Abstract
Nanotechnology presents the new aspect of material as nanomaterials (NMs) with unique properties such as the large surface area to the volume ratio compared to bulk types. Metal and polymer nanoparticles (NPs) are two major groups of NMs with various medicinal and non-medicinal applications. The rise of antibiotic resistance in microorganisms in general, and bacteria in particular, has necessitated the use of these NMs as novel antibacterial agents. In this regard, medicinal usage of natural polymers particularly cellulose, chitosan, and alginic acid are increasing due to their higher biocompatibility, biodegradability, and accessibility than to other biopolymers or synthetic polymers. Antibacterial activities of these polysaccharides can be improved by incorporation of silver NPs as nanocomposite (NC) forms. Therefore, in this review, recent advances related to nanoformulations of silver NPs with three biopolymers having antibacterial and biocompatibility properties have been discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
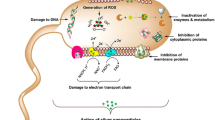
The development of new mechanisms by microorganisms for run away from efficient impacts of conventional antibiotics is a challenging issue (Rai et al. 2014; Taran et al. 2017b). For example, pathogenic bacteria such as methicillin-resistant Staphylococcus aureus (MRSA) can apply foreign penicillin-binding proteins (PBPs, PBP2a) to inactivate β-lactam antibiotics (Haghighat et al. 2013). Figure 1 illustrates other antibiotic resistance mechanisms in bacteria. These Gram-positive bacteria as opportunistic pathogens are related to surgical and chronic wound infections (Grigg et al. 2018). Therefore, in recent years, search for new antibacterial agents has been a main aim for many investigators. Antimicrobial applications of silver metal were decreased by the discovery of antibiotics. However, the emergence of silver nanoparticles (AgNPs) in the medical field has demonstrated effective antimicrobial effects (Alavi and Rai 2019; Kalwar and Shan 2018). These activities have resulted from unique properties of nanoparticles such as the large surface area to volume ratio and high aspect ratio of AgNPs compared to their bulk types (Asadi et al. 2019; Taran et al. 2016b). Depending on types of Gram-positive and Gram-negative bacteria, there are several interactions of released Ag+ ions with the cell wall and membrane components (Fig. 2). Also, these ions can bind to thiol (R-SH) groups of membrane proteins and inhibit respiration function of bacteria (Alavi and Karimi 2019b; Alavi et al. 2019b; Kalwar and Shan 2018).
Various mechanisms for antibiotic resistance in bacteria (Gupta et al. 2019)
The major disadvantage of AgNPs is their higher cytotoxicity or low biocompatibility in physiological conditions. In this way, various organic and inorganic materials were utilized to reduce or remove these unsuitable effects (Alavi and Karimi 2018a; Alavi and Karimi 2019a). One alternative is applications of synthetic and natural polymers as supporting biomaterials to augment biocompatibility and biodegradability. Polymers including polyvinyl chloride (PVC), polyvinyl alcohol (PVA), polyvinyl pyrrolidone (PVP), and polycaprolactone are common synthetic polymers with various biomedical applications (Dhote et al. 2019; Rolim et al. 2019; Tamayo et al. 2019). The high cost of preparation and also low biocompatibility were considered as disadvantages for these types of polymers (Binder 2019). In contrast, natural polymeric materials involving cellulose, chitosan, alginate sodium, polylactic acid (PLA), and collagen, with rich sources in nature, illustrate suitable biocompatibility and biodegradability (Hu et al. 2019). Among these polymers, cellulose, chitosan, and alginic acid polysaccharides have gained more attention because of higher accessibility compared to other natural polymers (Thomas et al. 2019). Plants and bacteria are the two main sources of cellulose (Sheikhi 2019). Chitosan was prepared by deacetylation of chitin material of the cell walls of fungi and the exoskeleton of crustaceans (Fazli Wan Nawawi et al. 2019). Moreover, alginic acid as another common polysaccharide polymer can be extracted from cell wall and biofilm parts related to brown algae and Pseudomonas aeruginosa, respectively (Priyan Shanura Fernando et al. 2019). Cellulose, chitosan, and alginic acid have been used in several studies as complementary biomaterials in NC synthesis having silver NPs with antibacterial abilities. Therefore, based on the above description, recent advances of AgNP complexes with cellulose and chitosan as abundant organic polymers have been reviewed. It is worth noting that there are several valuable investigations and reviews about antibacterial properties of each of these polymers as composites and nanocomposites forms (Jung et al. 2014; Khattak et al. 2019; Moon et al. 2007). However, there is lack of the comparative review about main aspects of antibacterial effects resulted from the coupling of AgNPs with these natural polysaccharides against pathogenic bacteria. In this way, this mini-review can be helpful to select suitable antibacterial agents based on nanoformulations of AgNPs with cellulose, chitosan, and alginic acid.
AgNP/cellulose
Cellulose polymer (C6H10O5)n can be extracted from plant components and specific bacteria biofilms by acetic treatments (Fig. 3a). Different treatments of this linear polymer lead to ether and ester derivatives of cellulose. From the aspect of nanomaterials, there are two main nanoforms of cellulose involving cellulose nanocrystals (CNCs) and nanofibers (CNFs). Cellulose nanowhiskers (CNWs) are related to CNC type with about 75% crystallinity. Spagnol and coworkers used succinic anhydride for surface modification of CNWs extracted from cotton fiber source. Then, AgNPs, poly(N-isopropylacrylamide), and PVA were embedded on modified CNWs by electrospinning and casting methods. The results of disc diffusion test showed higher antibacterial activities for prepared NCs by casting method with 11, 9, and 5 mm compared to electrospun NCs by 5, 7, and 4 mm for S. aureus, P. aeruginosa, and Escherichia coli, respectively (Spagnol et al. 2018).
As another functionalized nanoform of cellulose, dialdehyde CNFs were modified by AgNPs (average diameter size of 31.07 nm) in three steps by TEMPO (2,2,6,6-tetramethylpiperidine 1-oxyl radical), NaIO4, and [Ag(NH3)2]+ materials (Li et al. 2018a). It is worth mentioning that significant antibacterial performance of these NCs against E. coli and S. aureus has resulted from slow control release of Ag+ ions within 32 days (approximately 10%). Bacterial cellulose (BC) as another natural source of cellulose was used as an agent with abilities to reduce and stabilizing different concentrations of silver nitrate (0.01, 0.001, and 0.0001 M) to synthesize AgNP/BC films. These NC films illustrated inhibition zone diameters (IZDs) by 14 ± 2.11, 14 ± 0.61, 15 ± 1.58, and 15 ± 0.36 mm at higher concentration of AgNPs (0.01 M) than to 12 ± 0.44, 11 ± 0.20, 15 ± 0.73, and 13 ± 0.44 mm at lower amount (0.0001 M) for Pseudomonas aeruginosa, E. coli, S. aureus, and Klebsiella pneumoniae, respectively (Volova et al. 2018). For improvement in mechanical properties of AgNP-cellulose NCs, silica NPs were coupled with AgNPs as Ag-silica NCs in the matrix of cellulose fibers. These types of biocompatible NCs illustrated strong bacteriostatic and bactericidal effects on S. aureus and E. coli after 48 h (Smiechowicz et al. 2018). In another study, Ag-magnetite (Fe3O4) NCs were synthesized on polydopamine-decorated porous cellulose acetate microspheres as reducing and stabilizing materials. Antibacterial results of these NMs illustrated striking growth inhibition of E. coli with IZD of 10.3 mm compared with microspheres without AgNPs (Peng et al. 2018). Furthermore, antibacterial efficacy of Ag-TiO2-cellulose film against E. coli was higher under UV radiation than to dark condition because of TiO2 NP incorporation into these NCs (Li et al. 2018b).
As mentioned above, one way to modify cellulose is the use of TEMPO-mediated oxidation of hydroxyl groups on cellulose surface (Fig. 4). By this method, Ag+ ions can be reduced on the surface of cellulose fibers via ion-exchange reaction. For this case, bacterial cellulose pellicles were coated by AgNPs for preparation of wound dressing with 99.2% and 100% antibacterial effects on S. aureus and E. coli, respectively (Wu et al. 2018). It is worth noting that the shape and size of AgNPs can determine their properties specifically antibacterial activities (Rad et al. 2018). For example, silver nanorods (diameter size range of 80–135.3 nm) were produced by reducing and stabilizing abilities of CNCs without any functionalization approach. IZDs with 15.2 ± 0.5 mm for Bacillus subtilis were more than S. aureus, E. coli, and P. aeruginosa bacteria (Shaheen and Fouda 2018).
AgNP/chitosan
N-Acetyl-D-glucosamine of chitin polymer in exoskeletons of arthropods is treated by alkaline materials to synthesize chitosan polymer with a linear arrangement of D-glucosamine and N-acetyl-D-glucosamine (Fig. 3b). In addition to acceptable antibacterial activities, mechanical properties should also be improved for wound dressing based on chitosan hydrogel. In this regard, lithium hydroxide/potassium hydroxide/urea mixture was used instead of glacial acetic acid for the preparation of chitosan hydrogels followed by reduction of Ag+ ions (1, 2, and 3 g/100 ml of an aqueous solution of AgNO3) in by trisodium citrate to produce chitosan-AgNP hydrogels. S. aureus and E. coli showed 99.94% and 99.86% inhibition rates, respectively, under the treatment of these hydrogels after 24 h (Xie et al. 2018). Chitosan and PVA polymers were utilized to reduce and stabilize 0.02 M of silver nitrate at three volumes of 5, 1, and 0.1 ml. IZD values in the case of S. aureus, Salmonella enterica, E. coli, Salmonella typhi, and K. pneumoniae were 21 ± 1, 17 ± 1, 15 ± 0.5, 10 ± 0.1, and 17 ± 0.4 mm, respectively, for the highest concentration of sliver salt at chitosan-AgNCs with an average diameter of about 190 nm (Hajji et al. 2019). In a similar study, reduction of silver ions and stabilization of AgNPs in chitosan matrix were improved by gelatin polymer. In this synthesis method, tannic acid as a type of polyphenol substances had the cross-linking role for increasing of hydrogel stability. In addition to suitable Young’s modulus and tensile strength, antibacterial results for this hydrogel against E. coli were more than S. aureus. These properties were associated with accelerated wound healing in experimental rabbits after 15 days compared to Aquacel®Ag foam (Ye et al. 2019). Different amounts of AgNO3 (0.1, 0.05, 0.02, and 0.01 M) were incubated with acetic acid-treated chitosan solution on Petri dishes to synthesize NC films. FTIR results proved a prominent contribution of amine groups of chitosan in interaction with AgNPs. These NCs indicated both significant biodegradability, wound healing, and antibacterial activities. The results of antibacterial efficacies were meaningful for all the mentioned concentrations of AgNO3 against planktonic forms of E. coli, S. aureus, Staphylococcus epidermidis, and P. aeruginosa (Hernández-Rangel et al. 2019). Suitable mechanical stability of hydrogel composed of chitosan and AgNPs is an essential property in physiological conditions. Due to improvement in this property, several materials such as graphene were used as filler in a hydrogel formulation. In this way, enhanced mechanical ability with antibacterial effects on E. coli and S. aureus were observed for silver-PVA-chitosan-graphene hydrogel (Nešović et al. 2018). In another investigation, AgNP-chitosan-polymethyl methacrylate (PMMA) NCs were deposited layer-by-layer on sulfur prevulcanized natural rubber (SPNR) due to surface modification of SPNR having low surface friction and high antibacterial activities. The results of this work proved low cytotoxicity against fibroblast cells and meaningful antibacterial effects on E. coli and S. aureus for coated films by NCs compared to uncoated SPNR (Suteewong et al. 2019). It is noteworthy to mention that these films may be utilized in the production of gloves. Other application of Ag-chitosan NCs is the improvement of the dental barrier membrane as a remedy for periodontitis infection. The membrane impregnated with AgNPs into chitosan-polyurethane nanofibrous showed significant antibacterial activities in the case of Porphyromonas gingivalis ATCC 33277 with biocompatibility in a lower concentration of AgNPs for standard fibroblast cell line (Lee et al. 2018). Moreover, in order to increase antibacterial potential of dental implant based on titanium, catechol-containing chitosan was loaded by AgNPs. In spite of significant antibacterial activities against S. aureus and E. coli, lower cytotoxicity was observed for fibroblast cells (Cheng et al. 2019). In a similar study, electrospun nanofibrous membrane of chitosan with fiber diameter of 200 nm was loaded with AgNPs. Antibacterial activity against S. aureus was observed after 4 days of incubation for higher concentration of incorporated AgNPs (60 mg of AgNO3) in this membrane (Shao et al. 2019). In addition, one-pot green synthesis of AgNPs through stabilizing/reducer abilities of chitosan demonstrated both antibacterial potential and biocompatibility. S. aureus and E. coli demonstrated 312.5 and 39.1 μg/ml of minimum bactericidal concentration (MBC), respectively (Wongpreecha et al. 2018). The AgNPs may be stabilized by using other biological macromolecules such as amino acids. In this way, lysine as α-amino acid with carboxylate group (COO−) was utilized as stabilizing agent due to formation of AgNPs in chitosan-lysine-AgNP NCs with hydrodynamic diameter of 275.5 nm. Antibacterial results of this study demonstrated sensitivity upon treatment of NCs for P. aeruginosa, B. subtilis, and S. aureus compared with E. coli (Vanitha Kumari et al. 2018).
AgNP/alginic acid
The chemical formula of alginic acid is (C6H8O6)n with two components of β-D-mannuronate and α-L-guluronate (Fig. 3c). This linear polysaccharide can form alginate salts with calcium and sodium as common medicinal forms (Yeung and Kennedy 2019). There are several methods for reduction and stabilization of Ag+ ions to form AgNPs. For synthesis and modification of organic and inorganic NPs particularly metal NPs, affordable and eco-friendly way is green synthesis using natural sources such as residues of plants, fungi, algae, and bacteria (Alavi and Karimi 2018b; Alavi et al. 2019a; Taran et al. 2016a, 2017a; Wypij et al. 2019). Extracted sodium alginate from Sargassum muticum with reducing and stabilizing functions was applied to synthesize AgNPs with an estimated size of 22 nm. These NCs demonstrated antipathogenic impact on P. aeruginosa, Micrococcus luteus, Bacillus cereus, and S. aureus (Belattmania et al. 2018). Ascorbic acid and sodium alginate were applied as reducing and stabilizing agents, respectively, to synthesize AgNPs. In this regard, green synthesized AgNP/sodium alginate had antibacterial effect on E. coli and S. aureus via formation of pores in the bacterial membrane. It is worth mentioning that this antibacterial mechanism with cell membrane clumping and blebs was reported previously for AgNPs (Alavi and Karimi 2018b). In contrast, NaBH4 was used as a reducer for the preparation of AgNPs on collagen-alginate biocomposites. Moreover, in this method, PVP was utilized for stabilization of AgNPs. Significant cytotoxicity on mouse embryonic fibroblasts (NIH3T3) and antibacterial activity against E. coli and S. aureus were observed for these NCs as dose-dependent behavior (Zhang et al. 2018). In another study, silver-hydroxyapatite was loaded into gelatin-alginate-PVA cryogels in order to form stable porous scaffold having antibacterial abilities. E. coli and B. subtilis showed prominent sensitivity under treatment of these scaffolds by IZDs of 24 mm and 22 mm, respectively (Kumar Saini et al. 2019).
AgNP/chitosan/cellulose
In some studies, chitosan and cellulose were applied as a biocompatible scaffold for tissue engineering having both physicochemical properties of these biopolymers. In the case of antiseptic scaffold preparation, AgNPs can be added to these biocomposites. NCs of chitosan/carboxymethyl cellulose with different percentages of loaded carboxylated CNW-AgNPs (1, 2, 5, 5, and 10%) were used as the scaffold for bone tissue engineering having antibacterial activities. These NCs showed 100% removal of E. coli MTCC 1610 at 10% of carboxylated CNW-AgNP (Hasan et al. 2018). For the synthesis of biofilms with suitable antibacterial and mechanical strength, 3, 5, and 10% of Ag-dialdehyde CNC solution were decorated on chitosan via solution casting method. Disc diffusion results for the highest concentration of carboxylated CNW-AgNP demonstrated maximum and minimum IZD values with 10.48 and 7.45 mm for Enterobacter cloacae clinical and P. aeruginosa standard strains, respectively (Dong and Li 2018). Chitosan and AgNPs were coated on filter paper as cellulose film due to the preparation of wound dressing with significant antibacterial activity against E. coli and S. aureus bacteria (Haider et al. 2018). Carboxy-CNCs (CCNCs) and chitooligosaccharide-CCNCs were applied as stabilizer biocomposites for green synthesis of silver NPs with antibacterial activity against K. pneumoniae, E. coli, and S. aureus pathogens. In this context, all three bacteria illustrated complete sensitivity to 0.003 μg/ml concentration of AgNP-chitooligosaccharide-CCNCs and AgNP-CCNCs compared to amoxicillin antibiotic (Ni et al. 2018).
Conclusions
The antibiotic resistance in bacteria is developing with fast pace which is a matter of grave concern. In this context, several antibacterial and wound healing nanoformulations of silver NCs, based on supporting biopolymers of chitosan, cellulose, and alginic acid, have been developed by the researchers. Recent investigations related to antibacterial and biocompatibility activities of AgNP-chitosan, AgNP-cellulose, AgNP-sodium alginate, and AgNP-chitosan-cellulose NCs have proved that these activities may be influenced by many factors such as types of nanoformulation (films, foams, and hydrogels) and concentration or volume ratio of each ingredient. Therefore, by controlling these parameters, suitable NCs can be obtained for development of wound dressings and tissue engineering scaffolds.
References
Alavi M, Karimi N (2018a) Antiplanktonic, antibiofilm, antiswarming motility and antiquorum sensing activities of green synthesized Ag–TiO2, TiO2–Ag, Ag–Cu and Cu–Ag nanocomposites against multi-drug-resistant bacteria. Artif Cells Nanomed Biotechnol:1–15
Alavi M, Karimi N (2018b) Characterization, antibacterial, total antioxidant, scavenging, reducing power and ion chelating activities of green synthesized silver, copper and titanium dioxide nanoparticles using Artemisia haussknechtii leaf extract. Artif Cells Nanomed Biotechnol 46(8):2066–2081
Alavi M, Karimi N (2019a) Biosynthesis of Ag and Cu NPs by secondary metabolites of usnic acid and thymol with biological macromolecules aggregation and antibacterial activities against multi drug resistant (MDR) bacteria. Int J Biol Macromol 128:893–901. https://doi.org/10.1016/j.ijbiomac.2019.01.177
Alavi M, Karimi N (2019b) Ultrasound assisted-phytofabricated Fe3O4 NPs with antioxidant properties and antibacterial effects on growth, biofilm formation, and spreading ability of multidrug resistant bacteria. Artif Cells Nanomed Biotechnol 47(1):2405–2423. https://doi.org/10.1080/21691401.2019.1624560
Alavi M, Rai M (2019) Recent advances in antibacterial applications of metal nanoparticles (MNPs) and metal nanocomposites (MNCs) against multidrug-resistant (MDR) bacteria. Expert review of anti-infective therapy (just-accepted)
Alavi M, Karimi N, Salimikia I (2019a) Phytosynthesis of zinc oxide nanoparticles and its antibacterial, antiquorum sensing, antimotility, and antioxidant capacities against multidrug resistant bacteria. J Ind Eng Chem 72:457–473
Alavi M, Karimi N, Valadbaeigi T (2019b) Antibacterial, antibiofilm, antiquorum sensing, antimotility, and antioxidant activities of green fabricated Ag, Cu, TiO2, ZnO, and Fe3O4 NPs via Protoparmeliopsis muralis Lichen aqueous extract against multi drug resistant bacteria. ACS Biomater Sci Eng 5:4228–4243. https://doi.org/10.1021/acsbiomaterials.9b00274
Asadi N, Taran M, Rad M, Alavi M (2019) Effects of glucose, metformin, and protein on formation of flower-like nanocomposites of struvite in infected artificial urine medium by methicillin-resistant Staphylococcus aureus (MRSA): new report. Nano Biomed Eng 11(1):91–97
Belattmania Z, Bentiss F, Jama C, Barakate M, Katif C, Reani A, Sabour B (2018) Biosynthesis and characterization of silver nanoparticles using sodium alginate from the invasive macroalga Sargassum muticum. BioNanoScience 8(2):617–623. https://doi.org/10.1007/s12668-018-0518-3
Binder WH (2019) The past 40 years of macromolecular sciences: reflections on challenges in synthetic polymer and material science. Macromol Rapid Commun 40(1):1800610
Cheng YF, Zhang JY, Wang YB, Li CM, Lu ZS, Hu XF, Xu LQ (2019) Deposition of catechol-functionalized chitosan and silver nanoparticles on biomedical titanium surfaces for antibacterial application. Mater Sci Eng C 98:649–656. https://doi.org/10.1016/j.msec.2019.01.019
Dhote VK, Dhote K, Pandey SP, Shukla T, Maheshwari R, Mishra DK, Tekade RK (2019) Fundamentals of polymers science applied in pharmaceutical product development. In: Basic fundamentals of drug delivery. Elsevier, pp 85–112
Dong F, Li S (2018) Wound dressings based on chitosan-dialdehyde cellulose nanocrystals-silver nanoparticles: mechanical strength, antibacterial activity and cytotoxicity. Polymers 10(6):673
Fazli Wan Nawawi WM, Lee K-Y, Kontturi E, Murphy RJ, Bismarck A (2019) Chitin nanopaper from mushroom extract: natural composite of nanofibers and glucan from a single biobased source. ACS Sustain Chem Eng 7:6492–6496. https://doi.org/10.1021/acssuschemeng.9b00721
Grigg C, Palms D, Stone ND, Gualandi N, Bamberg W, Dumyati G, Harrison LH, Lynfield R, Nadle J, Petit S, Ray S, Schaffner W, Townes J, See I (2018) Burden of invasive methicillin-resistant Staphylococcus aureus infections in nursing home residents. J Am Geriatr Soc 66(8):1581–1586. https://doi.org/10.1111/jgs.15451
Gupta A, Mumtaz S, Li C-H, Hussain I, Rotello VM (2019) Combatting antibiotic-resistant bacteria using nanomaterials. Chem Soc Rev 48(2):415–427
Haghighat S, Siadat SD, Sorkhabadi SMR, Sepahi AA, Mahdavi M (2013) Cloning, expression and purification of penicillin binding protein2a (PBP2a) from methicillin resistant Staphylococcus aureus: a study on immunoreactivity in Balb/C mouse. Avicenna J Med Biotechnol 5(4):204–211
Haider A, Haider S, Kang I-K, Kumar A, Kummara MR, Kamal T, Han SS (2018) A novel use of cellulose based filter paper containing silver nanoparticles for its potential application as wound dressing agent. Int J Biol Macromol 108:455–461. https://doi.org/10.1016/j.ijbiomac.2017.12.022
Hajji S, Khedir SB, Hamza-Mnif I, Hamdi M, Jedidi I, Kallel R, Boufi S, Nasri M (2019) Biomedical potential of chitosan-silver nanoparticles with special reference to antioxidant, antibacterial, hemolytic and in vivo cutaneous wound healing effects. Biochim Biophys Acta Gen Subj 1863(1):241–254
Hasan A, Waibhaw G, Saxena V, Pandey LM (2018) Nano-biocomposite scaffolds of chitosan, carboxymethyl cellulose and silver nanoparticle modified cellulose nanowhiskers for bone tissue engineering applications. Int J Biol Macromol 111:923–934. https://doi.org/10.1016/j.ijbiomac.2018.01.089
Hernández-Rangel A, Silva-Bermudez P, España-Sánchez BL, Luna-Hernández E, Almaguer-Flores A, Ibarra C, Garcia-Perez VI, Velasquillo C, Luna-Barcenas G (2019) Fabrication and in vitro behavior of dual-function chitosan/silver nanocomposites for potential wound dressing applications. Mater Sci Eng C 94:750–765. https://doi.org/10.1016/j.msec.2018.10.012
Hu S, Cai X, Qu X, Yu B, Yan C, Yang J, Li F, Zheng Y, Shi X (2019) Preparation of biocompatible wound dressings with long-term antimicrobial activity through covalent bonding of antibiotic agents to natural polymers. Int J Biol Macromol 123:1320–1330
Jung J, Cavender G, Zhao Y (2014) The contribution of acidulant to the antibacterial activity of acid soluble α- and β-chitosan solutions and their films. Appl Microbiol Biotechnol 98(1):425–435. https://doi.org/10.1007/s00253-013-5334-7
Kalwar K, Shan D (2018) Antimicrobial effect of silver nanoparticles (AgNPs) and their mechanism—a mini review. Micro Nano Lett 13(3):277–280
Khattak S, Wahid F, Liu L-P, Jia S-R, Chu L-Q, Xie Y-Y, Li Z-X, Zhong C (2019) Applications of cellulose and chitin/chitosan derivatives and composites as antibacterial materials: current state and perspectives. Appl Microbiol Biotechnol 103(5):1989–2006. https://doi.org/10.1007/s00253-018-09602-0
Kumar Saini R, Prasad Bagri L, Bajpai AK (2019) Nano-silver hydroxyapatite based antibacterial 3D scaffolds of gelatin/alginate/poly (vinyl alcohol) for bone tissue engineering applications. Colloids Surf B: Biointerfaces 177:211–218. https://doi.org/10.1016/j.colsurfb.2019.01.064
Lee D, Lee SJ, Moon J-H, Kim JH, Heo DN, Bang JB, Lim H-N, Kwon IK (2018) Preparation of antibacterial chitosan membranes containing silver nanoparticles for dental barrier membrane applications. J Ind Eng Chem 66:196–202. https://doi.org/10.1016/j.jiec.2018.05.030
Li J, Kang L, Wang B, Chen K, Tian X, Ge Z, Zeng J, Xu J, Gao W (2018a) Controlled release and long-term antibacterial activity of dialdehyde nanofibrillated cellulose/silver nanoparticle composites. ACS Sustain Chem Eng 7(1):1146–1158
Li Y, Tian J, Yang C, Hsiao B (2018b) Nanocomposite film containing fibrous cellulose scaffold and Ag/TiO2 nanoparticles and its antibacterial activity. Polymers 10(10):1052
Moon J-S, Kim H-K, Koo HC, Joo Y-S, H-m N, Park YH, Kang M-I (2007) The antibacterial and immunostimulative effect of chitosan-oligosaccharides against infection by Staphylococcus aureus isolated from bovine mastitis. Appl Microbiol Biotechnol 75(5):989–998. https://doi.org/10.1007/s00253-007-0898-8
Nešović K, Janković A, Kojić V, Vukašinović-Sekulić M, Perić-Grujić A, Rhee KY, Mišković-Stanković V (2018) Silver/poly(vinyl alcohol)/chitosan/graphene hydrogels—synthesis, biological and physicochemical properties and silver release kinetics. Compos Part B 154:175–185. https://doi.org/10.1016/j.compositesb.2018.08.005
Ni X, Wang J, Yue Y, Cheng W, Wang D, Han G (2018) Enhanced antibacterial performance and cytocompatibility of silver nanoparticles stabilized by cellulose nanocrystal grafted with chito-oligosaccharides. Materials (Basel) 11(8). https://doi.org/10.3390/ma11081339
Peng S, Gao F, Zeng D, Peng C, Chen Y, Li M (2018) Synthesis of Ag–Fe3O4 nanoparticles supported on polydopamine-functionalized porous cellulose acetate microspheres: catalytic and antibacterial applications. Cellulose 25(8):4771–4782. https://doi.org/10.1007/s10570-018-1886-0
Priyan Shanura Fernando I, Kim K-N, Kim D, Jeon Y-J (2019) Algal polysaccharides: potential bioactive substances for cosmeceutical applications. Crit Rev Biotechnol 39(1):99–113
Rad M, Taran M, Alavi M (2018) Effect of incubation time, CuSO4 and glucose concentrations on biosynthesis of copper oxide (CuO) nanoparticles with rectangular shape and antibacterial activity: Taguchi method approach. Nano Biomed Eng 10(1):25–33
Rai M, Kon K, Ingle A, Duran N, Galdiero S, Galdiero M (2014) Broad-spectrum bioactivities of silver nanoparticles: the emerging trends and future prospects. Appl Microbiol Biotechnol 98(5):1951–1961. https://doi.org/10.1007/s00253-013-5473-x
Rolim WR, Pieretti JC, Renó DLS, Lima BA, Nascimento MHM, Ambrosio FN, Lombello CB, Brocchi M, de Souza ACS, Seabra AB (2019) Antimicrobial activity and cytotoxicity to tumor cells of nitric oxide donor and silver nanoparticles containing PVA/PEG films for topical applications. ACS applied materials & interfaces
Shaheen TI, Fouda A (2018) Green approach for one-pot synthesis of silver nanorod using cellulose nanocrystal and their cytotoxicity and antibacterial assessment. Int J Biol Macromol 106:784–792
Shao J, Wang B, Li J, Jansen JA, Walboomers XF, Yang F (2019) Antibacterial effect and wound healing ability of silver nanoparticles incorporation into chitosan-based nanofibrous membranes. Mater Sci Eng C 98:1053–1063. https://doi.org/10.1016/j.msec.2019.01.073
Sheikhi A (2019) Chapter 9—emerging cellulose-based nanomaterials and nanocomposites. In: Karak N (ed) Nanomaterials and polymer nanocomposites. Elsevier, pp 307–351
Smiechowicz E, Niekraszewicz B, Kulpinski P, Dzitko K (2018) Antibacterial composite cellulose fibers modified with silver nanoparticles and nanosilica. Cellulose 25(6):3499–3517
Spagnol C, Fragal EH, Pereira AGB, Nakamura CV, Muniz EC, Follmann HDM, Silva R, Rubira AF (2018) Cellulose nanowhiskers decorated with silver nanoparticles as an additive to antibacterial polymers membranes fabricated by electrospinning. J Colloid Interface Sci 531:705–715
Suteewong T, Wongpreecha J, Polpanich D, Jangpatarapongsa K, Kaewsaneha C, Tangboriboonrat P (2019) PMMA particles coated with chitosan-silver nanoparticles as a dual antibacterial modifier for natural rubber latex films. Colloids Surf B: Biointerfaces 174:544–552
Tamayo L, Palza H, Bejarano J, Zapata PA (2019) Polymer composites with metal nanoparticles: synthesis, properties, and applications polymer composites with functionalized nanoparticles. Elsevier, pp 249–286
Taran M, Rad M, Alavi M (2016a) Biological synthesis of copper nanoparticles by using Halomonas elongata IBRC-M 10214. Ind Text 67:351
Taran M, Rad M, Alavi M (2016b) Characterization of Ag nanoparticles biosynthesized by Bacillus sp. HAI4 in different conditions and their antibacterial effects. J Appl Pharm Sci 6(11):094–099
Taran M, Monazah A, Alavi M (2017a) Using petrochemical wastewater for synthesis of cruxrhodopsin as an energy capturing nanoparticle by Haloarcula sp. IRU1. Progress Biol Sci 6(2):151–157
Taran M, Rad M, Alavi M (2017b) Antibacterial activity of copper oxide (CuO) nanoparticles biosynthesized by Bacillus sp. FU4: optimization of experiment design. Pharm Sci 23(3):198–206. https://doi.org/10.15171/PS.2017.30
Thomas MS, Koshy RR, Mary SK, Thomas S, Pothan LA (2019) Applications of polysaccharide based composites. In: Thomas MS, Koshy RR, Mary SK, Thomas S, Pothan LA (eds) Starch, chitin and chitosan based composites and nanocomposites. Springer International Publishing, Cham, pp 43–55
Vanitha Kumari G, Mathavan T, Srinivasan R, Jothirajan MA (2018) The influence of physical properties on the antibacterial activity of lysine conjugated chitosan functionalized silver nanoparticles. J Inorg Organomet Polym Mater 28(6):2418–2426. https://doi.org/10.1007/s10904-018-0944-2
Volova TG, Shumilova AA, Shidlovskiy IP, Nikolaeva ED, Sukovatiy AG, Vasiliev AD, Shishatskaya EI (2018) Antibacterial properties of films of cellulose composites with silver nanoparticles and antibiotics. Polym Test 65:54–68
Wongpreecha J, Polpanich D, Suteewong T, Kaewsaneha C, Tangboriboonrat P (2018) One-pot, large-scale green synthesis of silver nanoparticles-chitosan with enhanced antibacterial activity and low cytotoxicity. Carbohydr Polym 199:641–648. https://doi.org/10.1016/j.carbpol.2018.07.039
Wu C-N, Fuh S-C, Lin S-P, Lin Y-Y, Chen H-Y, Liu J-M, Cheng K-C (2018) TEMPO-oxidized bacterial cellulose pellicle with silver nanoparticles for wound dressing. Biomacromolecules 19(2):544–554
Wypij M, Świecimska M, Dahm H, Rai M, Golinska P (2019) Controllable biosynthesis of silver nanoparticles using actinobacterial strains. Green Process Synth 8(1):207–214
Xie Y, Liao X, Zhang J, Yang F, Fan Z (2018) Novel chitosan hydrogels reinforced by silver nanoparticles with ultrahigh mechanical and high antibacterial properties for accelerating wound healing. Int J Biol Macromol 119:402–412
Ye H, Cheng J, Yu K (2019) In situ reduction of silver nanoparticles by gelatin to obtain porous silver nanoparticle/chitosan composites with enhanced antimicrobial and wound-healing activity. Int J Biol Macromol 121:633–642
Yeung RA, Kennedy RA (2019) A comparison of selected physico-chemical properties of calcium alginate fibers produced using two different types of sodium alginate. J Mech Behav Biomed Mater 90:155–164
Zhang H, Peng M, Cheng T, Zhao P, Qiu L, Zhou J, Lu G, Chen J (2018) Silver nanoparticles-doped collagen–alginate antimicrobial biocomposite as potential wound dressing. J Mater Sci 53(21):14944–14952. https://doi.org/10.1007/s10853-018-2710-9
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Disclaimer
The content is solely the responsibility of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alavi, M., Rai, M. Recent progress in nanoformulations of silver nanoparticles with cellulose, chitosan, and alginic acid biopolymers for antibacterial applications. Appl Microbiol Biotechnol 103, 8669–8676 (2019). https://doi.org/10.1007/s00253-019-10126-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-10126-4