Abstract
In this study, we firstly reported the large-scale screening and isolation of endophytic fungi from nine wild and six cultivated soybeans in the cold regions of China. We totally isolated 302 endophytic fungal strains, of which 215 strains are isolated from the wild soybeans and 87 are identified from cultivated soybeans. Among these endophytic fungal strains, in the roots, stems, and leaves, 24.17% were isolated from roots, 28.8% were isolated from stems, and 47.01% were isolated from leaves, respectively. Most endophytic fungal strains isolated from the wild soybean roots were the species of Fusarium genus, and the fungal strains in the stems were the species of ascomycetes and Fusarium fungi, whereas most strains in the leaves were Alternaria fungi. To analyze the taxonomy of the obtained samples, we sequenced and compared their rDNA internal transcribed spacer (ITS) sequences. The data showed that 6 strains are putatively novel strains exhibiting ≤ 97% homology with the known strains. We next measured the secondary metabolites produced by the different strains and we found 11 strains exhibited high-performance synthesis of triterpenoids, phenols, and polysaccharides. Furthermore, we characterized their tolerance to abiotic stresses. The results indicated that 4 strains exhibited high tolerance to cadmium, and some strains exhibited resistance to acid, and alkali. The results of the study could facilitate the further exploration of the diversity of plant endophytic fungi and the potential applications of the fungi to practical agriculture and medicine industries.
Key points
• 302 endophytic fungal strains isolated from wild soybean and cultivated soybean
• 11 strains had high contents of triterpenoids, phenols, and polysaccharides
• 4 strains exhibited high Cd tolerance, and a few strains with strong tolerance to acid and alkali solution
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endophytic fungi have been of great interest during the last two decades as potential producers of biologically active products. They are a rich source of functional secondary metabolites that include flavonoids, terpenoids, steroids, phenol, phenyl propanoids, quinines, indole derivatives, amines, alkaloids, amides, pyrrolizidines, sesquiterpenes, diterpenes, lignans, isocoumarin derivatives, peptides, phenolicacids, chlorinated metabolites, and aliphatic compounds (Jalgaonwala et al. 2011). Endophytic fungi are microfungi that internally infect living plant tissues without causing any visible manifestation of disease and live in mutualistic association with plants for at least a part of their life cycle, and endophytic fungi inhabit plant tissues without destroying or producing substances that cause an infection to the host cell. Their coevolution means that the endophytes produce the same or similar compounds to those originating from the plant (Hyde 2010). These endophytic fungi reside within living tissues of the hosts and establish relationships ranging from symbiotic to pathogenic. The colonization of plants by these fungi varies considerably, and some medicinal plants and agricultural crops are reported to harbor more endophytes (Sun et al. 2010; Maulana et al. 2018).
When endophytes reside in plant hosts, their continuous metabolic interactions occur between the fungi and hosts. Consequently, genetic recombination between the host plants and the endophytic fungi can happen so that the endophytic fungi can produce metabolic products similar to those produced by the host plants (Amal et al. 2010; Jalgaonwala et al. 2011). The endophytes associated with the plants are known to acquire the potential to produce the active principles for which the host is known. There is mounting evidence that many bioactive compounds isolated from various endophytic fungi which are also the potential sources in the discovery of novel products. Fungi represent the core group of eukaryotic organisms that demonstrate a high capacity to produce numerous metabolites with antimicrobial activities and that have potential applications as drugs (Gunatilaka and AAL 2006).
Several bioactive compounds demonstrating antibacterial and antiviral activities have been isolated from fungi (Suryanarayanan et al. 2009; Khan et al. 2016). Mesalazine A, pestalamide A, and asparagine were extracted from the fermentation broth of Pestalotiopsis LN560 isolated from tea tree (Camellia sinensis), and these compounds could efficiently inhibit the replication of HIV-1 (Li et al. 2008a). Furthermore, Phomopsis euphorbiae, isolated from the petiole of Trewia nudiflora, exhibits a strong inhibitory effect against the replication of HIV-1 in cells, which is attributed to the phomoeuphorbin A and phomoeuphorbin C in the fermentation products of Phomopsis euphorbiae (Yu et al. 2008). Some studies have reported endophytic fungi that produce anticancer agents (Cheng et al. 2019a, b). Wang et al. (2008) reported four out of six compounds from Penicillium sp. exhibited high cytotoxicity against human oral epidermoid carcinoma cells (KB) and HepG2. Song et al. (2010) isolated two endophytic fungi strains of Tripterygium wilfordii and found their secondary metabolites generated significant inhibitory effects against the human breast cancer cells MCF-7 and U937. In addition, a polyketide, pestalotiopsones A–F, isolated from Pestalotiopsis sp. JCM2A4 is specifically cytotoxic to the tumor cell line L5178Y-R of mouse DBA/2 lymphoma (Davis et al. 2010).
Besides, endophytic microbes and the host plants have symbiotic relationship and benefit to each other. Endophytic microbes are often functional in carrying nutrients from the soil into plants, modulate plant development, and increase plant tolerances to abiotic and biotic stresses, such as salt, drought, and diseases (White et al. 2019). The loss of endophytic microbes from crop plants during domestication and long-term cultivation could be remedied by transfer of endophytes from wild relatives of crops to crop species. Study of the diversity of endophytic fungi is important with respect to their characterization, species diversity, and also bioactive molecules. Using traditional taxonomic tools and also molecular taxonomy, many endophytic fungi isolated from diverse group of plants have been identified. Molecular tools used to study endophytic fungi are sequences of the 5.8S gene and flanking internal transcribed spacers (ITS1 and ITS2) of the rDNA, 18S, and 28S rRNA genes.
Soybeans are an important resource for edible oils and proteins. However, in comparison with other crops, studies on endophytes in soybean, especially in wild soybean plants, are still limited. Impullitti and Malvick (2013) obtained 12 endophyte genera using culture-dependent (CD) colony isolation method and isolated 6 endophyte genera using culture-independent (CI) colony isolation method. Many of the fungal endophytes identified in the above studies are known to be soybean pathogens, e.g., Fusarium and Alternaria; however, they were not able to cause symptoms of disease. It remains to be determined whether those endophytes are latent pathogens or non-pathogenic forms that benefit the host plants. Wild soybean (Glycine soja) originated in China and is the original ancestor of cultivated soybean. Under complex natural habitats, wild soybeans have abundantly genetic and ecological diversities (Dong 2008; Hu et al. 2011). Therefore, endophytic fungi in wild soybeans may be more diverse than those in other soybean species and we speculate that wild soybeans may harbor diverse endophytic fungi which produce special secondary metabolites that we need.
In the present study, we isolated endophytic fungi strains from wild soybeans in different habitats in Heilongjiang Province where it is the coldest region in China in winter. The diversities of endophytic fungi from wild soybeans and cultivated soybean were determined and compared by ITS sequence analysis. The phylogenetic relationship of the selected strains and their secondary metabolites and their biological functions in plant resistance to abiotic stresses were investigated. The findings of the present study could facilitate the isolation of novel functional endophytic strains which generate secondary metabolites from wild soybeans for potential medicinal and industrial applications.
Materials and methods
Isolation of endophytic fungi
Fifteen plant strains, including nine wild soybeans and six cultivated soybeans, were collected from different regions (Additional File 1 and Additional File 2) in Heilongjiang Province (in mid-July), China. The selected healthy roots, stems, and leaves of different soybean plants were washed under running water to remove dirt, and then were subject to surface-sterilized sequentially in 75% ethanol for 1 min and 1% NaOCl for 7 min, and were rinsed in sterile water for three times. The roots and stems were cut into 0.5-cm pieces and the leaves were cut into 0.5-cm2 discs. The roots and stem pieces and leaf discs were plated on 2% malt extract agar (MEA, Difco Inc.) supplied with 50 mg/l chloramphenicol to inhibit bacterial growth. The cultures were incubated at 25 °C in the dark. After 5 days, the fungal colonies were transferred into fresh potato dextrose agar (PDA) for growth and isolation.
Observation of colonization of endophytic fungi from different plants
The mycelium and sporulation structures of endophytic fungi can be stained to be bright blue by gossypol blue dye. The color made it relatively easy for us to determine the fungi colonization sites. To observe the endophytic fungi in plant tissues, we immersed the surface-sterilized soybean leaves, stems, and root tissues in 5% KOH solution (50 °C), decolorized for 2 days, washed with sterile water, and then bleached lightly with 3% H2O2 for 1 min, and then washed with 2% HCl for two times. Afterward, the plant samples were stained in 0.05% cotton blue (dissolved in lactic acid) for 2 h and were rinsed with sterile water. The tissues were cut into 0.5-cm2 slices and were observed under an Olympus BX51 optical microscope (Olympus, Germany).
Morphological identification of endophytic fungi
The morphological characteristics including the colony size, color, transparency, surface state, and edge (neat, irregular, radial, etc.) were recored by a Canon 60D camera (18–200 mm; Japan). The microstructures of endophyte mycelium including the surface state and the microstructure of mycelium were observed by the optical microscope. Colony microscopical observation was carried out by using axioskop2plus FL (Zeiss, Germany) and Axioplan 2 imaging MOT software.
Taxonomic analyses of endophytic fungi
The genomic DNA samples of the isolated endophytic fungi were extracted using the CTAB method. Primers, ITS1 (5′-CTTGGTCATTTAGACGAAGTAA-3′) and ITS4 (5′-GCATATCAATAAGCGGAGGA-3′), were used to amplify the ITS (internal transcribed spacer) sequences. PCR reactions were performed in a 50-μl reaction volume. The thermal cycling program was as follows: 3-min initial denaturation at 94 °C, followed by 35 cycles of 40-s denaturation at 94 °C, 50-s primer annealing at 55 °C, and 1-min extension at 72 °C. The PCR products were analyzed by gel electrophoresis, and the appropriate PCR products were purified and sequenced (Sangon Biotech, Shanghai, China). Each sequence was used as a query sequence to search for similar sequences using Blast (https://blast.ncbi.nlm.nih.gov/Blast.cgi) from GenBank.
Analysis of secondary metabolites produced by the endophytic fungi
The endophytic fungi of interest were cultured and fermented to analyze accumulation of the flavonoid, triterpenoid, total phenol, and polysaccharide in endophytic fungal hyphae and fermentation broth, and to screen endophytic strains with high yields of secondary metabolites. Three 0.8-cm endophytic fungi colonies in diameter on agar medium were picked to inoculate 150-ml potato dextrose broth (PDB) media in 250-ml flasks for fermentation. All fermentation flasks were shaken at 160 rpm for 10 days. After fermentation, the culture broths were separated from the mycelia by filtration. The culture filtrates were dried at 60 °C. The filtered culture broths were concentrated 10-fold using a rotary evaporator. The dried filtrates and concentrated broths were used for secondary metabolite analyses.
The polysaccharides in the dried filtrates and concentrated broths were determined using the anthrone-sulfuric acid method. The dried filtrates (0.20 g) were boiled in 5 ml distilled water for 30 min twice. The filtered solution was combined and diluted to 25 ml. Two milliliters of water and 6.5 ml of anthrone-sulfuric acid (1.0 g anthrone in 500 ml concentrated sulfuric acid) were added into 0.5 ml of extracted solution. After incubation at room temperature for 10 min, the absorption values of the solutions were measured at a wavelength of 625 nm in a fluorenscence spectrometer.
Total flavonoids in the dried filtrates and concentrated broths were analyzed using the previous method (Chen et al. 2011). To determine the flavonoids in solid hyphae, the dried hyphae were ground into powder using a mortar, and 0.20 g of the sample was resuspended in 10 ml of 50% ethanol in a 50-ml flask. The suspensions were sonicated for 90 min and then were filtered. The filtrates were diluted to 25 ml. To determine the concentration of flavonoids in the fermentation broth, 5 ml of the concentrated fermentation broth was diluted to 10 ml with absolute ethanol. The absorbance values of the solutions were measured at a wavelength of 550 nm.
Determination of total triterpenoids, oleanolic acid, and betulinic acid of endophytic fungi
Fifty milligrams of dried hyphae were suspended in 4 ml of 95% ethanol for 24 h. The extraction and detection methods for total triterpenoids were performed according to our previous method (Yin et al. 2012), and the concentrations of triterpenoids, oleanolic, and betulinic acids were determined using high-performance liquid chromatography (HPLC) according to the previous method (Yin et al. 2017).
Extraction and determination of polyphenols in endophytic fungi
To determine the concentrations of polyphenols in solid hyphae, 0.20 g of dried hyphae powder was transferred in 7 ml of 60% ethanol in 50-ml flasks. The suspension was sonicated for 45 min and centrifugated, and then, the supernatant was transferred into a fresh tube. The process was repeated for three times. Afterward, the supernatant was evaporated to dry at 70 °C. The solid material was resuspended in 15 ml of ddH2O. The extraction and determination of total phenolic substances in endophytic fungi were performed according to the method of Li et al. 2008b.
Abiotic resistance of endophytic fungi
Tolerance to cadmium and salt
To determine resistance of endophytic fungi to heavy metal stress, we inoculated two fungi colonies (0.8 cm in diameter) into 50 ml of PDB media containing 0 mg/l, 50 mg/l, 100 mg/l, 200 mg/l, and 400 mg/l CdCl2 or 5% NaCl in 100-ml flasks. The flasks were incubated at 160 rpm at 25 °C for 10 days. Then, the broths were filtered, and the mycelia dried at 60 °C.
Resistance to acidic and alkaline conditions
Two fungal colonies (0.8 cm in diameter) were inoculated into 50 ml PDB media with pH 3, 7, or 9 (adjusted using NaOH or HCl). The flasks were incubated at 160 rpm at 25 °C for 10 days. Then, the broths were filtered, and the mycelia dried at 60 °C.
Strain resistance to acidic and alkaline conditions
The experiments were conducted in triplicate: 50 ml PDB media in pH 3, 7, or 9 (adjusted using NaOH or HCl) were inoculated with two 0.8-cm-diameter agar of grown fungi. The methods of growing and weighing are similar to the ones applied above. Three biological repeats per treatment.
Results
Isolation and identification of endophytic fungi from wild and cultivated soybeans
We totally isolated 302 endophytic fungal strains from the roots (r), stems (s), and leaves (1) of 15 soybean materials including 9 wild soybean (Y1~Y6, BYS, SYS, WD) and 6 cultivated soybean (Z1~Z6) (Additional File 3 and Fig. 1A, B). Of these strains, 215 were from wild soybeans and 87 were from cultivated soybeans. Averagely, we isolated 26.9 endophytic fungal strains per wild soybean line and 14.5 endophytic fungal strains per soybean line, indicating that wild soybean hosted more diverse endophytic fungi. In addition, 43 and 40 strains of endophytic fungi were isolated from Y5 and Y6, respectively. Y5 with small brown grain was obtained from Ningan County (annual accumulated temperature: 2500 °C/year); Y6 with large brown grain was obtained from Xunke County (annual accumulated temperature: 2100 °C/year) (Fig. 1B). In the two regions, the climate is relatively cold, and the plants are more resistant to temperature stress, which may be the primary reason for the more diverse endophytic fungi. The analysis also revealed that 24.17%, 28.8%, and 47.01% of the endophytic fungi were isolated from the roots, stems, and leaves of the soybean materials, respectively, indicating that the leaves contained more endophytic fungi (Additional File 3).
Microstructural characteristics of different endophytic fungi
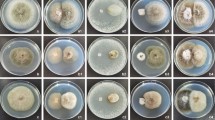
The morphology and the identified microstructural characteristics of different endophytic fungi were observed, and found that the characteristics of endophytic fungi from different materials were significantly different (Additional file 4 and Additional file 5). Their colonies showed different colors in the medium, including red (SYS2), pink (WDS5, Y6R1), yellow (Y5L13), brown (Y6S3), gray (YSYS1), and white (Y5L1) (Fig. 2). Some colonies grow to 7–10 days and have obvious metabolite synthesis, showing water drop or oil drop. In addition, it was found that the microstructure of different endophytic fungi was significantly different. Some strains produced spores of different shapes, hyphae had septa, some fungi did not produce spores, and hyphae did not have septa and branches. Such as Y6L2, the conidiophore is solitary, rarely branched, light brown, separated, straight, or curving, 53.4–96.7 × 3.8–6.2 μm; the conidia are solitary, rarely short chain (2–3 spores), brown, obovate or nearly elliptic, with 2–5 diaphragms, 1–4 longitudinal and oblique diaphragms, slightly constricted at the separation, 35.26–55.5 (43.8) × 9.7–18.0 (13.3) μm; the beak is columnar, and the boundary between the conidia and the body is unclear obviously, light brown, undivided, 5.1–26.3 × 2.5–3.5 μM. According to this, it was preliminarily identified as Alternaria (Fig. 3). Some endophytic fungi hyphae are septate and branched. Conidiophore branched or unbranched. There are two forms of conidia: small conidia are oval to columnar with 1–2 septum, such as Z3 and Y2R14; large conidia are sickle or long columnar, with more diaphragms, such as Y5R4, they were preliminarily identified as Fusarium sp. (Fig. 3). The microstructural characteristics of other strains can be found in Additional file 5. The results showed that endophytic fungi from wild and cultivated soybeans were abundant and varied, which may have the potential to synthesize active products and have good research value.
Colonization of endophytic fungi in soybean plants
Gossypol blue staining revealed that the endophytic fungi in wild soybean colonized mainly the veins, glandular hairs, and leaf intercellular spaces (Fig. 4A, B). In addition, according to the cross section staining results, the endophytic fungi were rich in the stems of wild soybean and were distributed in the intercellular spaces (Fig. 4C–F). Furthermore, according to the results of the root epidermal staining, root epidermal cells were more conducive for fungal, bacterial, and other microbial infections due to contact with soil. Therefore, the internal sections of the root epidermal tissue cells were blue, indicating that the epidermal cells contained endophytic fungi (Fig. 4G, H).
ITS sequence analysis of endophytic fungi
Based on the results of the colony morphology analyses of the isolated strains, analysis of ITS sequences was performed on the 162 endophytic fungi of interest, and a phylogenetic tree was constructed. The taxonomic units of the endophytic fungi were diverse, implying that wild soybean contained a wide range of endophytic fungal resources. The colonization sites of fungi with relatively close evolutionary distances were similar, such as YSYS5, YSYS6, and YSYS9, which were derived from the stems of the wild soybean YSY. Gibberella sp. and Fusarium sp. had a large taxonomic distance between them and endophytic fungal strains with relatively long evolutionary distances, such as Y5L7 and Y5L9, were isolated from similar parts of the same material, which suggested that abundant endophytic fungi were not isolated from such parts. Endophytic fungi, including WDS1, BYSS2, and Y5L7 from WD (pea), Y (wild), and BY (semi-wild) materials, had similar in taxonomic status, which further indicated that similar species of endophytic fungi in different plants had similar functions (Additional File 6).
The 162 fungi ITS sequence of wild soybeans obtained using BLAST indicated that the strains were divided into 25 genera. Among them, 73 strains were assigned to Fusarium sp. and accounted for 45.06% of all the isolated strains that were distributed in the roots, stems, and leaves, including wild soybean (Y), semi-wild soybean (BYS), wild pea (WD), and cultivated soybean (Z). Therefore, the endophytic fungi of the genus Fusarium were the dominant fungi based on their number. In addition, there were 20 and 24 Epichocum sp. and Gibberella moniformis, respectively (Additional file 6).
According to the BLAST alignment results, strains with a ≤ 97% similarity were potentially new strains. In the present study, six new suspected strains were observed (Additional File 3), which included WDL2 (Dothideomycetes sp., 95% similarity), Z4 (Dothideomycetes sp., 92% similarity), Y2S1 (Gibberella monitors, 91% similarity), Y5L13 (Pleosporales sp., 95% similarity), Y5S9 (Fusarium sp., 97% similarity), and BYSL3 (Gibberella moniformis, 97% similarity) (Additional File 6). The discovery of these new endophytes provides new microbial resources. They may have more important features or new functions. In this study, some of the special strains are preserved in Culture Preservation Center (Wuhan, China), including DEPOSIT No. of WDL2 of the typical culture preservation center in China is CCTCC M 2014518. DEPOSIT No. of WDS2 (Phomopsis sp.) is CCTCC M 2019394. The ITS sequences of five new strains were submitted to GeneBank. Their accession numbers are as follows:WDL2 (MT678832); Z4 (MT678833); Y2S1 (MT678834); Y5S9 (MT678835); BYSL3 (MT678836).
Screening of functional strains of endophytic fungi in wild soybean
Mycelial growth in different endophytic fungi in wild soybean
The wild soybean endophytic fungal strains Y2R14, Y5R4, and Y5R11 exhibited relatively low mycelial growth. The Y6S8, Y2S9, and Y1S9 strains had the highest growth rates, weighing 5.457 g, 4.655 g, and 4.487 g, respectively (Fig. 5). The average weight of the three strains with high mycelial growth was 4.632 g, nearly 20 times higher than the average in the low growth rate strains, indicating that the growth rates of different endophytic fungi in wild soybean were significantly different. The three strains with the lowest growth rates were all isolated from the roots of Y2 and Y5 wild soybean strains, including Y2R14, Y5R4, and Y5R11 strains. The three strains with the highest growth rates were all isolated from the stems of the wild soybean Y1 strain, Y2 strain, and Y6 strain (Fig. 5). The results of the analysis indicated that there were significant differences in the growth rates of endophytic fungi colonized in different parts. In addition, the three strains with low growth rates were all Fusarium oxysporum strains.
The 42 strains of endophytic fungi from wild soybean were isolated and purified, and the accumulation of secondary metabolites in the endophytic fungal hyphae and the fermentation broth was analyzed. The high-content polysaccharide strains were Y2S9, WDL3, and WDL2, with a maximum concentration of 85.51 mg/g (Fig. 6A); high-content flavonoid strains were Y1S9 and Y6S8, respectively; high-content triterpenoid strains were Y6S8, Y2R1, and Y6R2, respectively (Fig. 6B), and WDS7 had the highest total phenolic amount, at 22.65 mg/g (Fig. 6C). Among the fermentation broths of endophytic fungal, the strains with high flavonoid contents were Y1S8 and SYS4, and their contents were 3.11 mg/l and 4.81 mg/l, respectively (Fig. 7A). The strains with high total triterpenoid contents were YSYL2, Y1S8, and WDL3, at 17.46 mg/l, 35.00 mg/l, and 42.98 mg/l, respectively (Fig. 7B). Different habitats may be the main reason for the differences in rates of accumulation of secondary metabolites. In the present study, 11 strains of high-yield secondary metabolites were screened from wild soybeans, which laid a foundation for the potential functions and the screening of novel drug candidates of endophytic fungi from wild soybean.
Screening strains of endophytic fungi from wild soybean against cadmium
Ten strains with high contents of secondary metabolites and five common strains were selected for cadmium (Cd) toxicity experiments. Different strains exhibited different responses to the Cd, and similar strains also exhibited different responses to different CdCl2 concentrations (Fig. 8), and the performances were divided into three groups. In the first group, the growth mycelium growth rates first increased and then decreased with an increase in CdCl2 concentration, such as in ZY1S-8 (A), S20-1 (B), Y5L11 (C), SY2S2-1 (E), Y2R5 (G), S20-2 (J), SY6R-15 (K), Z3 (M), and YSYL3 (N). In the second group, the growth mycelium growth rates decreased within a specific range with an increase in CdCl2 concentration, such as in Y1S5 (F), YSYS1 (L), and Y6L3 (O). In the third group, the growth rates of hyphae increased gradually with an increase in CdCl2 concentrations (0–400 mg/l), for example, in WDR5 (D), Y2R14 (H), and Y5R11 (I) strains.
There were significant differences in the tolerances of the strains to CdCl2. YSYS1 (L) exhibited the least tolerance to Cd (< 50 mg/l) whileY2R14 (H), WDR5 (D), Y1S5 (F), and Y5R11 (C) still tolerated CdCl2 at a concentration of 400 mg/l. Notably, after the addition of CdCl2, hyphae growth increased in some strains, and the amounts of dried mycelium were significantly higher than in the control groups. Although CdCl2 inhibits the growth of hyphal balls, the number of hyphal balls increased after the addition of low concentrations of CdCl2, resulting in an overall increase in mycelial growth in the media (Fig. 8).
Four endophytic fungal strains highly resistant to Cd toxicity (which can survive ≤ 400 mg/l CdCl2) were screened. Based on the Cd2+ concentrations in hyphae, the WDR5 (D) absorption rate was 81.527% while the Y2R14 (H) Cd2+ absorption rate was 82.789%. There were no Cd-tolerant strains among the five strains with low concentrations of secondary metabolites, with the YSYS1 (L) strain exhibiting the least tolerance. These results suggested that high-level secondary metabolite strains were more likely to survive under heavy metal environments, and the tolerance of endophytic fungi to heavy metal toxicity was potentially correlated with secondary metabolite abundance.
Screening of salt-tolerant endophytic fungi from wild soybean
The results of salt tolerance screening for endophytic fungi revealed that strains A (ZY1S-8), C (Y5L11), L (YSYS1), and O (Y6L3) could grow in PDB with 5% NaCl, with growth in media increasing by 21.39~13.95%, while growth in other strains decreased under similar conditions. The decreases in the growth of the D, E, F, H, K, M, N, and J strains, with growth being lower than the growth in the control by 31.55~52.56%, were the most significant. The growth of strains B, G, and L was relatively high, while the NaCl stress had minimal effects on the growth (Table 1).
Screening of anti-acid and anti-alkali endophytic fungi in wild soybean
There were considerable differences in mycelial growth among different strains under similar acidic or basic environments, when compared with a control group cultured at pH 7. Growth in seven strains increased under pH 3, with the most significant increases observed in YSYS1 (L), SY2S2-1 (E), and ZY1S-8 (A) strains, which grew 19.27, 2.36, and 1.64 times more, respectively, than the control. In the alkaline condition (pH 9), the mycelial growth in Y6L3 (O) and Y2R5 (G) strains increased when compared with the control group, with the growth in Y2R5 (G) strain, which was 38.87% higher than in the control, being the most significant. Under pH 3 and 9, mycelium growth increased in YSYL3 (N), Y1S5 (F), Y2R14 (H), and Y5R11 (I) strains, with the most significant increase observed in the Y5R11 (I) strain, which was 5.83 times higher than that of the control at pH 7. In addition, under pH 3 and pH 9, Z3 (M) strain growth rates decreased by 74.03% and 54.81% than that of the control, respectively (Table 2).
Discussion
Plant-associated habitats are dynamic environments in which many factors influence the structure and species composition of microbial communities that colonize roots, stems, branches, and leaves (Rubini et al. 2005). Endophytic microorganisms may enhance plant fitness by reducing herbivory or colonization by phytopathogens (Azevedo 2000), promoting plant growth, or enhancing tolerance to heavy metals and drought (Xiao et al. 2010; Sun et al. 2010; Sujit et al. 2019). The genus Fusarium, which is described as an endophyte and/or a phytopathogen in many plant species, was the dominant group, suggesting that the fungi has a key role in plant development. Nine endophytic fungi were isolated from the roots of Dendrobium moniliforme. Among the isolated fungi, the dominant fungus was Fusarium sp., and R11 and R13 isolates can synthesize IAA and promote plant growth (Sujit et al. 2019). Isolations from 204 root segments from 15 wild rice plants yielded 58 isolates. Using a threshold of 90% similarity, 35 potentially different sequences (phylotypes) were found among 186 positive clones. Phylogenetic analysis showed that frequently detected clones were classified as Basidiomycota, and 60.2% of total analyzed clones were affiliated with unknown taxa. The findings indicate a complex and rich endophytic fungal consortium in wild rice roots (Yuan et al. 2010).
In the present study, 302 endophytic fungi were isolated from the 15 soybean materials, 215 isolates from wild soybean, and 87 isolates from cultivated soybean, which indicated that wild soybeans contained more endophytic fungi. In addition, 42 and 40 endophytic fungi were isolated from Y5 and Y6 strains, respectively, which were more than in other wild-type soybeans. The reason for the higher numbers of isolates could be the different habitats, with Y5 and Y6 originating from Ning’an County and Xunke County, respectively. The annual cumulative temperature was more low and the growth period was more short, which made the plants more resistant to stress and increased the number of endophytic fungi. The 162 fungal were divided into 25 genera. Among them, 73 strains were assigned to Fusarium sp., and they accounted for 45.06% of the isolated strains, which were distributed in the roots, stems, and leaves in wild soybean (Y) and cultivated soybean (Z). Therefore, the endophytic fungi of the genus Fusarium were the dominant fungi. Secondly, the numbers of Epichocum sp. and Gibberella moniformis were 20 and 24, respectively. The findings indicate a complex and rich endophytic fungal consortium in wild soybean, thus offering a potential bioresource for establishing a novel model of soybean-fungal mutualistic interactions.
Endophytic fungi colonize their hosts without any external disease symptoms, except when the host is under stress (Rudgers et al. 2004; Rubini et al. 2005). In addition, they can produce a variety of complex bioactive products (Davis et al. 2010; Wellensiek et al. 2013). Over the past two decades, numerous antibacterial, insecticidal, cytotoxic, and anticancer compounds have been isolated from endophytic fungi (Amal et al. 2010; Mitchell et al. 2010; Zhao et al. 2010; Shao et al. 2010; El-Sayed et al. 2019). The compositions of endophytic fungi from wild soybeans from different habitats were considerably different. Almost all the strains with high concentrations of secondary metabolites were derived from wild soybeans, which grew in harsh environments. The numbers of endophytic fungi and the levels of production of secondary metabolites were relatively high. For example, Y6S8, which had high levels of secondary metabolite production, was derived from wild soybean roots in Xunke County forest area in Heilongjiang. The annual accumulated temperature in the area was 2000–2100 °C and the frost-free period was short, with only 85–115 days. However, the Z3 endophytic fungus from the cultivated soybean grew in the experimental area in Acheng District, which had good cultivation conditions. The annual accumulated temperature was above 2800 °C, the soil was relatively fertile, and the water was adequate. The differences in the growth environments of the wild soybean and the cultivated soybean could be the major reason for the differences in the concentrations of secondary products observed among the endophytic fungi.
The endophytic fungi were highly diverse, and the types of secondary metabolites produced were also diverse (Kumar et al. 2017; El-Maali et al. 2018 Maulana et al. 2018; Tawfike et al. 2018). Fractionation of the EtOAc-soluble fraction of the liquid fermentation broth of an endophytic fungus, Annulohypoxylon ilanense, led to the isolation of a novel α-pyrone, ilanpyrone, along with three previously identified compounds. Their cytotoxicity against cell lines was evaluated using a tetrazolium assay. Two compounds exhibited moderate-to-weak cytotoxic effects against MCF-7, NCI-H460, and SF-268 cell lines in vitro (Cheng et al. 2019a, b).
Analysis of the secondary metabolites from endophytic fungi obtained wild soybean revealed that the 60 wild soybean endophytic fungi contained numerous secondary metabolites, including polysaccharides, triterpenoids, flavonoids, polyphenols, and phenolic substances. The concentrations of secondary metabolites in different strains varied considerably in the hyphae and fermentation broths of various endophytic fungi. The results showed that nine endophytic fungal strains with high concentrations of secondary metabolites were selected for the functional assessments and the screening of subsequent strains, and the endophytic fungi were also important sources of novel bioactive and medicinal substances.
It was reported that endophytic fungus LSE10 was isolated from the cadmium hyperaccumulator Solanum nigrum L. It was used as a biosorbent for biosorption of cadmium from the aqueous solution. The results showed that the maximum biosorption capacity was 247.5 mg/g (2.2 mmol/g) which was much higher than those of other adsorbents, including biosorbents and activated carbon (Xiao et al. 2010). In our present study, 15 strains exhibited different levels of resistance to heavy metal (Cd) toxicity, salt stress, and acid and alkali stress. Y2R14. RWDL4-1, Y1S5, and Y5R11 strains were the most tolerant to Cd ions and could still grow at a CdCl2 concentration of 400 mg/l. The Cd-tolerant strains were high-order metabolite strains, suggesting that the accumulation of secondary metabolites enhances their Cd tolerance. The salt tolerance of strain C (Y5L11) was significantly higher than that of the control group, and the acid and alkali resistance results showed that YSYS1 (L), SY2S2-1 (E), ZY1S-8 (A), and S20-2 (J) were acid-resistant strains, and strain Y2R5 (G) had high alkali tolerance.
Terrestrial plants can harbor endophytic fungi, which may alter plant physiology that would, in turn, influence interactions with herbivorous insects and other microorganisms (Clifton et al. 2018; Aboobaker et al. 2019). In cucumber, non-pathogenic F. oxysporum may induce host resistance against Pythium ultimum through a combination of antibiosis and mycoparasitism, in addition to inducing plant defense responses (Benhamou 2002). In addition, the crude extracts from Penicillium skrjabinii fungal isolates exhibit antimicrobial activity against Streptococcus aureus and Escherichia coli at concentrations of 0.03 and 0.09 mg/ml, respectively, with the major active ingredient being dibutyl phthalate (Aboobaker et al. 2019). Sclerotinia disease 15-2 for soybean, Phytophthora 1 race for soybean, blast fungus P-46-2 for rice, and corn leaf spot p51-2 are the major pathogens of the crops in Heilongjiang province. Four endophytic fungi exhibited strong inhibitory effects against rice blast disease P-46-2 and soybean sclerotinia 15-2, and endophytic fungi could be used to develop antibacterial agents (data not listed). In addition, we isolated six novel strains in the present study whose ITS sequences were ≤ 97% homologous to other strains based on BLAST analysis. The five novel strains could have novel molecular structures and could be sources of novel bioactive compounds. We will further study the characteristics and functions of these new strains in the future.
Wild soybean is the ancestor of cultivated soybean. It grows in poor, arid, even saline-alkali soil and other harsh environment (Cao et al. 2016). At present, some studies have been conducted to excavate and identify the resistance genes of wild soybean, for example, the important and special roles of Gshdz4, GsERF7, and GsERF6 from wild soybean in enhancing bicarbonate tolerance and responding to osmotic stress (Cao et al. 2016; Yu et al. 2016; Feng et al. 2020). However, few studies have involved the isolation and utilization of endophytic fungi of wild soybean in the cold region of northeast China. At the same time, there are also many reports on the mining and utilization of endophytic fungi in coastal plants or other medical plant, including many endophytic fungi with the function of anti-tumor drug synthesis, the endophytic fungi with heavy metal adsorption capacity, and the endophytic fungi with promoting plant growth and antibacterial function (Xiao et al. 2010; Aboobaker et al. 2019). The main purpose of this study is expected that these strains will play an important role in the medicinal value, the enrichment and treatment of heavy metal strains in industrial wastewater, and the improvement of saline-alkali soil in Northeast China. Therefore, the endophytic fungi isolated in this study have important application prospects.
Data availability
All supporting data are included as additional files.
References
Aboobaker Z, Viljoen A, Chen W, Crous PW, Maharaj VJ, Vuuren S (2019) Endophytic fungi isolated from Pelargonium sidoides DC: antimicrobial interaction and isolation of a bioactive compound. S Afr J Bot 122:535–542. https://doi.org/10.1016/j.sajb.2019.01.011
Amal HA, Abdessamad D, Julia K (2010) Fungal endophytes from higher plants: a prolific source of phytochemicals and other bioactive natural products. Fungal Divers 41:1–16. https://doi.org/10.1007/s13225-010-0034-4
Azevedo JL (2000) Endophytic microorganisms: a review on insect control and recent advances on tropical plants. Electron J Biothchn 3:e4. https://doi.org/10.4067/S0717-34582000000100004
Benhamou N (2002) Ability of nonpathogenic Fusarium oxysporum strain Fo47 to induce resistance against Pythium ulimum infection in cucumber. Appl Environ Microbiol 68:4044–4060. https://doi.org/10.1128/AEM.68.8.4044-4060.2002
Cao L, Yu Y, Duan H, Chen C, Duan X, Zhu P, Chen R, Li Q, Zhu Y, Ding X (2016) A novel Glycine soja homeodomain-leucine zipper (HD-Zip) I gene, Gshdz4, positively regulates bicarbonate tolerance and responds to osmotic stress in Arabidopsis. BMC Plant Biol 16(1):184.6. https://doi.org/10.1186/s12870-016-0872-7
Chen W, Wang X, Zhao Y (2011) Determination of flavonoids in Kudingcha of Wuzhishan, Hainan. J of Qiongzhou Uni 18:14–16. https://doi.org/10.3969/j.issn.1008-6722.2011.05.005
Cheng MJ, Wu MD, Chen JJ, Hsieh SY, Yuan GF, Chen IS (2019a) Secondary metabolites from the endophytic fungus of annulohypoxylon ilanense. Chem Nat Compd 49:523–525. https://doi.org/10.1007/s10600-013-0658-1
Cheng MJ, Yang SS, Wu MD, Chang HH, Kuo YH, Hsieh SY, Chen JJ, Wu HC (2019b) Isolation and structure elucidation of secondary metabolites from an endophytic fungus Annulohypoxylon ilanense. Nat Prod Commun 72:1–4. https://doi.org/10.1177/1934578X19857906
Clifton EH, Jaronski ST, Coates BS, Hodgson EW, Gassmann AJ (2018) Effects of endophytic entomopathogenic fungi on soybean aphid and identification of Metarhizium isolates from agricultural fields. PLoS One 13:e0194815. https://doi.org/10.1371/journal.pone.0194815
Davis RA, Carroll AR, Andrews KT (2010) Pestalactams AC: novel caprolactams from the endophytic fungus Pestalotiopsis sp. Org Biomol Chem 8:1785–1790. https://doi.org/10.1039/b924169h
Dong YS (2008) Research progress on wild soybeans in China. Journal of Jilin Agricultural University 30:394–400 CNKI:SUN:JLNY.0.2008-04-007
El-Maali NA, Mohrram AM, El-Kashef H, Gamal K (2018) Novel resources of Taxol from endophytic and entomopathogenic fungi: isolation, characterization and LC-Triple mass spectrometric quantification. Talanta 190:466–474. https://doi.org/10.4067/S0717-34582000000100004
El-Sayed ER, Ahmed AS, Abdelhakim HK (2019) A novel source of the cardiac glycoside digoxin from the endophytic fungus Epicoccum nigrum: isolation, characterization, production enhancement by gamma irradiation mutagenesis and anticancer activity evaluation. J Appl Mictobiol 10:14510–14762. https://doi.org/10.1111/jam.14510
Feng X, Feng P, Yu H, Yu X, Sun Q, Liu S, Minh TN, Chen J, Wang D, Zhang Q, Cao L, Zhou C, Li Q, Xiao J, Zhong S, Wang A, Wang L, Pan H, Ding X (2020) GsSnRK1 interplays with transcription factor GsERF7 from wild soybean to regulate soybean stress resistance. Plant Cell Environ 43:1192–1211. https://doi.org/10.1111/pce.13726
Gunatilaka, AAL (2006) Natural products from plant-associated microorganisms: distribution, structural diversity, bioactivity, and implications of their occurrence. J Nat Prod 69:509–526. https://doi.org/10.1021/np058128n
Hu XM, Zhang BX, Zhu YM (2011) Research and utilization of wild soybean resources. Aanhi Agri Sci 39:13311–13313 CNKI:SUN:AHNY.0.2011-22-023
Hyde K (2010) A case for re-inventory of Australia’s plant pathogens. Persoonia 15:50–60. https://doi.org/10.3767/003158510x548668
Impullitti AE, Malvick DK (2013) Fungal endophyte diversity in soybean. J Appl Microbiol 114(5):1500–1506. https://doi.org/10.1111/jam.12164
Jalgaonwala RE, Mohite BV, Mahajan RT (2011) A review: natural products from plant associated endophytic fungi. World J Microbiol Biotechnol 1:21–32. https://doi.org/10.3767/003158510x548668
Khan AL, Al-Harrasi A, Al-Rawahi A, Al-Farsi Z, Al-Mamari A, Waqas M, Lee IJ (2016) Endophytic fungi from Frankincense tree improves host growth and produces extracellular enzymes and indole acetic acid. PLoS One 11:e0158207. https://doi.org/10.1371/journal.pone.0158207
Kumar S, Nalli Y, Qadri M, Riyaz-Ul-Hassan S, Satti NK, Gupta V, Ali A (2017) Isolation of three new metabolites and intervention of diazomethane led to separation of compound 1 & 2 from an endophytic fungus, Cryptosporiopsis sp. depicting cytotoxic activity. Med Chem Res 26:2900–2908. https://doi.org/10.1007/s00044-017-1989-4
Li E, Tian R, Liu S (2008a) Pestalotheols A.D., bioactive metabolites from the plant endophytic fungus Pestalotiopsis theae. Nat Prod 71:664–668. https://doi.org/10.1021/np700744t
Li J, Li GQ, Sulitan A (2008b) Ultrasonic extraction of total flavonoids from Tamarix pubescens. Biotechnol 18:38–42
Maulana AF, Turjaman M, Sato T et al (2018) Isolation of endophytic fungi from tropical forest in Indonesia[J]. Symbiosis
Mitchell AM, Strobel GA, Moore E (2010) Volatile antimicrobials from Muscodor crispans, a novel endophytic fungus. Microb 156:270–277. https://doi.org/10.1099/mic.0.032540-0
Rubini MR, Silvaribeiro RT, Pomella AWV, Maki CS, Welington LA, Santos DRD (2005) Diversity of endophytic fungal community of cacao (theobroma cacao L.) and biological control of crinipellis perniciosa, causal agent of witches' broom disease. Int J Biol Sci:24–33. https://doi.org/10.7150/ijbs.1.24
Rudgers JA, Strauss SY, Wendel JF, Rudgers JA, Strauss SY, Wendel JF ( 2004) Trade-offs among anti-herbivore resistance traits: insights from Gossypieae (Malvaceae). Am J Bot 91(6):871–880
Shao C, Wang C, Zheng C (2010) A new anthraquinone derivative from the marine endophytic fungus Fusarium sp. (Nob77). Nat Prod Res 24:81–85. https://doi.org/10.1080/14786410902836701
Song P, Hong W, Wu CZ (2010) Study on the antibacterial activity of Tripterygium wilfordii endophyte. Chin Agri Sci Bull 26:262–266 CNKI:SUN:ZNTB.0.2010-05-057
Sujit S, Roshani, Shrestha S, Maharjan S, Selosse M-A, Pant B (2019) Isolation and characterization of plant growth-promoting endophytic fungi from the roots of dendrobium moniliforme[J]. Plants. https://doi.org/10.3390/plants8010005
Sun LN, Zhang YF, He LY, Chen ZJ, Wang QY, Qian M (2010) Genetic diversity and characterization of heavy metal-resistant-endophytic bacteria from two copper-tolerant plant species on copper mine wasteland. Bioresour Technol 101:501–509. https://doi.org/10.1016/j.biortech.2009.08.011
Suryanarayanan TS, Thirunavukkarasu N, Govindarajulu MB, Sasse F, Jansen R, Murali TS (2009) Fungal endophytes and bioprospecting. Fungal Biol Rev 23:9–19. https://doi.org/10.1016/j.fbr.2009.07.001
Tawfike AF, Abbott G, Young L, Edrada-Ebel R (2018) Metabolomic-guided isolation of bioactive natural products from Curvularia sp., an endophytic fungus of Terminalia laxiflora. Planta Med 84:182–190. https://doi.org/10.1055/s-0043-118807
Wang FW, Hou ZM, Wang CR (2008) Bioactive metabolites from Penicillium sp. an endophytic fungus residing in Hopea hainanensis. J Microbiol Biotechnol 24:21–43. https://doi.org/10.1007/s11274-008-9720-8
Wellensiek BP, Rajesh R, Bashyal BP (2013) Inhibition of HIV-1 replication by secondary metabolites from endophytic fungi of desert plants. Virology 7:72–80. https://doi.org/10.2174/1874357920130624002
White JF, Kingsley KL, Zhang Q, Verma R, Obi N, Dvinskikh S, Elmore MT, Verma SK, Gond SK, Kowalski KP (2019) Review: Endophytic microbes and their potential applications in crop management. Pest Manag Sci 75(10):558–2565. https://doi.org/10.1002/ps.5527
Xiao X, Luo S, Zeng G, Wei W, Wan Y, Chen L (2010) Biosorption of cadmium by endophytic fungus microsphaeropsis sp. lse10 isolated from cadmium hyperaccumulator solanum nigrum l. Bioresour Technol 101:1668–1674. https://doi.org/10.1016/j.biortech.2009.09.083
Yin J, Ren CL, Zhan YG, Li CX, Xiao JL, Qiu W (2012) Distribution and expression characteristics of triterpenoids and osc genes in white birch (Betula platyphylla suk.). Mol Biol Rep 39:2321–2328. https://doi.org/10.1007/s11033-011-0982-0
Yin J, Li X, Zhan Y, Li Y, Qu Z, Sun L (2017) Cloning and expression of bpmyc4 and bpbhlh9 genes and the role of BpHLH9 in triterpenoid synthesis in birch. BMC Plant Biol 17:214. https://doi.org/10.1186/s12870-017-1150-z
Yu BZ, Zhang GH, Du ZZ (2008) Phomoeuphorbins AD, azaphilones from the fungus Phomopsis euphorbiae. Phytochem 69:2523–2526. https://doi.org/10.1016/j.phytochem.2008.07.013
Yu Y, Liu A, Duan X, Wang S, Sun X, Duanmu H, Zhu D, Chen C, Cao L, Xiao J, Li Q, Nisa Z, Zhu Y, Ding X (2016) GsERF6, an ethylene-responsive factor from Glycine soja, mediates the regulation of plant bicarbonate tolerance in Arabidopsis. Planta 244(3):681–698.7. https://doi.org/10.1007/s00425-016-2532-4
Yuan ZL, Zhang CL, Lin FC, Kubicek CP (2010) Identity, diversity, and molecular phylogeny of the endophytic mycobiota in the roots of rare wild rice (Oryza granulate) from a nature reserve in Yunnan, China. Appl Environ Microbiol 76:1642–1652. https://doi.org/10.1128/AEM.01911-09
Zhao ZZ, Wang QS, Wang KM (2010) Study of the antifungal activity of Bacillus valli smortis ZZ185 in vitro and identification of its antifungal components. Bioresouce Technol 101:292–293. https://doi.org/10.1016/j.biortech.2009.07.071
Acknowledgements
This study was supported by Heilongjiang Academy of Agricultural Sciences. We wish to thank Dr. Y.C Lai and Pr. Y.G Zhan for the technical assistance.
Funding
This work was supported by the Natural Science Foundation of China (31101171), Natural Science Foundation Project of Heilongjiang Province (C2015008), and Project of Applied Technology Research and Development of Harbin (2015RQXJ019).
Author information
Authors and Affiliations
Contributions
J.L. Xiao designed this experiment, collected wild soybean resources, and wrote manuscripts. J.G. Sun, X. Zhou, and Y. Gong completed the isolation, purification, and ITS sequence identification of different endophytes. J.G. Sun completed the resistance screening experiment of endophyte strains. X. Zhou finished the analysis of the secondary product content of different endophyte strains. L.C. Jiang completed location of endophytic fungi in different parts of soybean materials. B. Pang participated in the writing of the manuscript and the analysis of the data. L. Zhang participated in the screening of heavy metal-resistant strains, X.D. Ding participated in the design of experiments and the guidance of experimental methods, and J. Yin participated in the design of experiments and the writing and modification of manuscripts. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This article does not contain any studies concerned with experimentation on human or animals.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 1058 kb).
Rights and permissions
About this article
Cite this article
Xiao, Jl., Sun, JG., Pang, B. et al. Isolation and screening of stress-resistant endophytic fungus strains from wild and cultivated soybeans in cold region of China. Appl Microbiol Biotechnol 105, 755–768 (2021). https://doi.org/10.1007/s00253-020-11048-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-11048-2