 1989; Richards and Kilberg 2006). Although this appears to be directly correlated, the scenario is convoluted because the clinically used L-asparaginase formulations from E. coli and Erwinia chrysanthemi have side glutaminase activity that entails the depletion of both asparagine and glutamine from the blood serum. Asparagine synthetase requires glutamine as an amino-group donor for the synthesis of asparagine; due to its depletion, asparagine synthetase should not be able to synthesize enough asparagine to meet the nutritional requirement of cancer cells (Lomelino et al. 2017). Chien et al. investigated the asparaginase-sensitive (B-ALL) and resistant cell lines (KHYG1) and suggested that the high standard expression level of asparaginase synthetase together with increased expression of glutamine synthetase and asparagine synthetase in the resistant cell lines aids in replenishing both the amino acids. The study inferred asparagine synthetase regulatory pathways to be a vital determinant in the sensitivity cells towards Asparaginase (Chien et al. 2015). Recently, Sun et al. identified an essential role of SLC1A3, an aspartate, and glutamate transporter in the development of resistance against L-asparaginase in case of solid tumours (Sun et al. 2019).
4.3.2 Dual Activity of L-asparaginase
There has been a prolonging debate on the associated glutaminase activity of L-asparaginase and its role in the cytotoxicity of cancer cells. This dual activity of L-asparaginase has long been known for eliciting various side effects in the ALL patients that markedly decrease the efficacy of the treatment (Miller and Balis 1969; Killander et al. 1976; Warrell Jr et al. 1982). On the contrary, some reports suggest the glutaminase activity equally crucial as the asparaginase activity for achieving the complete cytotoxicity of cancer cells (Panosyan et al. 2004; Offman et al. 2011; Willems et al. 2013; Parmentier et al. 2015). In the recent past, Chan et al. attempted to untangle these conflicting views by investigating the in vitro anti-leukemic effect of a glutaminase-deficient mutant (Q59L) of L-asparaginase on asparagine synthetase (ASNS) positive and negative cancer cells. Their study suggested L-asparaginase without the glutaminase activity to be sufficiently effective against the ASNS-negative cancer cells.
In contrast, the treatment of ASNS-positive cancer cells mandatorily required the glutaminase side activity of L-asparaginase (Chan et al. 2014). Paradoxically, when the investigation was carried out in vivo, the glutaminase activity of L-asparaginase was found to be equally necessary for the anti-leukemic effect against the ASNS-negative cancer cells (Chan et al. 2019). However, concerning the clinically used L-asparaginase derived from E. coli and Erwinia chrysanthemi, the glutaminase activity is attributed with numerous side effects that entail detrimental effects in patient’s health. Therefore, it is not easy to reach an unambiguous conclusion on whether the accompanied glutaminase activity of L-asparaginase should be elevated or reduced. It was considering the associated side effects, namely, hyperglycemia, pancreatitis, thrombosis, encephalopathy, myelosuppression, hypertriglyceridemia, and hepatic toxicity; scientists hypothesize glutaminase-free L-asparaginase to be better and safe for the treatment of ALL (Banerji 2015; Hijiya and Van Der Sluis 2016; Battistel et al. 2020). Accordingly, proactive research towards developing a better variant of L-asparaginase is persuaded by the scientific community by employing several genetic and protein engineering approaches (Einsfeldt et al. 2016; El-Sharkawy et al. 2016; Pola et al. 2018; Ardalan et al. 2018; Farahat et al. 2020; Saeed et al. 2020). Besides this, new and novel L-asparaginase lacking the dual activity is actively being inspected from different sources that can be adopted as an alternative to the clinically used asparaginase (Huang et al. 2014; Darvishi et al. 2019; Mazloum-Ravasan et al. 2020).
4.3.3 Stability in the Bloodstream
Apart from the limitations mentioned above, L-asparaginase has concerns related to its stability and susceptibility to proteolysis cleavage (Stecher et al. 1999; Patel et al. 2009). Moreover, being a foreign protein, recurrent immunological allergic reactions have been observed to be elicited in patients when administered during the treatment regime (Hijiya and Van Der Sluis 2016). These pharmacokinetic and immunological limitations have repercussions on the stability of L-asparaginase, which ensues early clearance of the drug from the body. Thus far, the PEGylated form of L-asparaginase (Oncaspar®) has been approved by the FDA, which is employed as an alternative when patients show hypersensitivity to E. coli and E. chrysanthemi L-asparaginase formulations. The conjugation with polyethylene glycol (PEG) increases the overall molecular mass that promotes the prolonged circulation of the drug in the blood (Soares et al. 2002; Kotzia et al. 2007; Meneguetti et al. 2019). However, opposing data on the polyethylene glycol conjugation (PEG) has been reported with clinical immunological and antigenic reactions. PEG has been widely employed as a therapeutic drug conjugate with a molecular weight of ≤40 kDa, which is close to the kidney’s glomerular filtration threshold (50 kDa). Also, its non-biodegradable nature further apprehends the pharmacokinetics of the therapeutic drug. The clinical studies on the metabolism of low-molecular-weight PEG have shown the formation of by-products that have severe toxic effects like acidosis (Hamidi et al. 2006; Zhang et al. 2014; Bader and Wardwell 2014; Ekladious et al. 2019; Thi et al. 2020). Therefore, to ameliorate these issues, studies are prompted to develop new bio- and nanotechnological strategies that can bring about an improved form of L-asparaginase as a therapeutic drug.
4.4 Marine Environment: Bioprospecting for Novel L-asparaginase
“It is in the exploration of this vast deep-sea region that the finest field for submarine discovery yet remains.” — Edward Forbes (1859).
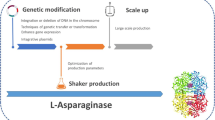
Marine environment, the “blue heart” of the Earth, covers a vast area of the planet with enormous biodiversity. Despite being the most extensive ecosystem, it has long been remained substantially unexplored, perhaps due to the harsh underwater environment that encompasses high pressure, temperature, and salinity. Nevertheless, the last couple of decades have presented an escalating curiosity towards the uncharted potential of marine microorganisms for biomolecules with novel properties (Krishna et al. 2013; Izadpanah Qeshmi et al. 2014; Bhargavi and Jayamadhuri 2016; Cheng et al. 2020). Since these microorganisms thrive in the unique marine conditions, the generation of biomolecules with uncommon features is anticipated. These biomolecules namely enzymes could be a better alternative to their terrestrial analogues. Thus, active research is persuaded towards inspecting the novel features of enzymes derived from marine microorganisms and their various applications in therapeutics and the food industry. A general scheme of isolation, screening and production optimization of marine microorganisms is illustrated in Fig. 4.2.
4.4.1 Marine L-asparaginase Producers
From way back, terrestrial macro- and microorganisms have been extensively explored and unceasingly been employed as an enzyme source. Similarly, the clinically adopted L-asparaginase formulations are derived from terrestrial microorganisms discredited for their associated toxicities and other immunological limitations. Therefore, it is obligatory to inspect other sources of L-asparaginase that could be a better alternative to the presently exploited forms. The marine environment that has intrigued many researchers holds excellent potential for obtaining L-asparaginase with biochemically and therapeutically novel and desired features. In the past few years, several reports on the marine L-asparaginase producers have been registered in the literature, including numerous actinomycetes, bacteria, and fungi (Dhevagi and Poorani 2006; Bhargavi and Jayamadhuri 2016; Shakambari et al. 2016; Vala et al. 2018). Among the actinomycetes, L-asparaginase producing Streptomyces sp. has been abundantly found in the marine environment, whereas Bacillus is the predominant L-asparaginase-producing marine bacterial species. Penicillium and Aspergillus sp. are the most isolated L-asparaginase-producing fungi from marine sources. Although different classes of microorganisms produce L-asparaginase, yet the bacterial source of enzymes is granted as a preferred choice over the others considering the ease in culturing, enzyme extraction, and the downstream processing.
These L-asparaginases from marine microorganisms are observed with desirable pharmaceutical features; however, more extensive investigation is required to formulate it as a better anti-leukemic enzyme that could be superior to the present forms of L-asparaginase.
4.4.2 Marine L-asparaginase: Biochemical Properties and Molecular Features
4.4.2.1 Effect of pH and Temperature
Marine L-asparaginases so far explored have been observed with some enviable biochemical properties as compared to their terrestrial counterparts. L-asparaginases derived from the most abundant bacterial sources, Bacillus and Pseudomonas sp. from the marine environment, are mostly produced extracellularly (Pradhan et al. 2013; Izadpanah Qeshmi et al. 2014; Badoei-Dalfard 2015). Being devoid of periplasmic space, the Gram-positive bacteria produce the enzymes and secrete in the culture medium, unlike the Gram-negative bacteria where the production is intracellular. These extracellularly secreted enzymes provide an additional advantage in purification and economic downstream processing (Vimal and Kumar 2017). The isolated marine L-asparaginases have been found to have pH optima in the range of 6–9 and optimum temperature in the range of 37–40 °C (Rahimzadeh et al. 2016; Lee et al. 2016; Shi et al. 2017). The pH and temperatures at which the marine L-asparaginase has been observed with the highest activity together with their respective stabilities are tabulated in Table 4.1. L-asparaginase with physiological pH and temperature optima have been observed in Bacillus PG02, Bacillus PG04, and Nocardiopsis alba NIOTVKMA08 with activity peaks at 35–40 °C (Meena et al. 2016; Izadpanah Qeshmi et al. 2015; Rahimzadeh et al. 2016; Shi et al. 2017) suggesting them as a suitable candidate for therapeutic purpose. In addition, marine asparaginases are found to be active in various concentrations of salt up to one molar. These marine microorganisms are thought to have adapted to the high salt concentration of marine environment making them halotolerant (Kamala and Sivaperumal 2017; Shaik et al. 2017).
4.4.2.2 Sensitivity Towards Metal Ions
Metal ions have been known to play a crucial role in the functioning and biological activity of enzymes. They can either work as an activator by helping in catalysis or can inhibit the enzyme activity. L-asparaginase derived from marine environment has also been investigated for its stability and activity in the presence of various monovalent and divalent metal ions. Among the metal ions tested, inhibition of L-asparaginase activity has been reported in the presence of Zn2+ and Hg2+ (Basha et al. 2009; Shakambari et al. 2016; Lee et al. 2016; Shi et al. 2017). On the other hand, Mg2+ has been shown to increase the asparaginase activity in marine Streptomyces sp., M. zeaxanthinifaciens S86, and Cobetia amphilecti AMI6 (Basha et al. 2009; Lee et al. 2016; Farahat et al. 2020). However, in contrast, Shi et al. reported inhibition of asparaginase activity by Mg2+ in P. barengoltzii CAU904, whereas enhanced activity in the presence of Ba2+ and Fe3+ was perceived (Shi et al. 2017). The effect of these metal ions on the activity of marine asparaginase at different concentrations has been tabulated in Table 4.2.
4.4.2.3 Molecular Features of Marine L-asparaginase
Studies have been prompted to gain a deep insight into the molecular structure of L-asparaginase derived from marine microorganisms. Molecular cloning of the L-asparaginase encoding gene has been reported by researchers with different gene lengths. A type I asparaginase from Mesoflavibacter zeaxanthinifaciens S86 was found to be homologous to E. coli and Yersinia pseudotuberculosis asparaginase. The enzyme of 344 amino acids length was coded by an ORF of 1035 bp (Lee et al. 2016). The recombinant L-asparaginase gene of P. barengoltzii CAU904 (1011-bp) encoded the enzyme of 39.1 kDa, and further analysis of the gene sequence revealed that the encoded asparaginase could be a novel Rhizobial-type L-asparaginase (Shi et al. 2017). Vala et al. (2018) investigated the L-asparaginase-producing gene in marine Aspergillus niger by PCR amplification and found a ~1 kb amplified gene fragment (Vala et al. 2018). In another study, 1179 bp L-asparaginase gene of A. terreus, isolated from the Red Sea coast of Egypt was amplified and cloned for further profound evaluation of the asparaginase gene sequence. BLAST analysis of the nucleotide sequence unveiled an intragenic sequence (intron) of 51 nucleotides within the open reading frame of asparaginase gene. Therefore, in order to get a complete uninterrupted open reading frame, cDNA was prepared and was used as a template for the Polymerase Chain Reaction (PCR) amplification. The amplified ORF was cloned and expressed under optimized conditions and found to code a protein of 42 kDa (Saeed et al. 2018).
Where some reported asparaginase with over 1 kb open reading frame, there are also reports on asparaginase with lower polynucleotide lengths. L-asparaginase biosynthetic gene of N. alba NIOT-VKMA08 has been found to be of 963 bp encoding a protein of 33.4 kDa (Meena et al. 2016). Similarly, an Open Reading Frame (ORF) of 990 bp has been documented in B. tequilensis NIOS4 producing a type I L-asparaginase of 36.6 kDa. Analysis of the polynucleotide with other asparaginases revealed 98% sequence identity with the ansA sequence of Bacillus subtilis. Moreover, the protein sequence analysis displayed a few substitutions of amino acids; however, the active-site residues were found to be conserved (Nayak et al. 2014). Recently, a 951 bp asparaginase gene was isolated and cloned from the marine C. amphilecti AMI6. The gene sequence was induced to express the corresponding asparaginase protein of approximately 37 kDa. Further analysis of the protein using dynamic light scattering revealed its homotetrameric structure with a native molecular weight of 140.7–154.4 kDa (Farahat et al. 2020).
4.5 In Vitro Anti-leukemic Studies on Marine L-asparaginase
Considerable investigation has been done to evaluate the in vitro anti-leukemic and cytotoxic activity of asparaginase derived from marine microorganisms. Table 4.3 lists the Km, IC50 values of marine-derived L-asparaginase along with their cytotoxic effects on different cancer cell lines. In vitro, cytotoxicity is assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay, which is based on the colourimetric measurement of cell viability. The mitochondrial enzyme, succinate dehydrogenase, catalyses the reduction of MTT (3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide) into formazan crystals which produces a violet colour. The intensity of the colour is directly proportional to the cell viability. Arjun et al. demonstrated the cytotoxic effect of the extracellularly produced L-asparaginase from marine Bacillus sp. TVS55. The enzyme was treated on HL-60 cancer cells, which resulted in ninety percent cell death compared to the control cells (Arjun et al. 2016). Similarly, another asparaginase derived from marine B. licheniformis displayed the proliferation inhibition of cancer cells in a dose-dependent manner together with manifestation of cell demise. The enzyme showed no toxicity towards the normal human cells (Alrumman et al. 2019). Related reports have been documented in the literature revealing the anti-leukemic potential of marine asparaginases. Recently, the anti-leukemic activity of marine B. velezensis L-asparaginase has been tested on breast cancer cell lines, MCF-7 and MDA-MB-231. The treated cancer cells displayed cell death evident by the detachment of the cells from the surface of the wells (Mostafa et al. 2019). Cytotoxicity of marine asparaginase has also been investigated on HeLa cell lines. Purified L-asparaginase from B. tequilensis PV9W showed cytotoxicity on HeLa cells at an IC50 value of 0.0360 IU per ml (Shakambari et al. 2016). Marine Aspergillus niger-derived L-asparaginase has shown effective anti-proliferative activity against a number of human cancer cell lines with an IC50 value of less than 1 IU per ml (Vala et al. 2018). These reports advocate the potential ability of marine-derived asparaginases as an anti-leukemic drug with lower inhibitory concentration compared to the clinically employed E. coli and Erwinia asparaginases.
4.6 Strategies for Enhancing the Stability and Reducing the Immunogenicity of L-asparaginase
L-asparaginase used clinically has low circulation time which demands repeated dosage of the drug; therefore, a stable formulation of asparaginase is intended with a prolonged half-life in the patient’s body. To mitigate this concern, various efforts have been made, and several nanoencapsulated or immobilized forms of this therapeutic enzyme have been developed. Several soluble and insoluble matrices have been employed for the immobilization of asparaginase, namely carboxymethyl cellulose (Sundaramoorthi et al. 2012), chitosan (Ates et al. 2018), silk fibroin (Wang and Zhang 2015), aluminium oxide (Agrawal et al. 2018), polyethylene glycol (Ramirez-Paz et al. 2018), and metal nanoparticles (Ulu et al. 2018; Al-Dulimi et al. 2020, b). Reports are available on asparaginase immobilization with liposomes, but results are not much satisfactory. Several studies have reported the chemical instability of nanoencapsulated form of L-asparaginase in liposomes. Polymer microspheres are highly robust self-assembled vesicles that can be used as an alternative to takeover this concern (Barenholz 2001; He et al. 2019; Aibani et al. 2020). Recently, researchers concentrated on the encapsulation of proteins with better nanoparticles than microspheres since these nanoparticles can be delivered subcutaneously, intramuscularly, and intravenously. Another effective solution to reduce the side effects of the enzyme is the trapping of L-asparaginase in red blood cells (Rossi et al. 2020). This helps in significantly reducing the immunogenicity along with providing protection against proteolysis.
However, formulations that have been produced so far are not being clinically employed, and still, studies are in progress to find new and effective formulations. Anti-cancer nanoparticles have become an attractive area of investigation for cancer therapeutics. The encapsulation of the enzyme in a polymer matrix can be a technique to reduce the clearance of asparaginase from the circulation. An investigation has been carried out on the bioconjugation of asparaginase for developing a formulation with enhanced pharmacokinetics and minimal immunogenicity profile. In this regard, PASylation® technology is under the spotlight. This technology is based on the conjugation of drugs, proteins, or peptides with polymers made up of proline, alanine, and serine (PAS). Such PAS-rich polymers maintain a stable random coil disordered structure with high solubility in the physiological environment.
Moreover, such drug conjugates impart long circulation time in the plasma and do not elicits any immune response. In 2017, a PASylation® technology-based asparaginase formulation with prolonged circulation time had been licensed by Jazz Pharmaceuticals plc in collaboration with XL-protein GmbH, a German biotech company (Gebauer and Skerra 2018; Ahmadpour and Hosseinimehr 2018). Recently, two new PEGylated formulations of asparaginase have been developed, the PEG-crisantaspases (Asparec® s) and Calaspargase Pegol. The clinical trials (phases II and III) of PEG crisantaspase have been initiated and would be employed as a second-line drug to the E. coli asparaginase. At the same time, Calaspargase Pegol received the FDA approval. This new formulation has higher stability due to the succinimidyl carbamate linker in place of an SS-connector of polyethylene glycol.
4.7 Drug Delivery System for L-asparaginase
4.7.1 Immobilization and Encapsulation in Nanomaterials
Considering its high demand in cancer treatment, efficient efforts are still undergoing for formulating an improved form of L-asparaginase. A number of nanomaterials have been investigated for asparaginase immobilization and encapsulation. L-asparaginase immobilized with starch on poly(methyl methacrylate) had shown an eightfold higher enzyme affinity and remained 60 percent active at 4 ° C for 30 days compared to the non-immobilized enzyme (Ulu et al. 2016). On the other hand, a study by the same author showed that the immobilized L-asparaginase on MCM-41-coated Fe3O4 magnetic nanoparticles retained over 40 percent of its enzyme activity even after used for 18 times. As compared to the free enzyme, the immobilized enzyme was found to be more stable at different pHs and temperatures studied. In addition, a residual activity near 50 percent and around 30 percent was observed when stored at 4 and 25 °C, respectively, for 28 days (Ulu et al. 2018). Agrawal et al. (2018) attempted to covalently immobilize E. coli L-asparaginase on aluminium oxide pellets and achieved a maximum immobilization yield of 85 percent. The immobilized enzyme was found to be stable at various temperatures and pHs with an enhanced activity, lower km value, and maintained its activity up to nine successive cycles of reuse (Agrawal et al. 2018). The same group in 2019 demonstrated the covalent immobilization of a glutaminase-free asparaginase on the functionalized oxide nanoparticles of aluminium and titanium. The developed nano-bioconjugates were shown to be effectively reusable even after nine successive uses. Aluminium oxide nanoparticle-conjugated asparaginase had shown a better affinity than the non-conjugated enzyme. The thermodynamic study of the nano-conjugated enzyme showed an enhanced rate of reaction. Moreover, unlike the free enzyme, the nano-conjugate was found to be 40 percent active when stored at 37 °C for 23 days. In vitro cytotoxicity study on human leukaemia, MOLT-4 cells revealed the inhibition of cell growth at 10 μg/ml concentration of the aluminium oxide asparaginase nano-conjugate (Agrawal and Kango 2019).
Yet, another immobilization platform is carbon nanotubes which have a large surface area and high drug loading capacity. Cristóvão et al. explored multi-walled carbon nanotubes (MWCNTs) for the immobilization of asparaginase by a simple adsorption method (Cristóvão et al. 2020). A double-emulsion–solvent evaporation technique has also been employed for the encapsulation of L-asparaginase in PLGA polymer. Treatment of mice tumour model with this nanoencapsulated asparaginase showed a significant decrease in the tumour volume with no sign of blood and liver toxicity (Singh et al. 2020). PLGA has also previously been employed for encapsulation of asparaginase by a similar method of double emulsification by de Brito et al. The group achieved a maximum encapsulation efficiency of over 85 percent and showed more than 20 percent higher activity compared to the free enzyme (de Brito et al. 2019).
Another method, ionotropic gelation method, has been employed for asparaginase encapsulation in chitosan nanoparticles with an entrapment efficiency of around 76 percent and loading capacity of approximately 50 percent. The encapsulated enzyme was observed with a prolonged in vitro half-life compared to the unbound enzyme (Bahreini et al. 2014).
The design of nanoformulated form of asparaginase involves a variety of key aspects like the choice of nanomaterial based on its biocompatibility and biodegradability (Keck and Müller 2013); it should be stable at storage temperature (4 °C) and can be sterilized. Scaling up is another crucial step in the development of the asparaginase nanoformulation (Paliwal et al. 2014; de Pachioni-Vasconcelos et al. 2016). Moreover, the size of the nanomaterial and its polydispersity are other pitfalls in small-scale production processes, such as film hydration method. Control over polydispersion is a critical factor which defines the heterogeneity of particles. Furthermore, the nanomaterial should be stable at the physiological conditions and should not bind to blood components leading to phagocytosis and clearance from the body before the drug release (Dawidczyk et al. 2014, 2015; Wicki et al. 2015; Apolinário et al. 2018). Notably, erythrocytes, the red blood cells, have been adopted as a biodegradable and biocompatible drug carrier in the anti-cancer research having high loading capacity. These bioinspired nanocarriers disguise and escape the immune system and have a long lifespan of 120 days in the circulation which merits their application as a drug delivery carrier (Luk et al. 2016; Pan et al. 2016).
4.7.2 Polymersomes as a Carrier of L-asparaginase
Polymersomes, similar to liposomes, are artificial vesicles formed from the self-assembly of amphiphilic block copolymers. These higher molecular weight vesicles have attracted the attention of researchers due to their fascinating properties of versatility, high stability in physiological environment, and encapsulation ability of both hydrophilic and hydrophobic drugs or therapeutic enzymes. Polymersomes form a platform for catalysis in an enclosed environment, thereby protecting the therapeutic enzyme from different physiological factors that affect its activity (Rideau et al. 2018; Vasile 2019). Though polymersomes have great potential as a drug delivery carrier, but due to their high-molecular-weight components, they are poorly permeable as compared to liposomes. Therefore, developing polymersomes with intrinsic permeability would enable its implications in cancer therapeutics. These synthetic nanoreactors can be developed by a number of techniques like nanoprecipitation, electroformation, film, and bulk hydration methods. However, “Polymerisation-induced self-assembly (PISA) ”has emerged as a new potent method for the synthesis of polymeric vesicles (Jiang et al. 2015; Phan et al. 2020). This approach provides a versatile platform for developing nanoreactors by simple mixing of monomers and enzymes. Using this approach, an intrinsically permeable polymersome has been prepared and loaded with functional enzymes (Blackman et al. 2017). Recently, the same preparation has been applied for encapsulation of asparaginase. The investigation demonstrated the synthesis of asparaginase loaded vesicles with size-specific permeability enabling an enclosed catalysis resulting in enhanced stability against proteolysis and decreased immunogenicity (Blackman et al. 2018).
Hybrid polymersomes have also been designed for the encapsulation of asparaginase. Bueno et al. reported the synthesis and development of high loading capacity (800 molecules per vesicles) hybrid asymmetric polymersomes (PMC25-PDPA70/PEO16-PBO22) loaded with asparaginase. The developed nanoreactors showed permeability and catalysis of asparagine from the confined environment resulting in cancer cell death while keeping the enzyme safe from the outside influences (Bueno et al. 2020).
Similarly, several other bioinspired strategies can be adopted to increase the permeability of polymersomes for enabling the diffusion of small molecules across the membrane (Varlas et al. 2019). In one way, channel proteins can be embedded in the membrane of polymersomes which helps in increasing the movement of small molecules in and out of the polymersomes (Schwarzer et al. 2018; Varlas et al. 2018). Another strategy could be the exploitation of layer-by-layer self-assembly for the synthesis of permeable polymersomes (Antipov et al. 2003). Table 4.4 enumerates the application of different constituent polymersomes for the encapsulation of asparaginase and other cancer therapeutic drugs.
4.8 Futuristic Therapeutics: Bioinspired Nanoparticles, Peptides, and Nanozymes
Enzymes present in biological system catalyse specific reactions that are required for the proper functioning of the animal, plant, and microbial metabolism. Enzymes have a wide variety of applications in biomedicine and industries; hence, strategies have been adopted for their large-scale production and purification. However, these steps are time-consuming and require a lot of resources. Keeping in mind the importance of these natural catalysts in immunoassays, diagnosis, and cancer treatment, scientists prompted their conception of mimicking the functioning of enzymes employing nanomaterials. Such nanostructures, called artificial enzymes or nanozymes, are developed to have enzyme activity and are relatively stable and cost-effective substitutes of the natural ones.
Nanozymes, nanoparticles made from inorganic materials with intrinsic catalytic activity, have emerged as an innovation in cancer therapy. Notably, “tumour microenvironment responsive nanocatalytic tumour therapy” has attracted the scientific community (Cao et al. 2018; Huang et al. 2019; Huo et al. 2019; Qian et al. 2019). In 2018, a nanozyme based on “nitrogen-doped porous carbon nanospheres” was developed. This nanozyme had multienzyme activities for oxidase, peroxidase, catalase, and superoxide dismutase. It catalysed the production of reactive oxygen species in the acidic tumour microenvironment with its peroxidase and catalase activity. On the other hand, catalase and superoxide dismutase activities of the nanozyme aided in scavenging free radicals in a neutral environment (Fan et al. 2018).
Recently, a biocompatible, multienzymatic nanozyme based on Au and Fe3O4 co-loaded with dendritic mesoporous silica nanoparticles has been reported to prompt a cascade of reactions in the tumour microenvironment (TME). The nanozyme catalysed the oxidation of glucose-producing hydrogen peroxide as a by-product which was in turn catalysed to decompose into hydroxyl radicals. These harmful radicals ultimately caused the induction of tumour cell death, validated by in vitro and in vivo experiments (Gao et al. 2019). Similar nanozyme preparations have been reported for their application in cancer treatment (Taleghani et al. 2019; Dong et al. 2020; Zhao et al. 2020; Sutrisno et al. 2020).
Nanozymes are indeed a smart module designed to intrinsically behave like a natural macromolecular enzyme in nanoscale. Nonetheless, the therapeutic efficacy of nanozymes is affected by certain factors in the tumour microenvironment like hypoxia, reduced endogenous concentration of hydrogen peroxide, etc. (Lin et al. 2018). In this respect, Xu et al. proposed an “immunomodulation-enhanced nanozyme-based tumour catalytic therapy” for the first time that would be able to harmonize the nanozyme with the tumour microenvironment (TME). The study reported intrinsic peroxide and catalase-like activities of “iron-manganese-silicate nanoparticles” conjugated with polyethylene glycol (PEG) loaded with tranforming growth factor-beta (TGF-β) inhibitor in the tumour microenvironment. Due to the acidic pH in the TME, the developed nanozyme can catalyse the decomposition of hydrogen peroxide to hydroxyl radicals and oxygen. In vitro and in vivo studies were undertaken to validate the antitumour activity of this impressive nanozyme (Xu et al. 2020).
From the last few years, therapeutic peptides have gained considerable attention of the researchers for its applications in drug delivery in cancer treatment. Proteins (enzymes) are known for their specificity and selectivity in catalysing reactions in biological systems. As mentioned earlier, their importance and applications in therapeutics, as well as the complexity in their large-scale production, have encouraged the scientists to explore the possibility of developing peptides with the same activity and functioning with less production pain. Peptides are small polymers of amino acid residues with uncomplicated structure. These peptides have been observed to inherent great biocompatibility, elicit fewer side effects, are cleared from the body, and have a relatively uncomplicated production strategy. Besides, they have the merit of facile modifications and synthesis in large scale (Gongora-Benitez et al. 2014; Ramsey & Flynn 2015). Peptides are competent in crossing the blood–brain barrier (BBB) by virtue of their small size, hence, benefits in drug delivery in the central nervous system as well (Bergmann et al. 2018; Jackman et al. 2018; Jafari et al. 2019). The current acclamation for peptides or anti-cancer peptides indeed has made speculations of their incorporation in the cancer treatment; however, they have not been widely adopted in therapeutics due to certain constraints. Compared to natural proteins, peptides are unable to achieve proper folding of their structure which substantially affects their activity. Anti-cancer peptides have less selectivity and affinity to their respective ligands or substrates; in addition, these molecules are highly susceptible to proteolytic degradation in the body circulation and thus are short-lived in the biological environment (Liu et al. 2018; Liscano et al. 2020). Therefore, research has been directed towards improving the characteristics of peptides for their better performance in the biological environment. Scientists have investigated the conjugation of nanoparticles to peptide for ameliorating their limiting properties. These peptide–nanoparticle conjugates have displayed enhanced structural and functional properties of peptides along with a characteristic of multivalency (Lauster et al. 2017; Pal et al. 2016; Bai Aswathanarayan et al. 2018). Multivalency strengthens the binding and selectivity of the anti-cancer peptide as a large number of molecules are available for interaction with the large target molecule; this allows for the successful multiple binding events to take place with strong binding strength. Binding of the interacting biological molecules is majorly based on weak non-covalent bonds; however, their combined individual interactions have strong binding strengths. Due to the increase in the number of interacting molecules, the cumulative binding is thus reinforced (Lauster et al. 2017; Jeong et al. 2020). Furthermore, the overall structure can be confirmed for the desired applications by customizing the nanoparticle scaffold (Mikolajczak and Koksch 2019; Ranjitha et al. 2019; Wilder et al. 2020). Consequently, these peptide–nanoparticle conjugates can be devised as an upcoming therapeutic drug delivery system for anti-cancer drugs. Examples of different naturally derived, modified, and synthetic peptides with anti-cancer properties are enumerated in Table 4.5.
Scientists have acquired inspiration from nature and the biological nanosystems to synthesize nanostructures that mimics the biological processes. The credibility of such a biomimetic nanosystem is based on different factors/parameters like the method of preparation, their shape, size, and texture. Over the years, a number of such delivery systems have been synthesized inspired by the cellular system and structure of bacteria, fungi, viruses, and mammalian cells (Farjadian et al. 2018; Sabu et al. 2018; Rao et al. 2020; Prasad 2016, 2017, 2019a, b; Prasad et al. 2016, 2018; Srivastava et al. 2021; Sarma et al. 2021). Virus-like particles (VLPs) and nanomaterials mimicking the viral membrane can be modified as desired with the chemotherapeutic drugs, ligands, and imaging agents. These nanosystems have no harmful predispositions and are utterly safe for biomedical applications (Wannasarit et al. 2019). These versatile nanosystems have great applications in the biomedicine due to their specificity, lesser toxicity, and most importantly, biocompatibility (Kozielski et al. 2016; Prasad et al. 2020; Saglam et al. 2021; Maddela et al. 2021).
4.9 Conclusion
L-asparaginase is an indispensable component of ALL and other blood cancer treatment. However, the clinically used formulation of asparaginase derived from E. coli and Erwinia have been known to elicit several side effects and immune responses when administered to the patients due to the glutaminase co-activity. Therefore, a wide variety of research has been directed towards an alternative form of this therapeutic enzyme. Recent years have seen an accelerated investigation on L-asparaginase derived from marine microorganisms which could be a potential alternative for the present asparaginase formulations. Marine-derived asparaginases have been observed to be glutaminase-free with requisite biochemical and kinetic properties. Evidently, in vitro studies have shown the remarkable anti-leukemic and cytotoxic properties of marine asparaginases against the cancer cells. These studies suggest the potential of marine asparaginases as a substitute for the current clinical forms.
Nevertheless, the physiological stability of asparaginase is another challenge that needs to be overcome. Several strategies have been adopted to prolong the circulation time of asparaginase. Initially, PEGylation was the method of choice for enhancing stability; however, considering the side effects of conjugated PEG polymers, studies have been shifted to alternative nanomaterials. Immobilization and encapsulation of asparaginase have shown tremendous enhancement of physiological as well as storage stability. A number of innovations have been observed recently in the nano-conjugated formulations of asparaginase with higher circulation half-life and reusability. The present chapter pinpoints the recent advents in the development of asparaginase formulations and the nanotechnological innovations that could be of great promise in the cancer therapeutics.
References
Abuchowski A, Kazo GM, Verhoest CR Jr, Van Es T, Kafkewitz D, Nucci ML, Viau AT, Davis FF (1984) Cancer therapy with chemically modified enzymes. I. Antitumor properties of polyethylene glycol-asparaginase conjugates. Cancer Biochem Biophys 7(2):175–186
Agrawal S, Kango N (2019) Development and catalytic characterization of L-asparaginase nano-bioconjugates. Int J Biol Macromol 135:1142–1150
Agrawal S, Sharma I, Prajapati BP, Suryawanshi RK, Kango N (2018) Catalytic characteristics and application of L-asparaginase immobilized on aluminum oxide pellets. Int J Biol Macromol 114:504–511
Ahmadpour S, Hosseinimehr SJ (2018) PASylation as a powerful technology for improving the pharmacokinetic properties of biopharmaceuticals. Curr Drug Deliv 15(3):331–341
Aibani N, Khan TN, Callan B (2020) Liposome mimicking polymersomes; A comparative study of the merits of polymersomes in terms of formulation and stability. Int J Pharmac X 2:100040
Al-Dulimi AG, Al-Saffar AZ, Sulaiman GM, Khalil KA, Khashan KS, Al-Shmgani HS, Ahmed EM (2020) Immobilization of L-asparaginase on gold nanoparticles for novel drug delivery approach as anti-cancer agent against human breast carcinoma cells. J Mater Res Technol 9(6):15394–15411
Alrumman SA, Mostafa YS, Al-izran KA et al (2019) Production and Anticancer Activity of an L-asparaginase from Bacillus licheniformis Isolated from the Red Sea, Saudi Arabia. Sci Rep 9:1–14. https://doi.org/10.1038/s41598-019-40512-x
Ammon HL, Weber IT, Wlodawer A, Harrison RW, Gilliland GL, Murphy KC, Sjölin L, Roberts J (1988) Preliminary crystal structure of Acinetobacter glutaminasificans glutaminase-asparaginase. J Biol Chem 263(1):150–156
Amylon MD, Shuster J, Pullen J et al (1999) Intensive high-dose asparaginase consolidation improves survival for pediatric patients with T cell acute lymphoblastic leukemia and advanced stage lymphoblastic lymphoma: A Pediatric Oncology Group study. Leukemia 13:335–342. https://doi.org/10.1038/sj.leu.2401310
Antipov AA, Sukhorukov GB, Möhwald H (2003) Influence of the ionic strength on the polyelectrolyte multilayers’ permeability. Langmuir 19(6):2444–2448
Apolinário AC, Magoń MS, Pessoa A Jr, Rangel-Yagui CDO (2018) Challenges for the self-assembly of poly (ethylene glycol)–poly (lactic acid)(PEG-PLA) into polymersomes: beyond the theoretical paradigms. Nano 8(6):373
ARENS A, RAUENBUSCH E, IRION E, WAGNER O, BAUER K, KAUFMANN W (1970) Isolation and properties of L-asparaginases from Escherichia coli. Hoppe-Seyler´ s Zeitschrift für Physiologische Chemie 351(1):197–212
Ardalan N, Mirzaie S, Sepahi A, Khavari-Nejad RA (2018) Novel mutant of Escherichia coli asparaginase II to reduction of the glutaminase activity in treatment of acute lymphocytic leukemia by molecular dynamics simulations and QM-MM studies. Medical hypotheses 112:7–17
Arjun JK, Aneesh B, Kavitha T, Hari Krishnan K (2016) Therapeutic L-asparaginase activity of bacteria isolated from marine sediments. Int J Pharmac Sci Drug Res 8(4):229–234
Aslanian AM, Kilberg MS (2001) Multiple adaptive mechanisms affect asparagine synthetase substrate availability in asparaginase-resistant MOLT-4 human leukaemia cells. Biochem J 358(1):59–67
Asselin BL et al (1999) Prognostic significance of early response to a single dose of Asparaginase in childhood acute lymphoblastic. Leukemia 21(1):6–12
Ates B, Ulu A, Köytepe S, Noma SAA, Kolat VS, Izgi T (2018) Magnetic-propelled Fe 3 O 4–chitosan carriers enhance L-asparaginase catalytic activity: a promising strategy for enzyme immobilization. RSC Adv 8(63):36063–36075
Avramis VI, Sencer S, Periclou AP, Sather H, Bostrom BC, Cohen LJ et al (2002) A randomized comparison of nativeEscherichia coli asparaginase and polyethylene glycol conjugated asparaginase for treatment of children with newly diagnosed standard-risk acute lymphoblastic leukemia: a Children’s Cancer Group study. Blood 99(6):1986–1994
Bader RA, Wardwell PR (2014) Polysialic acid: overcoming the hurdles of drug delivery. Ther Deliv 5:235–237. https://doi.org/10.4155/tde.13.153
Badoei-Dalfard A (2015) Purification and characterization of L-asparaginase from Pseudomonas aeruginosa strain SN004: production optimization by statistical methods. Biocatal Agric Biotechnol 4:388–397. https://doi.org/10.1016/j.bcab.2015.06.007
Bahreini E, Aghaiypour K, Abbasalipourkabir R et al (2014) Preparation and nanoencapsulation of L-asparaginase II in chitosan-tripolyphosphate nanoparticles and in vitro release study. Nanoscale Res Lett 9:1–13. https://doi.org/10.1186/1556-276X-9-340
Bai Aswathanarayan J, Rai Vittal R, Muddegowda U (2018) Anticancer activity of metal nanoparticles and their peptide conjugates against human colon adenorectal carcinoma cells. Artif Cells Nanomed Biotechnol 46(7):1444–1451
Balasubramanian MN, Butterworth EA, Kilberg MS (2013) Asparagine synthetase: Regulation by cell stress and involvement in tumor biology. Am J Physiol Endocrinol Metab 304(8):E789–E799. https://doi.org/10.1152/ajpendo.00015.2013
Banerji J (2015) Asparaginase treatment side-effects may be due to genes with homopolymeric Asn codons (Review-Hypothesis). Int J Mol Med 36:607–625. https://doi.org/10.3892/ijmm.2015.2285
Barenholz Y (2001) Liposome application: problems and prospects. Curr Opin Colloid Interface Sci 6(1):66–77
Basha NS, Rekha R, Komala M, Ruby S (2009) Production of extracellular anti-leukaemic enzyme lasparaginase from marine actinomycetes by solidstate and submerged fermentation: purification and characterisation. Trop J Pharm Res 8(4)
Battistel AP, da Rocha BS, dos Santos MT et al (2020) Allergic reactions to asparaginase: retrospective cohort study in pediatric patients with acute lymphoid leukemia. Hematol Transfus Cell Ther 43:9–14. https://doi.org/10.1016/j.htct.2019.10.007
Bergmann S, Lawler SE, Qu Y, Fadzen CM, Wolfe JM, Regan MS et al (2018) Blood–brain-barrier organoids for investigating the permeability of CNS therapeutics. Nat Protoc 13(12):2827–2843
Bhargavi M, Jayamadhuri R (2016) Isolation and screening of marine bacteria producing anti-cancer enzyme L-asparaginase. Am J Mar Sci 4:1–3. https://doi.org/10.12691/marine-4-1-1
Birolli WG, Lima RN, Porto ALM (2019) Applications of marine-derived microorganisms and their enzymes in biocatalysis and biotransformation, the underexplored potentials. Front Microbiol 10:1453. https://doi.org/10.3389/fmicb.2019.01453
Blackman LD, Varlas S, Arno MC, Fayter A, Gibson MI, O’Reilly RK (2017) Permeable protein-loaded polymersome cascade nanoreactors by polymerization-induced self-assembly. ACS Macro Lett 6(11):1263–1267
Blackman LD, Varlas S, Arno MC, Houston ZH, Fletcher NL, Thurecht KJ et al (2018) Confinement of therapeutic enzymes in selectively permeable polymer vesicles by polymerization-induced self-assembly (PISA) reduces antibody binding and proteolytic susceptibility. ACS Central Sci 4(6):718–723
Bonthron DT (1990) L-asparaginase II of Escherichia coli K-12: cloning, mapping and sequencing of the ansB gene. Gene 91:101–105. https://doi.org/10.1016/0378-1119(90)90168-Q
Broome JD (1963) Evidence that the L-asparaginase of guinea pig serum is responsible for its antilymphoma effects: I. properties of the L-asparaginase of guinea pig serum in relation to those of the antilymphoma substance. The Journal of experimental medicine 118(1):99
Broome JD (1965) Antilymphoma activity of L-asparaginase in vivo : clearance rates of enzyme prepara- tions from guinea pig serum and yeast in relation to their effect on tumor growth 1. J Natl Cancer Inst 35:967–974
Bueno CZ, Apolinário AC, Duro-Castano A, Poma A, Pessoa A Jr, Rangel-Yagui CO, Battaglia G (2020) L-asparaginase encapsulation into asymmetric permeable polymersomes. ACS Macro Lett 9:1471–1477
Cammack KA, Marlborough DI, Miller DS (1972) Physical properties and subunit structure of L-asparaginase isolated from Erwinia carotovora. Biochem J 126(2):361–379
Cao Z, Zhang L, Liang K, Cheong S, Boyer C, Gooding JJ et al (2018) Biodegradable 2D Fe–Al hydroxide for nanocatalytic tumor-dynamic therapy with tumor specificity. Adv Sci 5(11):1801155
Chan WK, Lorenzi PL, Anishkin A, Purwaha P, Rogers DM, Sukharev S et al (2014) The glutaminase activity of L-asparaginase is not required for anticancer activity against ASNS-negative cells. Blood 123(23):3596–3606
Chan WK, Horvath TD, Tan L, Link T, Harutyunyan KG, Pontikos MA et al (2019) Glutaminase activity of L-asparaginase contributes to durable preclinical activity against acute lymphoblastic leukemia. Mol Cancer Ther 18(9):1587–1592
Cheng TH, Ismail N, Kamaruding N et al (2020) Industrial enzymes-producing marine bacteria from marine resources. Biotechnol Rep 27:e00482. https://doi.org/10.1016/j.btre.2020.e00482
Chien WW, Le Beux C, Rachinel N et al (2015) Differential mechanisms of asparaginase resistance in B-type acute lymphoblastic leukemia and malignant natural killer cell lines. Sci Rep 5:19–21. https://doi.org/10.1038/srep08068
Choi YJ, Park SJ, Park YS, Park HS, Yang KM, Heo K (2018) EpCAM peptide-primed dendritic cell vaccination confers significant anti-tumor immunity in hepatocellular carcinoma cells. PLoS One 13(1):e0190638
Clementi A (1922) La désamidation enzymatique de l’asparagine chez les différentes espéces animales et la signification physio logique de sa presence dans l’organisme. Arch Int Physiol 19(4):369–398
Cristóvão RO, Almeida MR, Barros MA, Nunes JC, Boaventura RA, Loureiro JM et al (2020) Development and characterization of a novel L-asparaginase/MWCNT nanobioconjugate. RSC Adv 10(52):31205–31213
Darvishi F, Faraji N, Shamsi F (2019) Production and structural modeling of a novel asparaginase in Yarrowia lipolytica. Int J Biol Macromol 125:955–961. https://doi.org/10.1016/j.ijbiomac.2018.12.162
Das S, Lyla PS, Khan SA (2006) Marine microbial diversity and ecology: Importance and future perspectives. Curr Sci 90:1325–1335
Dawidczyk CM, Russell LM, Searson PC (2014) Nanomedicines for cancer therapy: state-of-the-art and limitations to pre-clinical studies that hinder future developments. Front Chem 2:69
Dawidczyk CM, Russell LM, Searson PC (2015) Recommendations for benchmarking preclinical studies of nanomedicines. Cancer Res 75(19):4016–4020
de Almeida Pachioni-Vasconcelos J, Lopes AM, Apolinário AC, Valenzuela-Oses JK, Costa JSR, de Oliveira Nascimento L et al (2016) Nanostructures for protein drug delivery. Biomater Sci 4(2):205–218
de Brito AEM, Pessoa A Jr, Converti A, de Oliveira Rangel-Yagui C, da Silva JA, Apolinário AC (2019) Poly (lactic-co-glycolic acid) nanospheres allow for high L-asparaginase encapsulation yield and activity. Mater Sci Eng C 98:524–534
Dellinger CT, Miale TD (1976) Comparison of anaphylactic reactions to asparaginase derived from Escherichia coli and from Erwinia culturs. Cancer 38(4):1843–1846
Demirgöz D, Pangburn TO, Davis KP, Lee S, Bates FS, Kokkoli E (2009) PR_b-targeted delivery of tumor necrosis factor-α by polymersomes for the treatment of prostate cancer. Soft Matter 5(10):2011–2019
Dhevagi P, Poorani E (2006) Isolation and characterization of L-asparaginase from marine actinomycetes. Indian J Biotechnol 5:514–520
Do TT, Do TP, Nguyen TN et al (2019) Nanoliposomal L-asparaginase and its antitumor activities in lewis lung carcinoma tumor-induced BALB/c mice. Adv Mater Sci Eng 2019. https://doi.org/10.1155/2019/3534807
Dolowy WC, Henson D, Cornet J, Sellin H (1966) Toxic and antineoplastic effects of L-asparaginase: study of mice with lymphoma and normal monkeys and report on a child with leukemia. Cancer 19(12):1813–1819
Dong S, Dong Y, Jia T, Liu S, Liu J, Yang D et al (2020) GSH-depleted nanozymes with hyperthermia-enhanced dual enzyme-mimic activities for tumor nanocatalytic therapy. Adv Mater 32(42):2002439
Dos Santos C, Hamadat S, Le Saux K, Newton C, Mazouni M, Zargarian L et al (2017) Studies of the antitumor mechanism of action of dermaseptin B2, a multifunctional cationic antimicrobial peptide, reveal a partial implication of cell surface glycosaminoglycans. PLoS One 12(8):e0182926
Duval M, Suciu S, Ferster A et al (2002) Comparison of Escherichia coli-asparaginase with Erwinia-asparaginase in the treatment of childhood lymphoid malignancies: results of a randomized European Organisation for Research and Treatment of Cancer - Children’s Leukemia Group phase 3 trial. Blood 99:2734–2739. https://doi.org/10.1182/blood.V99.8.2734
Einsfeldt K, Baptista IC, Pereira JCCV, Costa-Amaral IC, Costa ESD, Ribeiro MCM, ... Almeida RV (2016) Recombinant L-asparaginase from Zymomonas mobilis: a potential new antileukemic agent produced in Escherichia coli. PloS one 11(6):e0156692
Ekladious I, Colson YL, Grinstaff MW (2019) Polymer–drug conjugate therapeutics: advances, insights and prospects. Nat Rev Drug Discov 18:273–294. https://doi.org/10.1038/s41573-018-0005-0
El-Sharkawy AS, Farag AM, Embaby AM et al (2016) Cloning, expression and characterization of aeruginosa EGYII L-Asparaginase from Pseudomonas aeruginosa strain EGYII DSM 101801 in E. coli BL21(DE3) pLysS. J Mol Catal B Enzym 132:16–23. https://doi.org/10.1016/j.molcatb.2016.06.011
Epp O, Steigemann W, Formanek H, Huber R (1971) Crystallographic evidence for the tetrameric subunit structure of L-asparaginase from Escherichia coli. Eur J Biochem 20:432–437
Evans WE (1982) Anaphylacfoid Reactions to Escherichia coli and Erwinia Asparaginase in children with leukemia and lymphoma. Am Cancer Soc 49:1378–1383
Fan K, Xi J, Fan L, Wang P, Zhu C, Tang Y et al (2018) In vivo guiding nitrogen-doped carbon nanozyme for tumor catalytic therapy. Nat Commun 9(1):1–11
Farahat MG, Amr D, Galal A (2020) Molecular cloning, structural modeling and characterization of a novel glutaminase-free L-asparaginase from Cobetia amphilecti AMI6. Int J Biol Macromol 143:685–695. https://doi.org/10.1016/j.ijbiomac.2019.10.258
Farjadian F, Moghoofei M, Mirkiani S, Ghasemi A, Rabiee N, Hadifar S et al (2018) Bacterial components as naturally inspired nano-carriers for drug/gene delivery and immunization: Set the bugs to work? Biotechnol Adv 36(4):968–985
Fu CH, Sakamoto KM (2007) PEG-asparaginase. Expert opinion on pharmacotherapy 8(12):1977–1984
Gao S, Lin H, Zhang H, Yao H, Chen Y, Shi J (2019) Nanocatalytic tumor therapy by biomimetic dual inorganic nanozyme-catalyzed cascade reaction. Adv Sci 6(3):1801733
Gebauer M, Skerra A (2018) Prospects of PASylation® for the design of protein and peptide therapeutics with extended half-life and enhanced action. Bioorg Med Chem 26(10):2882–2887
Ghosh S, Chaganti SR, Prakasham RS (2012) Polyaniline nanofiber as a novel immobilization matrix for the anti-leukemia enzyme L-asparaginase. J Mol Catal B Enzym 74:132–137. https://doi.org/10.1016/j.molcatb.2011.09.009
Gongora-Benitez M, Tulla-Puche J, Albericio F (2014) Multifaceted roles of disulfide bonds. Peptides as therapeutics. Chem Rev 114(2):901–926
Guarecuco R, Williams RT, Baudrier L, La K, Passarelli MC, Ekizoglu N et al (2020) Dietary thiamine influences L-asparaginase sensitivity in a subset of leukemia cells. Sci Adv 6(41):eabc7120
Haley EE, Fischer GA, Welch AD (1961) The requirement for L-asparagine of mouse leukemia cells L5178Y in culture. Cancer Res 21(4):532–536
Hamidi M, Azadi A, Rafiei P (2006) Pharmacokinetic consequences of pegylation. Drug delivery 13(6):399–409
He H, Ye J, Wang Y et al (2014) Cell-penetrating peptides meditated encapsulation of protein therapeutics into intact red blood cells and its application. J Control Release 176:123–132. https://doi.org/10.1016/j.jconrel.2013.12.019
He H, Lu Y, Qi J, Zhu Q, Chen Z, Wu W (2019) Adapting liposomes for oral drug delivery. Acta Pharm Sin B 9(1):36–48
Hermanova I, Zaliova M, Trka J, Starkova J (2012) Low expression of asparagine synthetase in lymphoid blasts precludes its role in sensitivity to L-asparaginase. Exp Hematol 40(8):657–665
Hijiya N, Van Der Sluis IM (2016) Asparaginase-Associated toxicity in children with acute lymphoblastic leukemia. Leuk Lymphoma 57:748–757. https://doi.org/10.3109/10428194.2015.1101098
Hill JM, Roberts J, Loeb E, Khan A, MacLellan A, Hill RW (1967) L-asparaginase therapy for leukemia and other malignant neoplasms: remission in human leukemia. JAMA 202(9):882–888
Hlozkova K, Pecinova A, Alquezar-Artieda N, Pajuelo-Reguera D, Simcikova M, Hovorkova L et al (2020) Metabolic profile of leukemia cells influences treatment efficacy of L-asparaginase. BMC Cancer 20:1–13
Ho PP, Milikin EB, Bobbitt JL, Grinnan EL, Burck PJ, Frank BH et al (1970) Crystalline L-asparaginase from Escherichia coli B I. Purification and chemical characterization. J Biol Chem 245(14):3708–3715
Horowitz B, Madras BK, Meister A, Old LJ, Boyes EA, Stockert E (1968) Asparagine synthetase activity of mouse leukemias. Science 160(3827):533–535. https://doi.org/10.1126/science.160.3827.533
Huang L, Liu Y, Sun Y, Yan Q, Jiang Z (2014) Biochemical characterization of a novel L-Asparaginase with low glutaminase activity from Rhizomucor miehei and its application in food safety and leukemia treatment. Applied and environmental microbiology 80(5):1561–1569
Huang Y, Ren J, Qu X (2019) Nanozymes: classification, catalytic mechanisms, activity regulation, and applications. Chem Rev 119(6):4357–4412
Huo M, Wang L, Wang Y, Chen Y, Shi J (2019) Nanocatalytic tumor therapy by single-atom catalysts. ACS Nano 13(2):2643–2653
Irion ECKART, Voigt WH (1970) Electron microscopy of L-asparaginase from Escherichia coli. Hoppe Seylers Z Physiol Chem 351(9):1154–1156
Izadpanah Qeshmi F, Javadpour S, Malekzadeh K et al (2014) Persian Gulf is a bioresource of potent L-asparaginase producing bacteria: Isolation & molecular differentiating. Int J Environ Res 8:813–818
Izadpanah Qeshmi F, Rahimzadeh M, Javadpour S, Poodat M (2015) Intracellular L-asparaginase from Bacillus sp. PG02: purification, biochemical characterization and evaluation of optimum pH and temperature. Am J Biochem Biotechnol
Izadpanah F, Homaei A, Fernandes P, Javadpour S (2018) Marine microbial L-asparaginase: biochemistry, molecular approaches and applications in tumor therapy and in food industry. Microbiol Res 208:99–112. https://doi.org/10.1016/j.micres.2018.01.011
Jackman JA, Costa VV, Park S, Real ALC, Park JH, Cardozo PL et al (2018) Therapeutic treatment of Zika virus infection using a brain-penetrating antiviral peptide. Nat Mater 17(11):971–977
Jafari B, Pourseif MM, Barar J, Rafi MA, Omidi Y (2019) Peptide-mediated drug delivery across the blood-brain barrier for targeting brain tumors. Expert Opin Drug Deliv 16(6):583–605
Jeong WJ, Bu J, Han Y, Drelich AJ, Nair A, Král P, Hong S (2020) Nanoparticle conjugation stabilizes and multimerizes β-Hairpin peptides to effectively target PD-1/PD-L1 β-sheet-rich interfaces. J Am Chem Soc 142(4):1832–1837
Jiang W, Zhou Y, Yan D (2015) Hyperbranched polymer vesicles: from self-assembly, characterization, mechanisms, and properties to applications. Chem Soc Rev 44(12):3874–3889
Kamala K, Sivaperumal P (2017) Biomedical applications of enzymes from marine actinobacteria. In: Advances in food and nutrition research, vol 80. Academic Press, pp 107–123
Kaneda Y, Tsutsumi Y, Yoshioka Y, Kamada H, Yamamoto Y, Kodaira H et al (2004) The use of PVP as a polymeric carrier to improve the plasma half-life of drugs. Biomaterials 25(16):3259–3266
Keck CM, Müller RH (2013) Nanotoxicological classification system (NCS)–a guide for the risk-benefit assessment of nanoparticulate drug delivery systems. Eur J Pharm Biopharm 84(3):445–448
Kennedy J, Marchesi JR, Dobson ADW (2008) Marine metagenomics: Strategies for the discovery of novel enzymes with biotechnological applications from marine environments. Microb Cell Factories 7:1–8. https://doi.org/10.1186/1475-2859-7-27
Khamessi O, Mabrouk HB, Othman H, ElFessi-Magouri R, De Waard M, Hafedh M et al (2018) RK, the first scorpion peptide with dual disintegrin activity on α1β1 and αvβ3 integrins. Int J Biol Macromol 120:1777–1788
Khodaverdi E, Tayarani-Najaran Z, Minbashi E, Alibolandi M, Hosseini J, Sepahi S et al (2019) Docetaxel-loaded mixed micelles and polymersomes composed of poly (caprolactone)-poly (ethylene glycol)(PEG-PCL) and poly (lactic acid)-poly (ethylene glycol)(PEG-PLA): preparation and in-vitro characterization. Iran J Pharmac Res 18(1):142
Kidd JG (1953) Regression of transplanted lymphomas induced in vivo by means of normal guinea pig serum I. Course of transplanted cancers of various kinds in mice and rats given guinea pig serum, horse serum, or rabbit serum. J Exp Med 98(6):565–582
Killander D, Dohlwitz A, Engstedt L, Franzen S, Gahrton G, Gullbring B et al (1976) Hypersensitive reactions and antibody formation during L-asparaginase treatment of children and adults with acute leukemia. Cancer 37(1):220–228
Kim IW, Lee JH, Kwon YN, Yun EY, Nam SH, Ahn MY et al (2013) Anticancer activity of a synthetic peptide derived from harmoniasin, an antibacterial peptide from the ladybug Harmonia axyridis. Int J Oncol 43(2):622–628
Kiriyama Y, Kubota M, Takimoto T, Kitoh T, Tanizawa A, Akiyama Y, Mikawa H (1989) Biochemical characterization of U937 cells resistant to L-asparaginase: the role of asparagine synthetase. Leukemia 3(4):294–297
Kotzia GA, Lappa K, Labrou NE (2007) Tailoring structure–function properties of L-asparaginase: engineering resistance to trypsin cleavage. Biochem J 404(2):337–343
Kozielski KL, Rui Y, Green JJ (2016) Non-viral nucleic acid containing nanoparticles as cancer therapeutics. Expert Opin Drug Deliv 13(10):1475–1487
Krishna KK, Bhumika V, Thomas M et al (2013) Oceanospirillum nioense sp. nov., a marine bacterium isolated from sediment sample of Palk bay, India. Antonie Van Leeuwenhoek 103:1015–1021. https://doi.org/10.1007/s10482-013-9881-9
Lang S (1904) Uber desamidierung im Tierkorper. Beitr Chem Physiol Pathol 5:321–345
Lauster D, Glanz M, Bardua M, Ludwig K, Hellmund M, Hoffmann U et al (2017) Multivalent peptide–nanoparticle conjugates for influenza-virus inhibition. Angew Chem Int Ed 56(21):5931–5936
Lazarus H, McCoy TA, Farber S et al (1969) Nutritional requirements of human leukemic cells. Asparagine requirements and the effect of L-asparaginase. Exp Cell Res 57:134–138. https://doi.org/10.1016/0014-4827(69)90377-2
Lee SJ, Lee Y, Park GH, Umasuthan N, Heo SJ, De Zoysa M et al (2016) A newly identified glutaminase-free L-asparaginase (L-ASPG86) from the marine bacterium Mesoflavibacter zeaxanthinifaciens. J Microbiol Biotechnol 26(6):1115–1123
Lee JK, Kang SM, Wang X et al (2019) HAP1 loss confers L-asparaginase resistance in ALL by downregulating the calpain-1-Bid-caspase-3/12 pathway. Blood 133:2222–2232. https://doi.org/10.1182/blood-2018-12-890236
Levine DH, Ghoroghchian PP, Freudenberg J, Zhang G, Therien MJ, Greene MI et al (2008) Polymersomes: a new multi-functional tool for cancer diagnosis and therapy. Methods 46(1):25–32
Li S, Byrne B, Welsh J, Palmer AF (2007) Self-assembled poly (butadiene)-b-poly (ethylene oxide) polymersomes as paclitaxel carriers. Biotechnol Prog 23(1):278–285
Lin T, Zhao X, Zhao S, Yu H, Cao W, Chen W et al (2018) O2-generating MnO2 nanoparticles for enhanced photodynamic therapy of bladder cancer by ameliorating hypoxia. Theranostics 8(4):990
Liscano Y, Oñate-Garzón J, Delgado JP (2020) Peptides with dual antimicrobial–anticancer activity: strategies to overcome peptide limitations and rational design of anticancer peptides. Molecules 25(18):4245
Liu X, Wu F, Ji Y, Yin L (2018) Recent advances in anti-cancer protein/peptide delivery. Bioconjug Chem 30(2):305–324
Liu WJ, Wang H, Peng XW, Wang WD, Liu NW, Wang Y, Lu Y (2018) Asparagine synthetase expression is associated with the sensitivity to asparaginase in extranodal natural killer/T-cell lymphoma in vivo and in vitro. Onco Targets Ther 11:6605
Lomelino CL, Andring JT, McKenna R, Kilberg MS (2017) Asparagine synthetase: function, structure, and role in disease. J Biol Chem 292:19952–19958. https://doi.org/10.1074/jbc.R117.819060
Louzao I, van Hest JC (2013) Permeability effects on the efficiency of antioxidant nanoreactors. Biomacromolecules 14(7):2364–2372
Luk BT, Fang RH, Hu CMJ, Copp JA, Thamphiwatana S, Dehaini D et al (2016) Safe and immunocompatible nanocarriers cloaked in RBC membranes for drug delivery to treat solid tumors. Theranostics 6(7):1004
Maddela NR, Chakraborty S, Prasad R (2021) Nanotechnology for advances in medical microbiology. Springer Singapore (ISBN 978-981-15-9915-6). https://www.springer.com/gp/book/9789811599156
Maita T, Matsuda G (1980) The primary structure of L-asparaginase from Escherichia coli. Hoppe-Seyler´ s Zeitschrift für Physiologische Chemie 361(1):105–118
Maita T, Morokuma K, Matsuda G (1974) Amino acid sequence of L-asparaginase from Escherichia coli. J Biochem 76(6):1351–1354
Mashburn LT, Wriston JC (1964) Tumor inhibitory effect of L-asparaginase from Escherichia coli. Arch Biochem Biophys 105:450–452
Matsueda S, Itoh K, Shichijo S (2018) Antitumor activity of antibody against cytotoxic T lymphocyte epitope peptide of lymphocyte-specific protein tyrosine kinase. Cancer Sci 109(3):611–617
Mazloum-Ravasan S, Madadi E, Fathi Z et al (2020) The effect of Yarrowia lipolytica L-asparaginase on apoptosis induction and inhibition of growth in Burkitt’s lymphoma Raji and acute lymphoblastic leukemia MOLT-4 cells. Int J Biol Macromol 146:193–201. https://doi.org/10.1016/j.ijbiomac.2019.12.156
Meena B, Anburajan L, Vinithkumar NV et al (2016) Molecular expression of L-asparaginase gene from Nocardiopsis alba NIOT-VKMA08 in Escherichia coli: a prospective recombinant enzyme for leukaemia chemotherapy. Gene 590:220–226. https://doi.org/10.1016/j.gene.2016.05.003
Meneguetti GP, Santos JHPM, Obreque KMT, Barbosa CMV, Monteiro G, Farsky SHP et al (2019) Novel site-specific PEGylated L-asparaginase. PLoS One 14(2):e0211951
Mikolajczak DJ, Koksch B (2019) Peptide–gold nanoparticle conjugates as artificial carbonic anhydrase mimics. Catalysts 9(11):903
Miller HK, Balis ME (1969) Glutaminase activity of L-asparagine amidohydrolase. Biochem Pharmacol 18(9):2225–2232
Mostafa Y, Alrumman S, Alamri S et al (2019) Enhanced production of glutaminase-free L-asparaginase by marine Bacillus velezensis and cytotoxic activity against breast cancer cell lines. Electron J Biotechnol 42:6–15. https://doi.org/10.1016/j.ejbt.2019.10.001
Nayak S, Porob S, Fernandes A, Meena RM, Ramaiah N (2014) PCR detection of ansA from marine bacteria and its sequence characteristics from Bacillus tequilensis NIOS4. J Basic Microbiol 54(2):162–168
Neuman RE, Mccoy TA (1956) Dual requirement of Walker carcinosarcoma 256 in vitro for asparagine and glutamine. Science (80- ) 124:124–125. https://doi.org/10.1126/science.124.3212.124
Nguyen TH, Nguyen VD (2017) Characterization and applications of marine microbial enzymes in biotechnology and probiotics for animal health. In Advances in food and nutrition research (Vol. 80, pp. 37–74). Academic Press
North ACT, Wade HE, Cammack KA (1969) Physicochemical studies of L-asparaginase from Erwinia carotovora. Nature (London) 224:594–595
Offman MN, Krol M, Patel N, Krishnan S, Liu J, Saha V, Bates PA (2011) Rational engineering of Lasparaginase reveals importance of dual activity for cancer cell toxicity. Blood, The Journal of the American Society of Hematology 117(5):1614–1621
Old LJ, Boyse EA, Campbell HA, Brodey RS, Fidler J, Teller JD (1967) Treatment of lymphosarcoma in the dog with L-asparaginase. Cancer 20(7):1066–1070
Pachioni-Vasconcelos JDA, Apolinário AC, Lopes AM, Pessoa A Jr, Barbosa LRS, Rangel-Yagui CDO (2020) Compartmentalization of therapeutic proteins into semi-crystalline PEG-PCL polymersomes. Soft Mater 19(2):222–230
Pal I, Brahmkhatri VP, Bera S, Bhattacharyya D, Quirishi Y, Bhunia A, Atreya HS (2016) Enhanced stability and activity of an antimicrobial peptide in conjugation with silver nanoparticle. J Colloid Interface Sci 483:385–393
Paliwal R, Babu RJ, Palakurthi S (2014) Nanomedicine scale-up technologies: feasibilities and challenges. AAPS PharmSciTech 15(6):1527–1534
Pan D, Vargas-Morales O, Zern B, Anselmo AC, Gupta V, Zakrewsky M et al (2016) The effect of polymeric nanoparticles on biocompatibility of carrier red blood cells. PLoS One 11(3):e0152074
Panosyan EH, Seibel NL, Martin-Aragon S, Gaynon PS, Avramis IA, Sather H, ... Avramis VI (2004) Asparaginase antibody and asparaginase activity in children with higher-risk acute lymphoblastic leukemia: Children’s Cancer Group Study CCG-1961. Journal of pediatric hematology/oncology 26(4):217–226
Parmentier JH, Maggi M, Tarasco E et al (2015) Glutaminase activity determines cytotoxicity of L-asparaginases on most leukemia cell lines. Leuk Res 39:757–762. https://doi.org/10.1016/j.leukres.2015.04.008
Patel N, Krishnan S, Offman MN, Krol M, Moss CX, Leighton C et al (2009) A dyad of lymphoblastic lysosomal cysteine proteases degrades the antileukemic drug L-asparaginase. J Clin Invest 119(7):1964–1973
Peng X, Zhou C, Hou X, Liu Y, Wang Z, Peng X et al (2018) Molecular characterization and bioactivity evaluation of two novel bombinin peptides from the skin secretion of Oriental fire-bellied toad, Bombina orientalis. Amino Acids 50(2):241–253
Petersen MA, Hillmyer MA, Kokkoli E (2013) Bioresorbable polymersomes for targeted delivery of cisplatin. Bioconjug Chem 24(4):533–543
Peterson RG, Handschumacher RE, Mitchell MS (1971) Immunological responses to L-asparaginase. J Clin Investig 50(5):1080–1090
Phan H, Taresco V, Penelle J, Couturaud B (2020) Polymerisation-induced self-assembly (PISA) as a straightforward formulation strategy for stimuli-responsive drug delivery systems and biomaterials: recent advances. Biomater Sci
Pieters R, Hunger SP, Boos J et al (2011) L-asparaginase treatment in acute lymphoblastic leukemia. Cancer 117:238–249. https://doi.org/10.1002/cncr.25489
Pola M, Rajulapati SB, Potla Durthi C et al (2018) In silico modelling and molecular dynamics simulation studies on L-asparaginase isolated from bacterial endophyte of Ocimum tenuiflorum. Enzym Microb Technol 117:32–40. https://doi.org/10.1016/j.enzmictec.2018.06.005
Pradhan B, Dash SK, Sahoo S (2013) Screening and characterization of extracelluar L-asparaginase producing Bacillus subtilis strain hswx88, isolated from Taptapani hotspring of Odisha, India. Asian Pac J Trop Biomed 3:936–941. https://doi.org/10.1016/S2221-1691(13)60182-3
Prasad R (2016) Advances and applications through fungal nanobiotechnology. Springer, International Publishing Switzerland (ISBN: 978-3-319-42989-2)
Prasad R (2017) Fungal nanotechnology: applications in agriculture, industry, and medicine. Springer Nature Singapore Pte Ltd. (ISBN 978-3-319-68423-9)
Prasad R (2019a) Microbial nanobionics: Basic research and applications. Springer International Publishing (ISBN 978-3-030-16534-5). https://www.springer.com/gp/book/9783030165338
Prasad R (2019b) Microbial nanobionics: state of art. Springer International Publishing (ISBN 978-3-030-16383-9). https://www.springer.com/gp/book/9783030163822
Prasad R, Pandey R, Barman I (2016) Engineering tailored nanoparticles with microbes: quo vadis. WIREs Nanomed Nanobiotechnol 8:316–330. https://doi.org/10.1002/wnan.1363
Prasad R, Jha A, Prasad K (2018) Exploring the realms of nature for nanosynthesis. Springer International Publishing (ISBN 978-3-319-99570-0). https://www.springer.com/978-3-319-99570-0
Prasad R, Siddhardha B, Dyavaiah M (2020) Nanostructures for antimicrobial and antibiofilm applications. Springer International Publishing (ISBN 978-3-030-40336-2). https://www.springer.com/gp/book/9783030403362
Prihanto AA, Wakayama M (2016) Marine microorganism: an underexplored source of L-asparaginase. Adv Food Nutrit Res 79:1–25
Qian X, Zhang J, Gu Z, Chen Y (2019) Nanocatalysts-augmented Fenton chemical reaction for nanocatalytic tumor therapy. Biomaterials 211:1–13
Rahimzadeh M, Poodat M, Javadpour S, Qeshmi FI, Shamsipour F (2016) Purification, characterization and comparison between two new L-asparaginases from Bacillus PG03 and Bacillus PG04. Open Biochem J 10:35
Ramirez-Paz J, Saxena M, Delinois LJ, Joaquín-Ovalle FM, Lin S, Chen Z et al (2018) Site-specific PEGylation crosslinking of L-asparaginase subunits to improve its therapeutic efficiency. BioRxiv 317040
Ramsey JD, Flynn NH (2015) Cell-penetrating peptides transport therapeutics into cells. Pharmacol Ther 154:78–86
Ranjitha VR, Muddegowda U, Ravishankar Rai V (2019) Potent activity of bioconjugated peptide and selenium nanoparticles against colorectal adenocarcinoma cells. Drug Dev Ind Pharm 45(9):1496–1505
Rao L, Tian R, Chen X (2020) Cell-membrane-mimicking nanodecoys against infectious diseases. ACS Nano 14(3):2569–2574
Ravuri J, Kumari K (2013) In vitro anticancer activity of marine bacteria isolated from Andhra Pradesh and Tamil Nadu coastal regions. Int J Chem Environ Biol Sci 1:3–5
Richards NG, Kilberg MS (2006) Asparagine synthetase chemotherapy. Annu Rev Biochem 75:629–654. https://doi.org/10.1146/annurev.biochem.75.103004.142520. PMID: 16756505; PMCID: PMC3587692
Rideau E, Dimova R, Schwille P, Wurm FR, Landfester K (2018) Liposomes and polymersomes: a comparative review towards cell mimicking. Chem Soc Rev 47(23):8572–8610
Roberts J, Prager MD, Bachynsky N (1966) The antitumor activity of Escherichia coli L-asparaginase. Cancer Res 26(10):2213–2217
Rossi L, Pierigè F, Bregalda A, Magnani M (2020) Preclinical developments of enzyme-loaded red blood cells. Expert Opin Drug Deliv
Sabu C, Rejo C, Kotta S, Pramod K (2018) Bioinspired and biomimetic systems for advanced drug and gene delivery. J Control Release 287:142–155
Saeed H, Ali H, Soudan H, Embaby A, El-Sharkawy A, Farag A, ... Ataya F (2018) Molecular cloning, structural modeling and production of recombinant Aspergillus terreus L. asparaginase in Escherichia coli. International journal of biological macromolecules 106:1041–1051
Saeed H, Hemida A, El-Nikhely N et al (2020) Highly efficient Pyrococcus furiosus recombinant L-asparaginase with no glutaminase activity: Expression, purification, functional characterization, and cytotoxicity on THP-1, A549 and Caco-2 cell lines. Int J Biol Macromol 156:812–828. https://doi.org/10.1016/j.ijbiomac.2020.04.080
Saglam N, Korkusuz, F, Prasad R (2021) Nanotechnology applications in health and environmental sciences. Springer International Publishing (ISBN: 978-3-030-64410-9). https://www.springer.com/gp/book/9783030644093
Sarma H, Joshi S, Prasad R, Jampilek J (2021) Biobased nanotechnology for green applications. Springer International Publishing (ISBN 978-3-030-61985-5). https://www.springer.com/gp/book/9783030619848
Schwarzer TS, Klermund L, Wang G, Castiglione K (2018) Membrane functionalization of polymersomes: alleviating mass transport limitations by integrating multiple selective membrane transporters for the diffusion of chemically diverse molecules. Nanotechnology 29(44):44LT01
Shaik M, Sankar GG, Iswarya M, Rajitha P (2017) Isolation and characterization of bioactive metabolites producing marine Streptomyces parvulus strain sankarensis-A10. J Gen Eng Biotechnol 15(1):87–94
Shakambari G, Birendranarayan AK, Angelaa Lincy MJ et al (2016) Hemocompatible glutaminase free L-asparaginase from marine Bacillus tequilensis PV9W with anticancer potential modulating p53 expression. RSC Adv 6:25943–25951. https://doi.org/10.1039/c6ra00727a
Shi R, Liu Y, Mu Q, Jiang Z, Yang S (2017) Biochemical characterization of a novel L-asparaginase from Paenibacillus barengoltzii being suitable for acrylamide reduction in potato chips and mooncakes. Int J Biol Macromol 96:93–99
Shrivastava A, Khan AA, Khurshid M et al (2016) Recent developments in L-asparaginase discovery and its potential as anticancer agent. Crit Rev Oncol Hematol 100:1–10. https://doi.org/10.1016/j.critrevonc.2015.01.002
Singh M, Hassan N, Verma D, Thakur P, Panda BP, Panda AK et al (2020) Design of expert guided investigation of native L-asparaginase encapsulated long-acting cross-linker-free poly (lactic-co-glycolic) acid nanoformulation in an Ehrlich ascites tumor model. Saudi Pharmac J 28(6):719–728
Soares AL, Guimaraes GM, Polakiewicz B, de Moraes Pitombo RN, Abrahão-Neto J (2002) Effects of polyethylene glycol attachment on physicochemical and biological stability of E. coli L-asparaginase. Int J Pharm 237(1–2):163–170
Srivastava S, Usmani Z, Atanasov AG, Singh VK, Singh NP, Abdel-Azeem AM, Prasad R, Gupta G, Sharma M, Bhargava A (2021) Biological nanofactories: Using living forms for metal nanoparticle synthesis. Mini- Reviews in Medicinal Chemistry 21(2): 245–265
Stams WA, den Boer ML, Beverloo HB, Meijerink JP, Stigter RL, van Wering ER et al (2003) Sensitivity to L-asparaginase is not associated with expression levels of asparagine synthetase in t (12; 21)+ pediatric ALL. Blood J Am Soc Hematol 101(7):2743–2747
Stecher AL, De Deus PM, Polikarpov I, Abrahao-Neto J (1999) Stability of L-asparaginase: an enzyme used in leukemia treatment. Pharm Acta Helv 74(1):1–9
Su N, Pan YX, Zhou M, Harvey RC, Hunger SP, Kilberg MS (2008) Correlation between asparaginase sensitivity and asparagine synthetase protein content, but not mRNA, in acute lymphoblastic leukemia cell lines. Pediatric blood & cancer 50(2):274–279
Sun J, Nagel R, Zaal EA, Ugalde AP, Han R, Proost N, ... Agami R (2019) SLC 1A3 contributes to Lasparaginase resistance in solid tumors. The EMBO journal 38(21):e102147
Sundaramoorthi C, Rajakumari R, Dharamsi ABHAY, Vengadeshprabhu K (2012) Production and immobilization of L-asparaginase from marine source. Int J Pharm Pharm Sci 4:229–232
Sutrisno L, Hu Y, Hou Y, Cai K, Li M, Luo Z (2020) Progress of iron-based nanozymes for antitumor therapy. Front Chem 8
Swain AL, Jaskolski M, Housset D et al (1993) Crystal structure of Escherichia coli L-asparaginase, an enzyme used in cancer therapy. Proc Natl Acad Sci U S A 90:1474–1478. https://doi.org/10.1073/pnas.90.4.1474
Taleghani AS, Ebrahimnejad P, Heidarinasab A, Akbarzadeh A (2019) Sugar-conjugated dendritic mesoporous silica nanoparticles as pH-responsive nanocarriers for tumor targeting and controlled release of deferasirox. Mater Sci Eng C 98:358–368
Tanner P, Onaca O, Balasubramanian V, Meier W, Palivan CG (2011) Enzymatic cascade reactions inside polymeric nanocontainers: a means to combat oxidative stress. Chem Eur J 17(16):4552–4560
Thi TTH, Pilkington EH, Nguyen DH et al (2020) The importance of Poly(ethylene glycol) alternatives for overcoming PEG immunogenicity in drug delivery and bioconjugation. Polymers (Basel) 12(2):298. https://doi.org/10.3390/polym12020298
Tong WH, van der Sluis IM, Alleman CJM et al (2013) Cost-analysis of treatment of childhood acute lymphoblastic leukemia with asparaginase preparations: The impact of expensive chemotherapy. Haematologica 98:753–759. https://doi.org/10.3324/haematol.2012.073510
Tsuchiya N, Hosono A, Yoshikawa T, Shoda K, Nosaka K, Shimomura M et al (2018) Phase I study of glypican-3-derived peptide vaccine therapy for patients with refractory pediatric solid tumors. Onco Targets Ther 7(1):e1377872
Ueno T, Ohtawa K, Mitsui K et al (1997) Cell cycle arrest and apoptosis of leukemia cells induced by L-asparaginase. Leukemia 11:1858–1861. https://doi.org/10.1038/sj.leu.2400834
Ulu A, Ates B (2017) Immobilization of l -asparaginase on carrier materials: a comprehensive review. Bioconjug Chem 28:1598–1610. https://doi.org/10.1021/acs.bioconjchem.7b00217
Ulu A, Koytepe S, Ates B (2016) Design of starch functionalized biodegradable P (MAA-co-MMA) as carrier matrix for l-asparaginase immobilization. Carbohydrate polymers 153:559–572
Ulu A, Noma SAA, Koytepe S, Ates B (2018) Magnetic Fe3O4@ MCM-41 core–shell nanoparticles functionalized with thiol silane for efficient L-asparaginase immobilization. Artif Cells Nanomed Biotechnol 46(sup2):1035–1045
Upadhyay KK, Mishra AK, Chuttani K, Kaul A, Schatz C, Le Meins JF et al (2012) The in vivo behavior and antitumor activity of doxorubicin-loaded poly (γ-benzyl l-glutamate)-block-hyaluronan polymersomes in Ehrlich ascites tumor-bearing BalB/c mice. Nanomedicine 8(1):71–80
Vala AK, Sachaniya B, Dudhagara D et al (2018) International Journal of Biological Macromolecules Characterization of L-asparaginase from marine-derived Aspergillus niger AKV-MKBU, its antiproliferative activity and bench scale production using industrial waste. Int J Biol Macromol 108:41–46. https://doi.org/10.1016/j.ijbiomac.2017.11.114
van Oppen LM, Abdelmohsen LK, van Emst-de Vries SE, Welzen PL, Wilson DA, Smeitink JA et al (2018) Biodegradable synthetic organelles demonstrate ROS shielding in human-complex-I-deficient fibroblasts. ACS Central Sci 4(7):917–928
Varlas S, Blackman LD, Findlay HE, Reading E, Booth PJ, Gibson MI, O’Reilly RK (2018) Photoinitiated polymerization-induced self-assembly in the presence of surfactants enables membrane protein incorporation into vesicles. Macromolecules 51(16):6190–6201
Varlas S, Foster JC, Georgiou PG, Keogh R, Husband JT, Williams DS, O’Reilly RK (2019) Tuning the membrane permeability of polymersome nanoreactors developed by aqueous emulsion polymerization-induced self-assembly. Nanoscale 11(26):12643–12654
Vasile C (2019) Polymeric nanomaterials: recent developments, properties and medical applications. Polymeric nanomaterials in nanotherapeutics, 1-66. Micro and Nano Technologies 235–259
Vimal A, Kumar A (2017) Biotechnological production and practical application of L-asparaginase enzyme. Biotechnol Genet Eng Rev 33:40–61. https://doi.org/10.1080/02648725.2017.1357294
Vina I, Karsakevich A, Bekers M (2001) Stabilization of anti-leukemic enzyme L-asparaginase by immobilization on polysaccharide levan. J Mol Catal B Enzym 11:551–558. https://doi.org/10.1016/S1381-1177(00)00043-6
Wade HE, Elsworth R, Herbert D, Keppie J, Sargeant K (1968) A new L-asparaginase with antitumour activity. Lancet 292(7571):776–777
Wahab RA, Elias N, Abdullah F, Ghoshal SK (2020) On the taught new tricks of enzymes immobilization: An all-inclusive overview. React Funct Polym 152:104613. https://doi.org/10.1016/j.reactfunctpolym.2020.104613
Wang F, Zhang YQ (2015) Bioconjugation of silk fibroin nanoparticles with enzyme and peptide and their characterization. Advances in protein chemistry and structural biology 98:263–291
Wannasarit S, Wang S, Figueiredo P, Trujillo C, Eburnea F, Simón-Gracia L et al (2019) A virus-mimicking pH-responsive acetalated dextran-based membrane-active polymeric nanoparticle for intracellular delivery of antitumor therapeutics. Adv Funct Mater 29(51):1905352
Warrell RP Jr, Arlin ZA, Gee TS, Chou TC, Roberts J, Young CW (1982) Clinical evaluation of succinylated Acinetobacter glutaminase-asparaginase in adult leukemia. Cancer Treat Rep 66(7):1479–1485
Wicki A, Ritschard R, Loesch U, Deuster S, Rochlitz C, Mamot C (2015) Large-scale manufacturing of GMP-compliant anti-EGFR targeted nanocarriers: Production of doxorubicin-loaded anti-EGFR-immunoliposomes for a first-in-man clinical trial. Int J Pharm 484(1–2):8–15
Wilder LM, Handali PR, Webb LJ, Crooks RM (2020) Interactions between oligoethylene glycol-capped AuNPs and attached peptides control peptide structure. Bioconjug Chem
Willems L, Jacque N, Jacquel A, Neveux N, Trovati Maciel T, Lambert M, ... Bouscary D (2013) Inhibiting glutamine uptake represents an attractive new strategy for treating acute myeloid leukemia. Blood, The Journal of the American Society of Hematology 122(20):3521–3532
Williams RT, Guarecuco R, Gates LA, Barrows D, Passarelli MC, Carey B et al (2020) ZBTB1 regulates asparagine synthesis and leukemia cell response to L-asparaginase. Cell Metab 31(4):852–861
Xie X, Zhou W, Hu Y, Chen Y, Zhang H, Li Y (2018) A dual-function epidermal growth factor receptor pathway substrate 8 (Eps8)-derived peptide exhibits a potent cytotoxic T lymphocyte-activating effect and a specific inhibitory activity. Cell Death Dis 9(3):1–16
Xu H, Chen CX, Hu J, Zhou P, Zeng P, Cao CH, Lu JR (2013) Dual modes of antitumor action of an amphiphilic peptide A(9)K. Biomaterials 34(11):2731–2737. https://doi.org/10.1016/j.biomaterials.2012.12.039. Epub 2013 Jan 23
Xu B, Cui Y, Wang W, Li S, Lyu C, Wang S et al (2020) Immunomodulation-enhanced nanozyme-based tumor catalytic therapy. Adv Mater 32(33):2003563
Zhang YQ, Tao ML, De Shen W et al (2004) Immobilization of L-asparaginase on the microparticles of the natural silk sericin protein and its characters. Biomaterials 25:3751–3759. https://doi.org/10.1016/j.biomaterials.2003.10.019
Zhang B, Shi W, Li J, Liao C, Yang L, Huang W, Qian H (2017) Synthesis and biological evaluation of novel peptides based on antimicrobial peptides as potential agents with antitumor and multidrug resistance-reversing activities. Chem Biol Drug Des 90(5):972–980
Zhang K, Tang X, Zhang J, Lu W, Lin X, Zhang Y, ... He H (2014) PEG–PLGA copolymers: Their structure and structure-influenced drug delivery applications. Journal of Controlled release 183:77–86
Zhao Y, Ding B, Xiao X, Jiang F, Wang M, Hou Z et al (2020) Virus-like Fe3O4@ Bi2S3 nanozymes with resistance-free apoptotic hyperthermia-augmented nanozymitic activity for enhanced synergetic cancer therapy. ACS Appl Mater Interfaces 12(10):11320–11328
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Chakravarty, N., Mathur, A., Singh, R.P. (2022). L-asparaginase: Insights into the Marine Sources and Nanotechnological Advancements in Improving Its Therapeutics. In: Sarma, H., Gupta, S., Narayan, M., Prasad, R., Krishnan, A. (eds) Engineered Nanomaterials for Innovative Therapies and Biomedicine. Nanotechnology in the Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-82918-6_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-82918-6_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-82917-9
Online ISBN: 978-3-030-82918-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)