Abstract
Based on our previous study evaluating the in vivo cure efficacy of chitosan on bovine mastitis, a more water-soluble chitosan-oligosaccharide (OCHT) with a high degree of deacetylation and low molecular weight was prepared to obtain high antibiotic efficacy. The growth of Staphylococcus aureus isolated from bovine mastitis was inhibited within 10 min of treatment with OCHT in concentrations ranging from 0.0001 to 0.5%. Additionally, electron microscopic observation indicated that the surface of the OCHT-treated bacteria was expanded, distorted, and lysed compared to that of the control bacteria. In mice, the proportion of monocytes was elevated, and the levels of interleukin-6 and interferon-γ sharply increased l h after the peritoneal inoculation of the OCHT (0.5 to 1 mg per mouse). Mice challenged intraperitoneally with S. aureus (2.5 × 108 colony forming units) after oral treatment with OCHT (0.5 to 2 mg per day) for 7 days showed a higher survival rate (70–100%) than that of the control (10%). We suggest that the OCHT prepared in this study is a potential agent for the prevention and treatment of bovine mastitis based on its strong antibacterial activity against S. aureus as well as the immunostimulative effect it exhibits on murine infection by S. aureus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mastitis is caused primarily by microbial intramammary infection (IMI) and is a costly disease for dairy producers (Ruegg 2003). Staphylococcus aureus can provoke clinical mastitis but is more frequently associated with subclinical infections that tend to become chronic and are difficult to eradicate using conventional antimicrobial therapies (Sears and McCarthy 2003). Bovine mastitis due to S. aureus infection constitutes a major challenge to dairy producers due to the frequent inability of both the immune response and antibiotics to prevent infection and destroy the pathogen in the intramammary environment. Further research must be conducted to develop new prophylactic and therapeutic agents to counteract the pathogenesis of intramammary S. aureus infection (Brouillette and Malouin 2005).
Chitosan is one of the most abundant natural polymers and has important industrial applications, including waste water purification (Castellanos-Perez et al. 1988), chelation of transition metals, coacervate formation for cell entrapment, and seed coating for improved yield due to its unique polycation nature (Hadwiger et al. 1984). In addition to its lack of toxicity and allergenicity, its biocompatibility, biodegradability, and bioactivity make chitosan very attractive for a wide range of biomaterial, pharmaceutical, and medical applications (Allan and Hadwiger 1979; Sudarshan et al. 1992; Illum 1998; Singla and Chawla 2001). Recently, increasing attention has been paid to the potential use of chitosan in a number of veterinary applications, including use as a wound healing agent, antimicrobial agent, bandage material, skin grafting template, homeostatic agent, and a delivery vehicle (Senel and McClure 2004). There have also been numerous reports documenting the effects of chitosan on inhibition of bacteria, such as S. aureus and Escherichia coli, and fungi (Allan and Hadwiger 1979; Sudarshan et al. 1992; Seo et al. 1992; Darmadji and Izumimoto 1994), on the prevention of immunosuppression after surgery (Kosaka et al. 1996), on wound healing in cows (Okamoto et al. 1996), and on improvement of cure rates in mastitic cows (Moon et al. 1998). Minami et al. (1997) also observed that the number of phagocytes and their oxidative activities in udder secretion increased only 1 day after chitosan administration in both normal and mastitic cows.
The antibacterial effect of chitosan-oligosaccharides (OCHT) has been shown to be greatly dependent on their polymerization or molecular weight (MW; Jeon and Kim 2000). In addition, water-soluble OCHT may be an advantageous antibacterial agent for use in in vivo systems compared to water-insoluble chitosan (Park et al. 2002). Due to the in vivo efficacy of chitosan on treatment of bovine mastitis in our previous study (Moon et al. 1998), this study was conducted to prepare water-soluble and low MW OCHT from chitosan by treatment with chitosanase. The antibacterial activity of OCHT against S. aureus isolated from a mastitic cow and its immunostimulative effects on Institute of Cancer Research (ICR) mice were also investigated.
Materials and methods
Preparation of the chitosan-oligosaccharides
Taehoon Bio. (Seoul, Korea) donated the chitosan powder (degree of deacetylation, 70–100%; average MW, 207,954 Da; viscosity, 5 cP), which was prepared from crab shells from the East Sea in the Republic of Korea. The chitosan solution was prepared by dispersing 140 g of chitosan powder into 997 ml of distilled water and 3 ml of acetic acid with continuous stirring. A saturated sodium bicarbonate solution was used to adjust the pH to 5.8.
The OCHT used for this study was prepared via enzymatic hydrolysis of chitosan using the method described by Kim (1998). Briefly, the chitosan solution [14% (w/v)] was hydrolyzed by chitosanase (1 unit/g chitosan) treatment at 55°C for 5 h and terminated by boiling at 100°C for 10 min. The mixture of OCHT was obtained by filtration using filter paper no. 2. Finally, the improved solubility in acetone was used to separate the OCHT based on the differences of MWs in the mixture so the OCHT with the highest antibacterial activity could be determined. The OCHT was precipitated with acetone, centrifuged (50,000×g, 30 s), and dried to obtain a water-soluble white powder with a quantitative yield of 65.8 g.
The properties of the OCHT prepared in this study were analyzed using Fourier-transformed infrared (FT-IR) spectra, which were obtained on FT/IR-300E and Micro-FT/IR (Model-300E, Jasco, Tokyo, Japan) using the potassium bromide (KBr) disk sandwiched technique (2 mg sample in 200 mg KBr). The %T mode was applied within an absorption wavelength ranging from 400∼4,000 to 1 cm−1 of the resolution. Commercial chitosan (C-3646, Sigma Chemical, St. Louis, MO, USA) was used as the standard control to examine the constitutional transformation of the chitosan (Taehoon Bio.) into OCHT. OCHT [0.5% (w/v)] dissolved in 1% acetic acid solution (w/v) was measured at 20°C using a physical viscometer (Model SM-HM Mc10, Frankfurt, Germany). The degree of deacetylation of the OCHT was determined using the method described by Kina et al. (1974). The distribution of MW of the OCHT was analyzed by gel permeation chromatography (Model LCSS-90, Jasco), using a system comprised of three coupled columns (Shodex OHpak SB-801, SB-802 and SB-803), a pump (Jasco PU-980), and an internal refractive index (RI) detector (Jasco RI-930). The samples were analyzed using a 0.1 M sodium chloride/0.2% acetic acid aqueous solution as eluant at a flow rate of 1 ml/min. Monodispersed pullulan (Jasco) of different MWs (5,800; 12,200; 23,700; 48,000; 100,000; 186,000; 380,000; 1,660,000) and a commercial OCHT kit from chitosan-dimer to chitosan-pentamer (Suisankagaku Industry, Tokyo, Japan; MWs, 322, 483, 645, 806) were used as calibration standards.
Antibacterial activity of the chitosan-oligosaccharides against S. aureus
S. aureus (American Type Culture Collection [ATCC] 29213) obtained from the National Veterinary Research and Quarantine Service (Anyang, Korea) as well as a field isolate that originated from bovine subclinical mastitis (Moon et al. 1998) were used for this experiment because they had the highest pathogenicity in our preliminary study. The S. aureus cells were cultured aerobically in nutrient broth (Difco, Detroit, MI, USA) at 37°C for 36 h and then adjusted to 2 × 107 colony forming units (CFU)/ml. After dilution with fresh nutrient broth, they were cultured with OCHT dissolved in 0.1 M phosphate-buffered saline (pH 7.2) at one of the following final concentrations: 0.5, 0.1, 0.01, 0.001, and 0.0001% (w/v). One milliliter from each of these cultures was separately sampled from the same vial at 5, 10, and 30 min, then 1, 4, and 18 h after inoculation, and diluted 1:100 in 0.1 M phosphate-buffered saline (pH 7.0). Each of these 50-μl mixtures was then spread onto plate count agar (Difco) and incubated at 37°C for 24 h and the viable colonies counted.
Observation of S. aureus treated with the chitosan-oligosaccharides by electron microscopy
The morphological features of S. aureus treated with OCHT were examined using scanning electron microscopy (SEM) and transmission electron microscopy (TEM) using the method described by Yun et al. (1999). Briefly, a suspension of S. aureus that had been incubated for 12 h on Luria-Bertani (LB) agar (Difco) was evenly distributed onto new LB agar plates that contained holes filled with 30 μl of 0.0005% OCHT solution. After incubation at 37°C for 36 h, the zones of inhibition on each plate were cut to a size of 0.5∼1 mm3, added to a 2.5% (w/v) glutaraldehyde solution in 100 mM phosphate buffer (pH 7.2) for 3 h, and then washed three times with 100 mM of phosphate buffer solution (pH 7.2) for 15 min. After being fixed with 1% osmium tetroxide (w/v) for 1 h and sequentially dehydrated with 30 to 100% ethanol, the specimens were immersed into isoamyl acetate and propylene oxide for observation by SEM (Hitachi, S-2500C, Tokyo, Japan) and TEM (Carl Zeiss, TEM 109, Oberkochen, Germany), respectively.
Immunostimulative activity of the chitosan-oligosaccharides in mice
ICR male mice (4–5 weeks old) were provided by the National Veterinary Research and Quarantine Services (Anyang). All mice were cared for according to the guidelines for good animal laboratory practices set by the National Veterinary Research and Quarantine Services. The immunostimulative effect of the OCHT treatment was examined in mice that were divided into three groups comprising of either 40 mice for cytokine analysis or 25 mice for hematological analysis. The mice were intraperitoneally injected with 0.5 or 1.0 mg of OCHT or a control (physiological saline). Mice undergoing cytokine analysis were injected once, and those being analyzed for hematological changes were injected daily for 5 and 10 days.
The amount of OCHT administered was determined based on a finding reported by another study in which the migratory activity of canine neutrophils was maximized when the concentration of chitin was 1.0 mg/ml in blood (Usami et al. 1994). Five mice per group were killed and blood collected for cytokine analysis at 0, 1, 2, 4, and 8 h after treatment with OCHT. Additionally, ten mice per group were bled for hematological analysis at 5 and 10 days after treatment with OCHT. Ethylenediamine tetraacetic acid-treated blood samples were examined using an automatic blood cell counter (Serono System 9018, Japan) and stained using the Giemsa staining method to allow for the differential counting of white blood cells under a microscope. The concentration of cytokines, including interleukin-1α (IL-1α), IL-2, and IL-6, interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α) in the serum was measured using the double sandwich enzyme-linked immunosorbent assay technique (Endogen, Woburn, MA, USA).
Curative and protective effect of the chitosan-oligosaccharide against S. aureus infection in mice
Five mice in each group were also intraperitoneally infected with 1.0 × 108 CFU of S. aureus, the minimum lethal dose determined in our preliminary study, to investigate the curative effect of the OCHT against S. aureus. These mice were then treated intraperitoneally with 0.5 ml of OCHT solution (0.05, 0.1, 0.5, and 5%) or a control (physiological saline solution) 1 h after infection (Isenberg et al. 1982). The survival status of the infected mice was assessed twice a day for 7 days.
The mice were divided into four groups of ten mice that each received 0.5, 1, and 2 mg OCHT daily or saline (for the control group) to investigate the preventive effect of OCHT against S. aureus. After oral administration of OCHT for 7 days, the mice were intraperitoneally challenged with a field isolate of S. aureus (2.5 × 108 CFU) that originated from bovine subclinical mastitis (Moon et al. 1998), then observed twice daily for 7 days. An amount of S. aureus 2.5 times greater than the minimum lethal dose was used because the mice were 1 week older than those used to determine the curative effect of OCHT, and challenge with 1.0 × 108 CFU S. aureus after daily consumption of the OCHT produced the same survival rate, 100%.
Statistical analysis
The number of viable S. aureus, white blood cell (WBC) data, and cytokine concentrations between different groups were compared using an independent samples t-test, and a paired samples t-test was used to analyze any significant differences in the data that originated from the same group but were determined at a different time. Other hematological data (excluding WBC) between the control and one of treatment groups were compared by Kruskal–Wallis one-way analysis of variance (ANOVA) by ranks, and significant differences in data that originated from the same group, but were determined at a different time, were analyzed by a Wilcoxon signed-ranks test. All statistical analyses were carried out using commercially available software (Analyse-it software). The level of significance was set at p < 0.05.
Results
Analysis for selection of the optimal chitosan-oligosaccharides
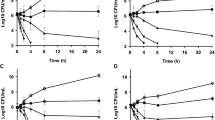
MWs of the different pilot OCHTs were analyzed by gel permeation chromatography based on a standard calibration curve generated using two kinds of standards, commercial pullulan (Jasco), and OCHTs (Suisankagaku Industry; Fig. 1a). The MW of chitosan, which had 70–100% deacetylation and 5 cP of viscosity, ranged from 49,012 to 235,448 Da (Fig. 1b). However, the MW of the OCHT changed to between 279 and 17,853 Da (Fig. 1c) and to between 300 and 12,796 Da (Fig. 1d) at 12 and 24 h after treatment with the chitosanase enzyme, respectively. The proportion of OCHTs with less than ten oligosaccharides was 74 and 87.6% at 12 and 24 h after enzymatic treatment, respectively. When analyzed by FT-IR, the same patterns of spectra (%T mode) among commercial chitosan (Sigma Chemical), raw chitosan (Taehoon Bio.), and three kinds of pilot OCHTs were observed (Supplementary Fig. 1), indicating that there was no constitutional transformation from the raw chitosan (Taehoon Bio.) into OCHT. Finally, 96% deacetylated OCHT with 2.02 cP viscosity and a mean MW of 1,088 Da ranging from 300 to 12,796 Da (supplemental Table. 1), consisting mostly of 1–16 oligosaccharides (98.8%), was selected for use in this study due to its water solubility and non-precipitation under alkaline conditions (pH > 8).
Gel permeation chromatography analysis of the OCHT of low MW prepared by the treatment of the raw chitosan (70–100% deacetylation, 5 cP of viscosity, and MW of 49,012 to 235,448 Da; Taehoon Bio.) with chitosanase. a MW calibration curve of calibration standards (monodispersed pullulan [Jasco] of different MW [a 1,660,000; b 380,000; c 186,000; d 100,000; e 48,000; f 23,700 g 12,200 h 5,800] and commercial OCHT from chitosan-pentamer to chitosan-dimer [Suisankagaku Industry; MW, i 806; j 645; k 483; l 322]), gel permeation chromatography analysis of the raw chitosan (deacetylation 96%, viscosity 2.02 cP, Taehoon Bio.; b) and the OCHT prepared in this study following enzymatic treatment for 12 h (c) and 24 h (d)
Antibacterial activity of the chitosan-oligosaccharides against S. aureus
In our study, three kinds of pilot OCHTs were produced and screened to evaluate their antibacterial effects against S. aureus. The viable number of both the reference strain of S. aureus (ATCC 29213) and an isolate from a mastitic cow was markedly reduced 10 min after treatment (p < 0.05), and no viable organisms were observed after 18 h when treated with various concentrations of the OCHT (0.5, 0.1, 0.01, 0.001, and 0.0001%), whereas the viable number of bacteria in the control group increased continuously with time. However, there was no statistical difference in antibacterial effect against S. aureus among the groups treated with different concentrations of OCHT (Table 1).
Electron microscopic features of S. aureus treated with the chitosan-oligosaccharides
The features of S. aureus grown in LB medium containing a sublethal dose of OCHT, as observed by SEM and TEM, were markedly different, both internally and externally, than those of the normal strains (Fig. 2a–d). The bacterial cells treated with chitosan were severely damaged, having a swollen cell wall and an irregular shape as determined by SEM. In addition, when viewed using TEM, the outer membrane of the cell was expanded and distorted and cells appeared to be lysed.
Hematological changes and cytokine production in mice induced by chitosan-oligosaccharides
It is important to consider suitable therapeutic levels of chitosan. The mice in our study showed no significant difference in the total number of leukocytes between the treatment and control group (Table 2). Although the number of neutrophils in mice treated with 0.5 mg OCHT was significantly reduced, the number of monocytes increased significantly in mice injected with OCHT at both 5 and 10 days after treatment compared to the control group (p < 0.05). The increase in the number of monocytes was greater in the group of mice treated with 0.5 mg of OCHT for 10 days than those treated for 5 days (p < 0.05).
The concentrations of various cytokines, including IL-1α, IL-2, IL-6, IFN-γ, and TNF-α, in mice treated with OCHT, were measured at different time intervals in our study. Among the cytokines tested, the concentration of IL-6 and IFN-γ significantly increased at 1 h, peaked 2 h after treatment (p < 0.01), and then, gradually dropped (p < 0.05). The concentrations of the two cytokines became similar to the initial concentrations before the treatment at around 4 to 8 h after administration (Fig. 3). However, the levels of IL-1α and TNF-α production were relatively low (less than 1.0 and 3.0 pg/ml, respectively) until 8 h after OCHT stimulation. In addition, no significant difference in the concentration of IL-2 was observed between the 0.5 and 1.0 mg OCHT treatment and control group (that is 20.2, 24.1, and 21.8 pg/ml 2 h after treatment, respectively; p > 0.05).
The changes in the concentration of IFN-γ (a) and IL-6 (b) in mice after the administration of the OCHT (0.5 and 1.0 mg) and the saline control. Each bar represents the mean ± standard error from five replicates. Significant differences between the groups were analyzed by independent sample t-tests and are indicated (a, asterisk, p < 0.01): a significant difference between control and one of treatment groups; asterisk significant difference between two treatment groups
Curative and protective effect of the chitosan-oligosaccharides against murine infection by S. aureus
The curative effect of OCHT against S. aureus, shown by the survival rate of mice, is summarized in Table 3. Although all mice in the control group treated with physiological saline died, 20–100% of the mice inoculated intraperitoneally with 0.25, 0.5, 2.5, and 25 mg of OCHT were still alive at 5 days posttreatment. Based on these results, we investigated the preventive effect of OCHT against S. aureus in mice. The mice were orally administered 0.5, 1, and 2 mg of OCHT once daily for 7 days, then intraperitoneally challenged with S. aureus (2.5 × 108 CFU). The survival rates in those treatment groups were 70–100%, compared to 10% for the mice in the control group (Table 4).
Discussion
The antimicrobial activity of chitosan against several bacteria has been recognized; however, this activity is influenced by a number of factors, including type of chitosan, degree of polymerization, and other various chemical and physical properties (Choi et al. 2001; No et al. 2002). The antibacterial effect of chitosan and chitosan oligomers has also been reported to be dependent on MW (Uchida et al. 1989; Jeon and Kim 2000; No et al. 2002) and degree of deacetylation (Saito et al. 1994). Some studies have suggested that chitosans with a low MW are more effective at inhibiting the growth of S. aureus, Escherichia coli, Saccharomyces cerevisiae, Candida albicans, and Fusarium culmorum than chitosans with high MWs (Yun et al. 1999; Jung et al. 2002). Therefore, in our study, the chitosan deacetylated from chitin was hydrolyzed by chitosanase to obtain a more purified form (Fig. 1), and the OCHT with the lowest MW was then separated from the mixture based on its high solubility in acetone. In a recent study, Chung et al. (2004) reported that the amount of chitosan adsorbed by different bacterial cells was the same order of magnitude determined for the antibacterial activity of chitosan on these bacteria and that chitosans with a high degree of deacetylation would result in greater amounts of adsorption. The OCHT prepared in this study was highly deacetylated (96%), and this may be one of the reasons for its relatively high antibacterial activity.
The growth of S. aureus isolated from bovine mastitis was inhibited within 10 min of treatment with OCHT (Table 1). Tsai et al. (2000) also reported that the chitosan mixture prepared by cellulase digestion of shrimp chitosan caused a rapid reduction in the number of the Staphylococcus species in raw milk. The OCHT prepared in this study showed inhibitory activity against S. aureus from a concentration of 0.0001%, which is much lower than concentrations reported to have inhibitory effects in other studies, including water-soluble chitin derivatives (0.6–2.5%), partially deacetylated chitins (0.13–0.5%), and chitosan oligomers (0.025–0.05%; Stossel and Leuba 1984; Saito et al. 1994; Yun et al. 1999). This concentration is also far lower than that reported in another study in which the minimum inhibitory concentration of chitosans against four species of Gram-negative bacteria (E. coli, Pseudomonas flurescens, Salmonella typhimurium, and Vibrio parahaemolyticus) and seven species of Gram-positive bacteria (Listeria monocytogenes, Bacillus megaterium, B. cereus, S. aureus, Lactobacillus plantarum, L. brevis, and L. bulgaricus) ranged from 0.05 to >0.1% (No et al. 2002). Discrepancies have also been reported in concentrations showing the antimicrobial activity of chitosan or its derivatives (Papineau et al. 1991; Sudarshan et al. 1992; Wang 1992; Yang et al. 2005).
Electron microscopic observation indicated that the surface of the OCHT-treated bacteria was expanded and distorted and that cells appeared to be lysed when compared to that of the control bacteria (Fig. 2). This finding is similar to that of a previous study conducted by Yun et al. (1999) who described that such morphological changes were presumably induced by chitosan and that the antibacterial activity of chitosan might work via the same mechanism. EI-Ghaouth et al. (1992) asserted that the cationically charged amino group in chitosan reacted with anionic components, such as N-acetyl muramic acid, sialic acid, and neuraminic acid, on the bacterial cell surface by electrostatic interaction altered cell permeability and that such interactions further prevented the entry of material or resulted in the leakage of material. The close relationship between the antibacterial activity of chitosan and the surface characteristics of the bacterial cell wall has also been demonstrated in a recent study in which Chung et al. (2004) reported that chitosan made changes to the structure of the cell wall and the permeability of the cell membrane and that both adverse effects resulted in bacterial death.
The proportion of monocytes in mice was elevated after peritoneal inoculation of OCHT in this study (Table 2). Monocytes constitute up to 10% of blood leukocytes and migrate into almost all tissues, where they develop into macrophages. Macrophages play a key role in the immune system as antigen presenting cells by ingesting and processing non-self antigens from infected bacteria and viruses. There have also been studies reporting that chitosan treatment may enhance the activity of polymorphonuclear leukocytes (PMN) and macrophages in cows (Usami et al. 1994) and that chitosan induced migration and activation of macrophages in dogs (Kosaka et al. 1996). Recently, Mori et al. (2005) reported that the mechanism of macrophage activation by chitin derivatives resulted in accelerated wound healing.
A previous study demonstrated that intraperitoneal administration of chitosan enhanced the oxidative activity of PMN, but did not affect their numbers in mice (Suzuki et al. 1984). In this study, the effect of chitosan on the change in the number of neutrophils was different between the two treatment groups of mice administered either 0.5 or 1 mg of OCHT (Table 2). The increase in the number of monocytes was more than 1.8-fold greater in groups of mice administered 0.5 or 1.0 mg OCHT compared to the control group. By contrast, the number of neutrophils in mice treated with 0.5 mg of OCHT was significantly reduced and followed by an increase in monocytes by 2.4- and 3.8-fold at 5 and 10 days after intraperitoneal injection. Therefore, the reduction in neutrophils should have been relative to the increase in monocytes because the total WBC counts were consistent.
Cytokines are small proteins that act as intercellular communication signals in hematopoiesis, stress, inflammation, immunity, and tissue repair (Belardelli and Ferrantini 2002). The identification of a large number of cytokines and disclosure of their pathophysiological mechanisms has opened innovative frontiers in diagnosis and therapy (Alluwaimi 2004). Lipopolysaccharide stimulation of a mammary gland epithelial cell line induced IL-6 in a dose-dependent manner (Okada et al. 1999). IL-6 expression was detected in mammary glands as early as 14 h postinfection with E. coli (Shuster et al. 1997). In this study, the levels of IL-6 and IFN-γ sharply increased 2 h after peritoneal inoculation of OCHT (0.5 to 1 mg per mouse; Fig. 3). It has been postulated that IL-6 facilitates transition of the inflammatory process from an influx of neutrophils to monocytes. A shift from neutrophils to monocytes is essential for suitable immune responses and to decrease the noxious effect of neutrophils (Kaplanski et al. 2003). A previous study demonstrated that it took approximately 6 h to induce apoptosis in 50% of macrophages when stimulated with chitosan (Mori et al. 2005). IFN-γ is an important mediator in the activation of recruited neutrophils and macrophages as well as the enhancement of their phagocytosis (Riollet et al. 2000; Wedlock et al. 2000).
In addition, Nishimura et al. (1984) revealed that, of the many chitin derivatives, 70% of deacetylated chitins promote the production of circulating antibodies, induce delayed-type hypersensitivity, enhance helper T cell activity, stimulate generation of alloreactive cytotoxic T lymphocytes, facilitate natural killer cell activity, and induce cytotoxic macrophages in mice. Similar studies have also reported that chitosan activates the immune system by enhancing production of IFN-γ, lysozyme (Fletcher and White 1973), IL-1, TNF-α, granulocyte macrophage colony stimulating factor, nitric oxide, and IL-6 from macrophages (Peluso et al. 1994; Tokro et al. 1998; Jeong et al. 2000; Feng et al. 2004). Thus, our data suggest that the OCHT prepared in this study may act as an immunopotentiator for the production of monocytes IFN-γ and IL-6, which can play important roles in the initial host defense against infectious disease in mice. However, the detailed mechanism of immune system activation and host resistance against S. aureus infection remains unclear in cows.
The protective and curative effects of the 0.5-mg OCHT treatment against S. aureus infection in mice showed a higher survival rate than other treatment groups in this study (Tables 3 and 4). No direct relationship between the administered amounts of chitosan and its efficacy was also found in other studies that demonstrated that, when the sarcoma 180 tumor cell-bearing BALB/c mice were treated with OCHT intraperitoneally once a day for a period of 14 days and antitumor effect was examined at doses of 50, 20, and 10 mg kg−1 day−1 chitosan, there was a significant difference in inhibition of tumor growth and increase in weight of immune organs, such as spleen and thymus, with the highest efficacy being found in the group treated with 20 mg of OCHT (Jeon and Kim 2001). The survival rate of white shrimp that received chitosan at a dose of 2, 4, and 6 μg/g (body weight) were significantly higher than that of the control group after 1–6 days. Conversely, there was no significant difference in survival rates among groups administered 2 to 6 μg/g of chitosan (Wang and Chen 2005). Further research is needed to determine suitable therapeutic levels of chitosan, including an administration pathway, dosage, and interval.
Using infection of laboratory animals with S. aureus originating from a mastitic cow as an experimental IMI model of bovine mastitis, we can expect chitosan to act as both an effective agent for prevention and a cure of bovine mammary gland infection by S. aureus. This was already demonstrated in our previous study, in which 56 cows with IMI from nine farms were selected and subjected to diets that contained 15–20 g of chitosan per day for 5–7 days. The cure rates of chitosan against S. aureus, coagulase-negative Staphylococci, Streptococci spp., other Gram-positive bacteria, and coliforms in bovine mastitis were 30.4, 42.8, 33.3, 66.6, and 54.6%, respectively (Moon et al. 1998). An important factor in the resistance against IMI is the ability of inflammatory cells to initiate and maintain an immune response. PMN and macrophages in the mammary gland are the major immune cells acting as the initial host defense against organisms that cause mastitis. Chitosan is also capable of activating these PMNs and macrophages in the host defenses to prevent infection. Additionally, chitin, chitosan, and their derivatives have shown extensive antibacterial activity against pathogenic microbes (Senel and McClure 2004). The protective and curative effects of the OCHT may be partially due to chitosan inducing accelerated wound healing resulting in a satisfactory healing surface, as well as the many other positive effects OCHT has on the immune system (Mori et al. 2005).
In conclusion, the water-soluble OCHT prepared in this study exhibits strong antibacterial activity against S. aureus isolated from mastitic cows and can act as an immunopotentiator for the activation of nonspecific immune cells by increasing the number of monocytes and stimulating the secretion of IFN-γ as well as IL-6 in mice. Therefore, our results indicate that the OCHT prepared in this study can be applied as an effective agent for the prevention and cure of bovine IMI by S. aureus after adjustment of its concentration and administration route to the mastitic cow.
References
Allan CR, Hadwiger LA (1979) The fungicidal effects of chitosan on fungi and varying in cell wall composition. Exp Mycol 3:285–287
Alluwaimi AM (2004) The cytokines of bovine mammary gland; prospects for diagnosis and therapy. Res Vet Sci 77:211–222
Belardelli F, Ferrantini M (2002) Cytokines as a link between innate and adaptive antitumor immunity. Trends Immunol 23:201–208
Brouillette E, Malouin F (2005) The pathogenesis and control of Staphylococcus aureus-induced mastitis: study models in the mouse. Microbes Infect 7:560–568
Castellanos-Perez N, Maldanando-Vega M, Fernandez-Villagomez G, Cafferal-Mendez S (1988) An evaluation of coagulating ability of chitosans from different crustacea species and fungi. In: Skjak-Break G, Anthonson T, Sandford P (eds) Chitin and chitosan. Elsevier, London, pp 567–576
Choi BK, Kim KY, Yoo YJ, Oh SJ, Choi JH, Kim CY (2001) In vitro antimicrobial activity of a chitooligosaccharide mixture against Actinobacillus actinomycetemcomitans and Streptococcus mutans. Int J Antimicrob Agents 18:553–557
Chung YC, Su YP, Chen CC, Jia G, Wang HL, Wu JCG, Lin JG (2004) Relationship between antibacterial activity of chitosan and surface characteristics of cell wall. Acta Pharmacol Sin 27:932–936
Darmadji P, Izumimoto M (1994) Effect of chitosan in meat preservation. Meat Sci 38:243–254
EI-Ghaouth A, Arul J, Asselin A, Benhamou N (1992) Antifungal activity of chitosan on two post-harvest pathogen of strawberry fruits. Phytopathology 82:398–402
Feng J, Zhao L, Yu Q (2004) Receptor-mediated stimulatory effect of oligochitosan in macrophages. Biochem Biophys Res Commun 317:414–420
Fletcher TC, White A (1973) Lysozyme activity in the plaice (Pleuronectes platessa L). Experientia 29:1283–1285
Hadwiger LA, Fristensky BW, Rigglman RC (1984) Chitosan, a natural regulator in plant-fungal pathogen interaction, increases crop yields. In: Zikakis JP (ed) Chitin, chitosan and related enzymes. Academic, New York, NY, pp. 291–302
Illum L (1998) Chitosan and its use as a pharmaceutical excipient. Pharm Res 15:1326–1331
Isenberg HD, Sampson-Scherer J, Cleeland R, Titsworth E, Beskid G, Christenson JG, DeLorenzo WF, Unowsky J (1982) Correlation of the results of antibiotic synergy and susceptibility testing in vitro with results in experimental mouse infections. Crit Rev Microbiol 10:1–76
Jeon YJ, Kim SK (2000) Production of chitooligosaccharide using an ultrafiltration membrane reactor and their antibacterial activity. Carbohydr Polym 41:133–141
Jeon YJ, Kim SK (2001) Potential immuno-stimulating effect of antitumoral fraction of chitooligosaccharide. J Chitin Chitosan 6:163–167
Jeong HJ, Koo HN, Oh EY, Chae HJ, Kim HR, Suh SB, Kim CH (2000) Nitric oxide production by high molecular weight water-soluble chitosan via nuclear factor-kB activation. Int J Immunopharmacol 22:923–933
Jung BO, Chung SJ, Lee GW (2002) Effect of molecular weight of chitosan on its antimicrobial activity. J Chitin Chitosan 7:231–236
Kaplanski G, Marin V, Montero-Julian F, Mantovani A, Farnarier C (2003) IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol 24:25–29
Kim HK (1998) Production of chitosan oligosaccharides and their antibacterial effect to pathogenic Escherichia coli. Pukyung National University, Busan, South Korea
Kina K, Tamura K, Ishibashi J (1974) The fungicidal effect of chitosan on fungi of varying cell wall composition. Bunseki Kagaku 23:1082–1100
Kosaka T, Kaneko Y, Nakada Y, Matsuura M, Tanaka S (1996) Effect of chitosan implantation on activation of canine macrophages and polymorphonuclear cells after surgical stress. J Vet Med Sci 58:963–967
Minami S, Egawa T, Ohira J, Okamoto Y, Matsuhashi A (1997) Effects of chitosan with intramammary administration on phagocytes in udder secretion. J Jpn Vet Med Assoc 50:143–146
Moon JS, Joo YS, Ku BK, J Kim Y, Park YH, Hahn TW (1998) A study on efficacy of chitosan on bovine mastitis. Korean J Vet Res 38:71–76
Mori T, Murakami M, Okumura M, Kadosaw T, Uede T, Fujinaga T (2005) Mechanism of macrophage activation by chitin derivatives. J Vet Med Sci 67:51–56
Nishimura K, Nishimura S, Nishi N, Saiki I, Tokura S, Azuma I (1984) Immunologic activity of chitin and its derivatives. Vaccine 2:93–99
No HK, Park NY, Lee SH, Meyers SP (2002) Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int J Food Microbiol 74:65–72
Okada H, Ohtsuka H, Kon Nai S, Kirisawa R, Yokomizo Y, Yoshino T, Rosol TJ (1999) Effects of lipopolysaccharide on production of interleukin-1 and interleukin-6 by bovine mammary epithelial cells in vitro. J Vet Med Sci 61:33–35
Okamoto Y, Minami S, Matsuhashi A, Hamada K, Yanagiya G, Ohira J, Fukumoto Y, Murakami C (1996) Effects of chitosan on bovine wound healing. J Jpn Vet Med Assoc 49:22–27
Papineau AM, Hoover DG, Knorr D, Farkas DF (1991) Antimicrobial effect of water-soluble chitosans with high hydrostatic pressure. Food Biotechnol 5:45–57
Park PJ, Kim SK, Lee HK (2002) Antimicrobial activity of chitooligosaccharides on Vibrio parahaemolyticus. J Chitin Chitosan 7:225–230
Peluso G, Petillo O, Ranieri M, Santin M, Ambrosio I, Calabro D, Avallone B (1994) Chitosan-mediated stimulation of macrophage function. Biomaterials 15:1215–1220
Riollet C, Ranard P, Poutrel B (2000) Cells and cytokines in inflammatory secretions of bovine mammary gland. Adv Exp Med Biol 480:247–258
Ruegg PL (2003) Investigation of mastitis problems on farms. Vet Clin North Am Food Anim Pract 19:47–73
Saito K, Shimojoh M, Fukushima K (1994) Growth inhibition of chitosan from squid pen against oral Streptococci. Annual Report of Chitin and Chitosan Research. Japanese Society for Chitin and Chitosan, Yokohama, Japan, pp 77–79
Sears PM, McCarthy KK (2003) Management and treatment of staphylococcal mastitis. Vet Clin North Am Food Anim Pract 19:171–185
Senel S, McClure SJ (2004) Potential applications of chitosan in veterinary medicine. Adv Drug Deliv Rev 56:1467–1480
Seo H, Mitsuhash K, Tanibe H (1992) Antibacterial and antifungal fiber blended by chitosan. In: Brine CJ, Sandford PA, Zikakis JP (eds) Advances in chitin and chitosan. Elsevier, London, pp 34–40
Shuster DE, Kehrli ME, Rainard P, Paape M (1997) Complement fragment C5a and inflammatory cytokines in neutrophil recruitment during intramammary infection with Escherichia coli. Infect Immun 65:3286–3292
Singla AK, Chawla M (2001) Chitosan: some pharmaceutical and biological aspects—an update. J Pharm Pharmacol 53:1047–1067
Stossel P, Leuba JL (1984) Effect of chitosan, chitin, some amino sugars on growth of various soil-borne phytopathogenic fungi. Phytopathol Z 111:82–90
Sudarshan NR, Hoove DG, Knorr D (1992) Antibacterial action of chitosan. Food Biotechnol 6:257–272
Suzuki K, Okawa Y, Hashimoto K, Suzuki S, Suzuki M (1984) Protecting effect of chitin and chitosan on experimentally induced murine candidiasis. Microbiol Immunol 28:903–912
Tokro A, Suzuki K, Mikami T (1998) Growth-inhibitory effect of hexa-N-acetylchitohexaose and chitohexaose against Meth-A solid tumor. Chem Pharm Bull 36:784
Tsai GJ, Wu ZY, Su WH (2000) Antibacterial activity of a chitooligosaccharide mixture prepared by cellulase digestion of shrimp chitosan and its application to milk preservation. J Food Prot 63:747–752
Uchida Y, Izume M, Ohtakara A (1989) Preparation of chitosan oligomers with purified chitosanase and its application. In: Skjak-Brak G, Anthonsen T, Sandford P (eds) Chitin and chitosan: source, chemistry, biochemistry, physical properties and application. Elsevier, London, pp 372–382
Usami Y, Okamoto Y, Minami S, Matsuhashi A, Kumazawa NH, Tanioka S, Shigemasa Y (1994) Migration of canine neutrophils to chitin and chitosan. J Vet Med Sci 56:761–762
Wang GH (1992) Inhibition and inactivation of five species of foodborne pathogens by chitosan. J Food Prot 55:916–919
Wang SH, Chen JC (2005) The protective effect of chitin and chitosan against Vibrio alginolyticus in white shrimp Litopenaeus vannamei. Fish Shellfish Immunol 19:191–204
Wedlock DN, Doolin EF, Parlane N, Lacy-Hulbert S, Woolford MW (2000) Effects of yeast expressed recombinant interleukin-2 and interferon-γ on physiological changes in bovine mammary glands and on bacterial activity of neutrophils. J Dairy Res 67:189–197
Yang TC, Chou CC, Li CF (2005) Antibacterial activity of N-alkylated disaccharide chitosan derivatives. Int J Food Microbiol 97:237–245
Yun YS, Kim KS, Lee YN (1999) Antibacterial and antifungal effect of chitosan. J Chitin Chitosan 4:8–14
Acknowledgments
This work was supported by a grant from the National Veterinary Research and Quarantine Services, Republic of Korea. Dr. Hye Cheong Koo and Dr. Yong Ho Park were supported by the Korea Research Foundation Grant (KRF-2006-005-J02903), the Technology Development Program for Agriculture and Forestry provided by the Ministry of Agriculture and Forestry (grant no. 305003031HD110), and the Research Institute of Veterinary Science, Department of Veterinary Microbiology, College of Veterinary Medicine and the BK21 Program for Veterinary Science, Seoul National University.
Jin-San Moon and Hee-Kyung Kim contributed equally to this study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Moon, JS., Kim, HK., Koo, H.C. et al. The antibacterial and immunostimulative effect of chitosan-oligosaccharides against infection by Staphylococcus aureus isolated from bovine mastitis. Appl Microbiol Biotechnol 75, 989–998 (2007). https://doi.org/10.1007/s00253-007-0898-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-007-0898-8