Abstract
Biofilm associated Pseudomonas aeruginosa infections are of major clinical concern due to treatment failure by conventional antibiotics. Referring to many reports, antimicrobial peptides (AMPs) would be act as a new promising agent to overcome the issue. In this regard, our study was aimed to evaluate the kinetics of melittin as a natural AMP, in simultaneously degrading and killing potent biofilm producing multidrug-resistant (MDR) P. aeruginosa isolates. The sensitivity of P. aeruginosa clinical isolates against routinely prescribed antibiotics was evaluated using disc diffusion and micro-dilution broth methods. Biofilm formation ability of the isolates was determined by colorimetric method. The biofilm formation kinetics was evaluated in five highly biofilm producer MDR isolates during 48 h. The efficiency of melittin to degradation of biofilm biomass and killing the bacteria within the biofilm were kinetically performed. The degradation activity of melittin on preformed biofilm and also its effect on the morphology of P. aeruginosa within the biofilm was investigated by field emission-scanning electron microscopy (FE-SEM). Melittin at the amount of 2 and 4 µg inhibited or killed all the examined strains in planktonic state while at 50 µg degraded the biofilm layer and killed all embedded bacteria after 24 and 48 h, respectively. FE-SEM results confirmed the biofilm removal and killing activities of melittin. Linear regression analysis verified the trend of melittin’s activities in a concentration and time dependent manner. In conclusion, it seems plausible that melittin should be further investigated in an animal model of biofilm associated burn infection as a new drug lead.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, the development of multidrug-resistant (MDR) bacteria particularly in burn infections has led to increased interest for new antibiotics from natural sources (Davies and Davies 2010).
Pseudomonas aeruginosa as a highly versatile opportunistic pathogen worldwide, is the major cause of morbidity and mortality in burn patients (Turner et al. 2014). Reference to many documented reports in many countries, the incidence of MDR P. aeruginosa is being increased (Falagas et al. 2006; Aloush et al. 2006; Hirsch and Tam 2010). Recent reports on the antibiotic sensitivity patterns of P. aeruginosa have highlighted the problem of antibiotic resistance in burn infection strains in comparison with other hospital isolates (Klockgether and Tümmler 2017; DiMuzio et al. 2014).
Pseudomonas aeruginosa persists in a biofilm, which further enhances the high antibiotic tolerance of the organism (Dean et al. 2011; Wood 2017). Heavy prescription of antibiotics has led to the worldwide spread of antibiotic-resistant bacteria in hospitals and communities which poses as a serious danger to human health (Ventola 2015).
At the moment, all of the prescribed antibiotics including fluoroquinolones, cephalosporins, aminopenicillins, colistin, carbapenems and tetracyclines, in single or combined therapy cannot guarantee the complete eradication of some P. aeruginasa burn infections (Høiby et al. 2010, 2005). This phenomenon is due to the mutations of the antibiotic targets that beside the biofilm barrier can lead to failure in treatment of different kinds of P. aeruginosa infections, such as burn and respiratory tract infections (Oliver et al. 2004).
Biofilm is one of the major concerns in the issue of antibiotic resistance in P. aeruginasa burn infections (Percival et al. 2015; Costerton et al. 1999). Biofilm can provide a protected niche in which the bacteria enveloped in an exopolysaccharide and extracellular protein secretion. Biofilm is often physically resistant to anti-microbial agents and host immune responses (Wood 2017; Limoli et al. 2015; Verstraeten et al. 2008).
It has been shown that biofilm formation can be a defensive reaction in the presence of antibiotics. While biofilms contain cells with a heterogeneous range of states (Balcázar et al. 2015), on average, bacteria in biofilms have a much higher antibiotic tolerance up to 1000-fold greater than their planktonic counterparts (de la Fuente-Núñez et al. 2013).
Eradication of bacteria within the biofilm is a challenging issue that is corresponded to alginate, as a physical barrier, and remarkable reduction in bacterial metabolism in mature biofilm as well (Ishida et al. 1998; Wood 2016).
These two reasons are the major limitations to the successful implementation of conventional antibiotics. In this vital condition, increasing the dose of prescribed antibiotics, not only fail to address the problem but may also induce significant toxicity. Colistin as a choice to treatment of P. aeruginosa burn infection is a good example where nephrotoxicity is a remarkable sequel (Hachem et al. 2007).
Annually, 17 million new biofilm associated infections are reported in the United States, and amongst this figure 550,000 people die (Wood 2017).
In this critical condition, antimicrobial peptides could be new promising agents to overcome this threatening challenge (Mandal et al. 2014). AMPs are evolutionarily conserved key effector molecules of the innate immune system that exist in a wide range of insects, animals, plants, and bacteria (Diamond et al. 2009). The venom of venomous animals also indicates a potential source of AMPs (da Mata et al. 2017).
AMPs usually have common characteristics including small size (12–60 amino acids), an excess positive charge ranging from + 2 to + 9, around 50% hydrophobicity, and the acquisition of different secondary structures in membranous environments (Jindal et al. 2014).
In comparison to conventional antimicrobial agents, AMPs have some attractive advantages including rapid killing and broad spectrum antimicrobial activity, typically not involving induction of bacterial resistance, and show synergistic activity when combined with classic antibiotics (Chung and Khanum 2017). The AMPs firstly bind to the membrane and subsequently invade it as a detergent or by degradation of the bacterium by a pore forming mechanism. In some cases, the AMPs can transfer themselves into the cytoplasm and interfere with replication, transcription, and translation activities (Gaspar et al. 2013).
Among AMPs, a series of drug candidates derived from antimicrobial peptides (i.e. Locilex, C16G2, Cefilavancin, NVB302, Dalvance, LTX-109, PXL01, and LL-37) which are in the different phases of clinical studies (Felício et al. 2017).
Among antimicrobial peptides, melittin was selected as a potent candidate to prove our hypothesis being that it can degrade biofilm layers and kill the containing bacteria. Melittin (C131H229N39O31) is an alpha helical cationic peptide consist of 26 amino acids (Terwilliger and Eisenberg 1982). The first 20 residues (N-terminal) of melittin are predominantly hydrophobic amino acids, whereas the carboxyl-terminal of the peptide is mostly composed of hydrophilic residues. This amphipathic entity allows the peptide to interact with phospholipid membranes (Lee et al. 2013).
Regarding the major concern of biofilm in terms of global and local antibiotic resistance, this study was aimed to evaluate the kinetics of antimicrobial peptide, melittin, in simultaneously degrading and killing potent biofilm producing MDR P. aeruginosa isolated from third degree burn patients.
Materials and Methods
Reagents and Media
Antibiotic discs including gentamicin, ceftazidime, ciprofloxacin, piperacillin–tazobactam, doripenem, levofloxacin, cephalothin, doxycycline, and cefepime were purchased from MAST Group Co. (UK). Antibiotic powders including doripenem, colistin, and ceftazidime were obtained from Sigma-Aldrich (St Louis, MO, USA). Muller Hinton Broth (MHB) and Agar (MHA) were purchased from Merck Co. (USA).
All the results are obtained from three independent replicates in three parallel trials otherwise indicated. Error bars represent the mean ± standard deviations.
Peptide Synthesis
The peptide, melittin, was synthesized at Mimotopes Company (Clayton, Victoria, Australia) using Fmoc chemistry with detailed procedures previously described by solid-phase method with C-terminal amidation. The purity of the ordered peptide was roughly greater than 95%. To validate the molecular weight of synthetic melittin, mass spectrometry was performed in positive ion mode on a Sciex API100 LC/MS instrument (PerkinElmer Co., Norwalk, CT, USA) by the company.
In Vitro Antibacterial Activity of Melittin Against Clinical Isolates
Thirty-three P. aeruginosa isolates were collected from third degree burn patients during the period between 2017 and 2018 at Shahid Motahari burn hospital, Tehran-Iran. The samples were collected from burn areas at least 48–72 h after patient hospitalization and detected based on the routine laboratory methods.
Antibiotic susceptibility assay was performed on all isolates using disk diffusion to determine the frequency distribution of antibiotic resistance. Inhibitory and bactericidal activities of melittin were examined on all isolates and compared with some conventional antibiotics in order to show the therapeutic value of melittin against multiple drug resistant bacteria.
Disc Diffusion Assay
Bacterial suspensions were prepared by spectrophotometry at 625 nm. Based on the McFarland method, optical density in the range of 0.08–0.1 is equivalent to 1.5 × 108 CFU/mL. In this study, to increase the accuracy of quantification, the OD of suspension was considered at 0.09. Disc diffusion assay was performed using gentamicin, ceftazidime, ciprofloxacin, piperacillin–tazobactam, doripenem, levofloxacin, cephalothin, doxycycline, and cefepime. Susceptibility testing was performed by the Kirby–Bauer disk diffusion method according to Clinical and Laboratory Standard Institute guidelines (CLSI) (Clinical and Laboratory Standards Institute guideline 2015).
Determination of MIC and MBC
Minimum inhibitory concentrations (MICs) were determined by a standard microtiter dilution method using Muller Hinton Broth (MHB) medium according to CLSI guidelines (Clinical and Laboratory Standards Institute guideline 2015). To compare the antibacterial activity of melittin with the most applied antibiotics, the MIC for colistin, doripenem and ceftazidime were also determined.
Briefly, the bacteria were grown overnight at 37 °C in the MHB. Melittin and the antibiotics were serially diluted in a 96 well polypropylene microplate, 1.5 × 105 bacteria added to each well and then incubated at 37 °C for 24 h. MBCs were determined at the end of the incubation period by subculture of a sample from each well with no visible growth onto MHA. The resultant colonies were counted after overnight incubation at 37 °C. MBC was determined as the lowest concentration of the peptide or antibiotics that kill 100% of the bacteria.
Biofilm Assay
The goal of this assay was tracing for potent biofilm producer strains among MDR isolates. Biofilm assay was performed based on the Segev-Zarko et al. protocol as detailed below (Segev-Zarko et al. 2015). All clinical isolates were cultured in 5 mL of TSB-glucose and incubated at 37 °C for 24 h. The suspensions were washed two times in TSB-glucose medium and their turbidity was adjusted to 0.5 McFarland (OD 0.08–0.1) at 625 nm using a spectrophotometer.
Then, 1.5 × 107 CFU was added to each well that was pre-filled with 100 µL TSB-glucose medium and incubated at 37 °C overnight. After incubation, the upper medium was aspirated entirely and the wells were washed three times with 250 µL of PBS to remove the suspension and the non-attached bacteria. Then 200 µL of methanol (99%) was added to each of the wells and incubated at room temperature (RT) for 15 min let dry.
The wells were stained with 200 µL of crystal violet (1%) for 5 min and washed three times with sterile deionized water. Finally, 200 µL of ethanol (95%) was added to each well and incubated at 37 °C for 30 min in a shaker incubator. The content of each well was transferred to the equivalent well in other microplate and then, the optical density (OD) was measured at 595 nm using a microplate spectrophotometer (EPOCH, BioTek Co., Winooski, VT, USA).
Kinetics of Biofilm Formation
Five MDR P. aeruginosa isolates were selected to follow the anti-biofilm activity of melittin. To get more accurate insight into the biofilm formation, kinetics study was performed on the selected strains. Briefly, biofilm assay were performed as aforementioned above but the OD of each sample was measured after 1, 3, 6, 24, and 48 h.
The Kinetics for Multiplication of the Bacteria in Biofilm Environment
Simultaneously, in a parallel group, the selected strains were cultured as detailed before and then the numbers of bacteria were counted in each well after 1, 3, 6, 18, 24, and 48 h. To do this, the upper solution was discarded and the wells were washed three times with saline solution. Afterward, saline (100 µL) was added to each well and the surfaces of the wells were scratched, from which 10 µL was cultured in MHA medium at 37 °C. The resultant colonies were counted after 24 h. The positive control was P. aeruginosa (ATCC 27853) in TSB-glucose medium.
Biofilm Degradation Kinetics
This assay was kinetically performed to determine the efficiency of melittin to degradation of biofilm biomass. Different amounts of melittin were examined in different time points to achieve the target amount and time in which the most degradation would be recorded. The bacterial biofilms were produced as aforementioned above. Then, the melittin was serially diluted ranging from 8 to 0.06 µg and added to the wells in a descendent manner. After 1, 3, 6, and 24 h the remaining biofilm was measured as described above.
Time-Kill Kinetics of Melittin Against Bacteria Within Biofilm
In this assay, the potency of melittin for killing bacteria in the surrounding biofilm was kinetically assessed. Different amounts of melittin were examined at different time points to achieve the target amount and time in which the all bacteria would be killed. The biofilms of the bacteria were produced as aforementioned above. Melittin at the amount of 50 µg added to the wells; after 0.5, 1, 3, 6, 24, 48, and 72 h the surface of each well was scratched and 10 µL of the suspension was cultured on MHA, incubated for 24 h, and the possible colonies were counted.
Morphological Assessment for Activity of Melittin by Field Emission Scanning Electron Microscopy
Field emission-scanning electron microscopy was used to visualize the effect of melittin on the biofilm removal of a MDR P. aeruginosa. At first, melittin (10 µg) was added to 1.5 × 107 bacteria and incubated at 37 °C for 24 h. Before incubation, sterile slides were aseptically put into the wells. Sample preparation for SEM was performed as previously described (El-Azizi et al. 2015). Briefly, the slides were gently washed three times with sterile distilled water and the sample was fixed in glutaraldehyde (0.1 M in PBS 1×) for 3 h at room temperature. The slides were then rinsed three times in fresh PBS buffer and post-fixed in 1.5% osmium tetroxide for 1 h. They were dehydrated in a series of ethanol solutions (30–100%). The specimens were then mounted on aluminum stubs, allowed to dry for 3 h, coated with gold nanoparticles, and examined in an FE-SEM instrument (MIRA3, TESCAN Co., Czech).
Results
In Vitro Antibacterial Activity
Disc Diffusion
Among 33 strains, 18.18% (6 cases) and 18.18% (6 cases) were resistant and sensitive to all examined antibiotics, respectively. The frequency of strains that were resistant to at least three antibiotics form three antibiotic classes was estimated as 39.4% (13 cases).The lowest resistance was seen against doripenem (Fig. 1).
The frequency distribution of sensitivity in all isolates against the examined antibiotic discs. The results showed that 18.8% of the isolates were resistant to all nine examined antibiotics. The frequency of strains that were resistant to at least three antibiotics form three antibiotic classes was estimated as 39.4% (13 cases). The lowest resistance was seen against doripenem. GM, CAZ, CIP, PTZ, DOR, LEV, KF, DOX, and CPM are abbreviated for gentamicin, ceftazidime, ciprofloxacin, piperacillin–tazobactam, doripenem, levofloxacin, cephalothin, doxycycline, and cefepime, respectively
MIC and MBC for Melittin, Colistin, Doripenem, and Ceftazidime
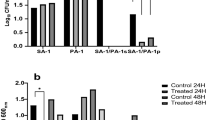
Melittin at the amounts of 2 and 4 µg inhibited or killed all the examined strains, respectively. The growth of 48% of the strains was inhibited at the concentration of 2.5 µg/mL (0.5 µg) and 31% of them were destroyed in the same amount.
Colistin, doripenem, and ceftazidime at the maximum concentration of 64 µg/mL were not able to inhibit or kill all of the isolates. There was a major difference between MIC and MBC of the antibiotics and melittin (p value < 0.05). The in vitro activitiy of melittin and the examined antibiotics against all of the P. aeruginosa isolates are summarized in Figs. 2 and 3.
The frequency distribution of MIC for melittin, colistin, doripenem, and cephtazidime. Reference to the results, the majority of strains was inhibited at lower amounts of melittin in comparison to other examined antibiotics. Melittin at the amounts of 2 µg inhibited the growth of all the examined strains. Colistin, doripenem, and ceftazidime at the maximum concentration of 64 µg/mL were not able to inhibit all of the isolates. There was a major difference between MIC of each of the antibiotics and melittin (p value < 0.05)
The frequency distribution of MBC for melittin, colistin, doripenem, and ceftazidime. In reference to the results, the majority of strains were killed at lower amounts of melittin in comparison to other examined antibiotics. Melittin at the amount of 4 µg killed all the examined strains. Colistin, doripenem, and ceftazidime at the maximum concentration of 64 µg/mL were not able to kill all of the isolates. There was a major difference between MBC of each of the antibiotics and melittin (p value < 0.05)
Comparing the MIC and MBC results for melittin with other examined antibiotics showed that melittin had an eight and fourfold greater inhibitory and killing activities than doripenem, respectively. These values for ceftazidime were 32- and 16-fold whereas for colistin was fourfold for both of MIC and MBC.
The Kinetics of Biofilm Formation
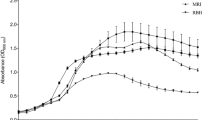
The highest OD for all examined isolates was showed after 24 h that ranged between 0.85 and 2. Biofilm formation was significantly time dependent up to 18 h in all isolates and ATCC strain (R2 = 0.92–0.99) as determined by linear regression assay. Slope of biofilm formation was rapidly elevated between 18 and 24 h followed by a remarkable decrease at 48 h (Fig. 4).
The kinetics of biofilm formation. The maximum OD was seen at 24 h in all examined bacteria. This data showed that the biofilm biomasses maturated at 24 h. Biofilm formation was significantly time dependent up to 18 h in all isolates and ATCC strain (R2 = 0.92–0.99) as determined by linear regression assay. Slope of biofilm formation was rapidly elevated between 18 and 24 h followed by a remarkable decrease at 48 h
The Kinetics of Bacterial Multiplication in Biofilm Environment
During biofilm formation at a determined time point, the numbers of the bacteria were counted. The results indicated that the slope of growth is slow but occurred in a time dependent manner up to 24 h. The numbers of bacteria were decreased from 24 to 48 h (Fig. 5).
The kinetics of bacterial multiplication during biofilm formation. The results indicated an increase in the number of bacteria in a gradual manner up to 24 h. Along with the kinetics study of biofilm formation, the numbers of bacteria were counted to determine the velocity of bacterial growth within the biofilm layers. The results showed a time dependent multiplication up to 24 h. The geometric mean of total count of bacteria was at the log of 7.65 after 24 h
Biofilm Degradation Kinetics of Melittin
Different ascending amounts of melittin from 0.06 up to 50 µg were prepared and added to pre-formed biofilm to evaluate the removal activity of melittin in a kinetics trend. The results showed that all the examined doses had removal activity in a time dependent manner. The amount of 50 µg had the greatest activity to degrade the biofilm layer in comparison to other doses (Fig. 6).
Killing Kinetics Assay of Melittin
Melittin at 50 µg reduced the amount of MDR and ATCC bacteria by a rate of 6 logs after 24 h incubation and eradicated all of them after 48 h (Figs. 6, 7).
Killing kinetic curve for the ability of melittin to kill the examined bacteria within the biofilm. The results showed eradication of all bacteria after 48 h. The results indicated that melittin at 50 µg completely eradicated all bacteria after 48 h. This issue indicated that melittin maintains its stability along this duration, penetrates to biofilm layers gradually, and then induces its killing activities
Scanning Electron Microscopy
Field emission-scanning electron microscopy was used to examine the biofilm removal activity of melittin. The results demonstrated that melittin at the amount of 10 µg started its biofilm degradation activity so that no biofilm layer was seen on the surface of slides. During this time, melittin could affect the bacterial membrane and cell shape as vesicle and squeezing, respectively. After 60 min, these effects were seen more and more and many of the bacteria disintegrated (Fig. 8).
The morphology of P. aeruginosa biofilm was investigated after 30 min treatment with 10 µg melittin by scanning electron microscopy. Analysis of FE-SEM results showed that melittin not only has a significant biofilm degradation activity for removal of biofilm layers but is also able to kill them via invading their membranes. Visualizing many vesicles on bacterial membranes and subsequent death demonstrates this mechanism. A1 and A2 control, B1 and B2 Biofilm formation, C1 and C2 P. aeruginosa biofilm treated with melittin for 30 and 60 min respectively. B biofilm, BD biofilm degradation, lys lysis, v vesicle, sq squeezing
Discussion
Our current understanding of the players of bacterial biofilm formation has being increased (Batoni et al. 2016) but there is still a great challenge in the development of anti-biofilm drugs. To date, no anti-biofilm drug has been registered for clinical trial yet. Penetration of antimicrobial agents into biofilm layers is a hard challenge (Percival et al. 2011) besides the issue of antibiotic resistance. Another issue for bacterial resistance within biofilm is the slow or inactivated metabolism in persister cells (Wood 2016). These have made the treatment of biofilm-related infections very problematic. In this condition, the need for a new promising anti-biofilm agent is urgently necessitated. In this regard, we tried to examine the anti-biofilm activity of melittin, a potent natural AMP.
The frequency distribution of resistant isolates against the examined antibiotic disks showed that 18.8% of isolates were resistant to nine conventional antibiotics. This issue could lead to devastating outcomes in the burn center that the isolates were collected from, in terms of the potential outbreak of XDR bacteria.
The advent of XDR strains in a burn center indicated that an outbreak is being developed. In this condition, treatment of these infections by an alternative antibiotic is very critical to inhibit the spread of XDR strains.
Based on the results, 20% of the isolates were resistant to doripenem. As recently documented, doripenem is a newly presented antibiotic amongst the carbapenem family. The moderate frequency of resistance to this antibiotic is a disappointing issue regarding its future application.
Reference to the results obtained from the MIC and MBC assays, melittin had a more inhibitory and lethal activity against the clinical isolates in comparison to colistin, doripenem, and ceftazidime. According to the MIC value, the most active agents against P. aeruginosa were melittin, colistin, doripenem and ceftazidime, respectively.
Our results gathered from disk diffusion, MIC, and MBC assays on the planktonic state of clinical isolates of burn infections suggest that melittin has a precious pharmaceutical value so much so that it is a good candidate for further examination in a mouse model of third degree burn infection.
Pompilo et al. in 2012 obtained the inhibitory activity of natural antimicrobial peptide BMAP-27 and BMAP-28 and also that of an engineered peptide in 25 P. aeruginosa isolates of cystic fibrosis patients at the range of 4–16, 4–32, and 4–32 µg/mL, respectively (Pompilio et al. 2012). In comparison, melittin in our study showed greater activity ranging 0.625–10 µg/mL (0.125–2 µg).
In the study of Sánchez-Gómez et al. in 2015, the antibacterial activity of a series of peptides and lipopeptides against planktonic P. aeruginosa PAO1 were totally lower than melittin (0.625–10 µg/mL) (Sánchez-Gómez et al. 2015).
In 2015, Beringer et al. showed the MIC and MBC of Rhesus θ-defensin-1 (RTD-1) on 29 P. aeruginosa isolates of cystic fibrosis patients at 8 and 64 µg/mL (Beringer et al. 2015). In comparison, melittin had approximately the same inhibitory activity and a 3.2-fold greater killing activity against burn infection P. aeruginosa isolates.
Based on the kinetics results for biofilm formation, it could be suggested that the biofilm is being produced in a time dependent manner up to 18 h. After this time, the conditions were ideal for the bacteria to form the maximum amount of biofilm during 6 h so that at the time of 24 h the biofilm reached its maximum maturation. Obtaining this critical time point is necessary to have a precise result in the study of biofilm degradation kinetics and also killing kinetics of melittin. Interestingly, the biomass dropped significantly during the next 24 h. This reduction may be due to accumulation of acidic byproducts and subsequent physical degradation of biofilm layers. It must be noted that this degradation may not happen in clinical conditions.
Production of different biomasses in different bacteria indicates the heterogenicity in biofilm formation ability of the strains. This issue may be due to different expression profiles of the genes involved in biofilm formation. The data reproduced in this manner can improve our insight to develop the experimental design of in vivo studies. From the point of clinical approach, these results indicate that the physicians have only 18 h to start the appropriate antibiotic regimens.
Along with the kinetics study of biofilm formation, the numbers of bacteria were counted to determine the velocity of bacterial growth within the biofilm layers. The results showed a time dependent multiplication up to 24 h. The total count of bacteria was at the log of 7.8 after 24 h.
Knowing this data can be very useful in killing kinetics experiments which can basically inform us how many bacteria exist at the biofilm maturation point.
The results of biofilm degradation and killing kinetics of melittin on the examined bacteria indicated that melittin at 50 µg degraded the biofilm layer remarkably after 24 h and completely eradicated all bacteria after 48 h. This issue indicated that melittin maintains its stability along this duration, penetrates to biofilm layers gradually, and induces its degradation and killing activities.
Hirt and Gorr in 2013 showed that GL13K as an engineered peptide at the amount of 70 µmol killed 99.9% of the bacteria within pre-formed P. aeruginosa biofilm during 4 h (Hirt and Gorr 2013) whereas melittin, in our study, eradicates all examined MDR isolates at 0.017 µmol for up to 48 h. Our data indicated that melittin in comparison to GL13K is more promising for eradication of bacteria in the depth of biofilm.
Sánchez-Gómez et al. in 2015 showed that the peptides and peptoids, when tested on biofilms grown under dynamic or static conditions, did not show promising antibacterial activity. Among them, LF11-215 and LF11-227 were the most potent peptides, since, at 10× MIC, they were able to decrease more than 2 logs (i.e. > 99%) of cell viability of biofilms in just 10 min. Furthermore, both peptides caused a 10,000-fold (4 logs) reduction in biofilm cell viability after 1 h of treatment at 10× MIC (Sánchez-Gómez et al. 2015). In comparison to our results, melittin at its MIC dose completely degraded the biofilm in MDR isolates for 6–24 h and also fully eradicated the examined bacteria after 48 h. These comparisons indicate that the stability of the examined peptides and peptoids were being decreased over time whereas melittin induced its activity gradually by maintaining its stability.
Beringer et al. in 2015 showed a modest killing activity of RTD-1 as an engineered peptide on pre-formed biofilm containing P. aeruginosa PAO1 strain (Beringer et al. 2015) whereas melittin completely killed the MDR isolates in a time dependent manner. The maximum effect of RTD-1 on decreasing biofilm abundance was obtained at an average of 74.2% whereas melittin gradually degraded the biofilm biomass during 24 h.
Pulido et al. in 2016 examined the anti-biofilm activity of RN3 (5-17P22-36), derived from RNAse 3 antimicrobial peptide, and showed 100% killing activity at 25 µm (Pulido et al. 2016) whereas melittin in our study demonstrated 100% killing activity at 0.017 µm, indicating a more significant activity than the abovementioned engineered peptide.
Melittin degraded about 90–95% of biofilm biomass at 25 MIC (50 µg) during 24 h while Mohamed et al. in 2017 showed that RR-4, an engineered AMP derived from LL-37, reduced 61% of biofilm mass at 32 MIC (Mohamed et al. 2017).
Analysis of FE-SEM results showed that melittin not only has a significant biofilm degradation activity for removal of biofilm layers but is also able to kill them via invading their membranes. Visualizing many vesicles on bacterial membranes and subsequent death demonstrates this mechanism.
Beringer et al. in 2015 demonstrated the effect of RTD-1 on the P. aeruginosa PA01 strain as aggregation. RTD-1 failed to kill them during 48 h, shown by SEM (Beringer et al. 2015) while melittin not only degrades the biofilm layer but can induce disintegration of the MDR bacteria, proved by FE-SEM demonstrations.
To the best of our knowledge, in reference to our thorough review on documented papers about natural anti-biofilm or engineered AMPs, there are no promising AMPs which can completely degrade the pre-established biofilm and/or eradicate the bacteria within the biofilm milieu (Batoni et al. 2016; Shang et al. 2017; Mishra and Wang 2017; Grassi et al. 2017; Thamri et al. 2017; Schillaci et al. 2014).
In the serious case of antibiotic resistance, the ability of some bacterial pathogens to produce biofilm aggravates this issue. Recently, AMPs have been made in hopes of not only overcoming the issue of MDR bacteria but dispersing the pre-formed biofilm or killing the internal bacteria.
To eradication of biofilm associated infections, both of biofilm degradation and killing activities are basically needed. A candidate antibiofilm agent must have a complete bactericidal activity against MDR bacteria. Since, melittin had these ideal advantages, this issue encouraged us to examine the in vivo efficacy of it as a topical antimicrobial agent in a mouse model of third degree burn infection in our future studies. Toxicity of melittin on normal cells is a disadvantage for future application of melittin as a drug but it cannot an important issue regarding to its therapeutic effects on third degree burn infections. This suggestion is originated from this fact that in this type of burn, all three layers of skin are destroyed so that the toxicity of melittin is not a limitation factor to its topical application. LL-37, for instance, is a natural toxic human peptide but it has been entered in phase II clinical trials as a topical agent to treatment of leg ulcers (Felício et al. 2017).
In conclusion, melittin successfully degraded all of pre-established biofilm and also eradicated all of P. aeruginosa bacteria within the biofilm in a concentration and time dependent manner. Altogether, pharmaceutical value of melittin, as a drug lead, could be examined in a mouse model of burn infection as a topical antimicrobial and anti-biofilm agent.
References
Aloush V, Navon-Venezia S, Seigman-Igra Y, Cabili S, Carmeli Y (2006) Multidrug-resistant Pseudomonas aeruginosa: risk factors and clinical impact. Antimicrob Agents Chemother 50(1):43–8
Balcázar JL, Subirats J, Borrego CM (2015) The role of biofilms as environmental reservoirs of antibiotic resistance. Front Microbiol 6:1216
Batoni G, Maisetta G, Esin S (2016) Antimicrobial peptides and their interaction with biofilms of medically relevant bacteria. Biochim Biophys Acta (BBA) Biomembr 1858(5):1044–1060
Beringer PM, Bensman TJ, Ho H, Agnello M, Denovel N, Nguyen A et al (2015) Rhesus θ-defensin-1 (RTD-1) exhibits in vitro and in vivo activity against cystic fibrosis strains of Pseudomonas aeruginosa. J Antimicrob Chemother 71(1):181–188
Chung PY, Khanum R (2017) Antimicrobial peptides as potential anti-biofilm agents against multidrug-resistant bacteria. J Microbiol Immunol Infect 50(4):405–410
Clinical and Laboratory Standards Institute guideline (2015) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard-10th Clinical and Laboratory Standards Institute CLSI guideline. M07-A10
Costerton JW, Stewart PS, Greenberg EP (1999) Bacterial biofilms: a common cause of persistent infections. Science 284(5418):1318–1322
da Mata ÉCG, Mourão CBF, Rangel M, Schwartz EF (2017) Antiviral activity of animal venom peptides and related compounds. J Venom Anim Toxins incl Trop Dis 23(1):3
Davies J, Davies D (2010) Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev 74(3):417–433
de la Fuente-Núñez C, Reffuveille F, Fernández L, Hancock RE (2013) Bacterial biofilm development as a multicellular adaptation: antibiotic resistance and new therapeutic strategies. Curr Opin Microbiol 16(5):580–589
Dean SN, Bishop BM, Van Hoek ML (2011) Susceptibility of Pseudomonas aeruginosa biofilm to alpha-helical peptides: d-enantiomer of LL-37. Front Microbiol 2:128
Diamond G, Beckloff N, Weinberg A, Kisich KO (2009) The roles of antimicrobial peptides in innate host defense. Curr Pharm Des 15(21):2377–2392
DiMuzio EE, Healy DP, Durkee P, Neely AN, Kagan RJ (2014) Trends in bacterial wound isolates and antimicrobial susceptibility in a pediatric burn hospital. J Burn Care Res 35(5):e304-e11
El-Azizi M, Farag N, Khardori N (2015) Antifungal activity of amphotericin B and voriconazole against the biofilms and biofilm-dispersed cells of Candida albicans employing a newly developed in vitro pharmacokinetic model. Ann Clin Microbiol Antimicrob 14(1):21
Falagas ME, Koletsi PK, Bliziotis IA (2006) The diversity of definitions of multidrug-resistant (MDR) and pandrug-resistant (PDR) Acinetobacter baumannii and Pseudomonas aeruginosa. J Med Microbiol 55(12):1619–1629
Felício MR, Silva ON, Gonçalves S, Santos NC, Franco OL (2017) Peptides with dual antimicrobial and anticancer activities. Front Chem 5:5
Gaspar D, Veiga AS, Castanho MA (2013) From antimicrobial to anticancer peptides. A review. Front Microbiol 4:294
Grassi L, Maisetta G, Maccari G, Esin S, Batoni G (2017) Analogs of the frog-skin antimicrobial peptide temporin 1 Tb exhibit a wider spectrum of activity and a stronger antibiofilm potential as compared to the parental peptide. Front Chem 5:24
Hachem RY, Chemaly RF, Ahmar CA, Jiang Y, Boktour MR, Rjaili GA et al (2007) Colistin is effective in treatment of infections caused by multidrug-resistant Pseudomonas aeruginosa in cancer patients. Antimicrob Agents Chemother 51(6):1905–1911
Hirsch EB, Tam VH (2010) Impact of multidrug-resistant Pseudomonas aeruginosa infection on patient outcomes. Expert Rev Pharmacoecon Outcomes Res 10(4):441–451
Hirt H, Gorr S-U (2013) Antimicrobial peptide GL13K is effective in reducing biofilms of Pseudomonas aeruginosa. Antimicrob Agents Chemother 57(10):4903–4910
Høiby N, Frederiksen B, Pressler T (2005) Eradication of early Pseudomonas aeruginosa infection. J Cyst Fibros 4:49–54
Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O (2010) Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents 35(4):322–332
Ishida H, Ishida Y, Kurosaka Y, Otani T, Sato K, Kobayashi H (1998) In vitro and in vivo activities of levofloxacin against biofilm-producing Pseudomonas aeruginosa. Antimicrob Agents Chemother 42(7):1641–1645
Jindal M, Le C, Mohd Yusof M, Sekaran S (2014) Net charge, hydrophobicity and specific amino acids contribute to the activity of antimicrobial peptides. J Health Transl Med 17(1)
Klockgether J, Tümmler B (2017) Recent advances in understanding Pseudomonas aeruginosa as a pathogen. F1000Research 6:1261
Lee M-T, Sun T-L, Hung W-C, Huang HW (2013) Process of inducing pores in membranes by melittin. Proc Natl Acad Sci USA 110(35):14243–14248
Limoli DH, Jones CJ, Wozniak DJ (2015) Bacterial extracellular polysaccharides in biofilm formation and function. Microbiol Spectr. https://doi.org/10.1128/microbiolspec.MB-0011-2014
Mandal SM, Roy A, Ghosh AK, Hazra TK, Basak A, Franco OL (2014) Challenges and future prospects of antibiotic therapy: from peptides to phages utilization. Front Pharmacol 5:105
Mishra B, Wang G (2017) Individual and combined effects of engineered peptides and antibiotics on Pseudomonas aeruginosa biofilms. Pharmaceuticals 10(3):58
Mohamed MF, Brezden A, Mohammad H, Chmielewski J, Seleem MN (2017) A short d-enantiomeric antimicrobial peptide with potent immunomodulatory and antibiofilm activity against multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Sci Rep. https://doi.org/10.1038/s41598-017-07440-0
Oliver A, Levin BR, Juan C, Baquero F, Blázquez J (2004) Hypermutation and the preexistence of antibiotic-resistant Pseudomonas aeruginosa mutants: implications for susceptibility testing and treatment of chronic infections. Antimicrob Agents Chemother 48(11):4226–4233
Percival SL, Hill KE, Malic S, Thomas DW, Williams DW (2011) Antimicrobial tolerance and the significance of persister cells in recalcitrant chronic wound biofilms. Wound Repair regen 19(1):1–9
Percival SL, McCarty SM, Lipsky B (2015) Biofilms and wounds: an overview of the evidence. Adv Wound Care 4(7):373–381
Pompilio A, Crocetta V, Scocchi M, Pomponio S, Di Vincenzo V, Mardirossian M et al (2012) Potential novel therapeutic strategies in cystic fibrosis: antimicrobial and anti-biofilm activity of natural and designed α-helical peptides against Staphylococcus aureus, Pseudomonas aeruginosa, and Stenotrophomonas maltophilia. BMC Microbiol 12(1):145
Pulido D, Prats-Ejarque G, Villalba C, Albacar M, González-López JJ, Torrent M et al (2016) A novel RNase 3/ECP peptide for Pseudomonas aeruginosa biofilm eradication that combines antimicrobial, lipopolysaccharide binding, and cell-agglutinating activities. Antimicrob Agents Chemother 60(10):6313–6325
Sánchez-Gómez S, Ferrer-Espada R, Stewart PS, Pitts B, Lohner K, de Tejada GM (2015) Antimicrobial activity of synthetic cationic peptides and lipopeptides derived from human lactoferricin against Pseudomonas aeruginosa planktonic cultures and biofilms. BMC Microbiol 15(1):137
Schillaci D, Cusimano MG, Spinello A, Barone G, Russo D, Vitale M et al (2014) Paracentrin 1, a synthetic antimicrobial peptide from the sea-urchin Paracentrotus lividus, interferes with staphylococcal and Pseudomonas aeruginosa biofilm formation. AMB Express 4(1):78
Segev-Zarko L-a, Saar-Dover R, Brumfeld V, Mangoni ML, Shai Y (2015) Mechanisms of biofilm inhibition and degradation by antimicrobial peptides. Biochem J 468(2):259–270
Shang D, Meng X, Zhang D, Kou Z (2017) Antibacterial activity of chensinin-1b, a peptide with a random coil conformation, against multiple-drug-resistant Pseudomonas aeruginosa. Biochem Pharmacol 143:65–78
Terwilliger TC, Eisenberg D (1982) The structure of melittin. II. Interpretation of the structure. J Biol Chem 257(11):6016–6022
Thamri A, Létourneau M, Djoboulian A, Chatenet D, Déziel E, Castonguay A et al (2017) Peptide modification results in the formation of a dimer with a 60-fold enhanced antimicrobial activity. PLoS ONE 12(3):e0173783
Turner KH, Everett J, Trivedi U, Rumbaugh KP, Whiteley M (2014) Requirements for Pseudomonas aeruginosa acute burn and chronic surgical wound infection. PLoS Genet 10(7):e1004518
Ventola CL (2015) The antibiotic resistance crisis: part 1: causes and threats. Pharm Ther 40(4):277
Verstraeten N, Braeken K, Debkumari B, Fauvart M, Fransaer J, Vermant J et al (2008) Living on a surface: swarming and biofilm formation. Trends Microbiol 16(10):496–506
Wood TK (2016) Combatting bacterial persister cells. Biotechnol Bioeng 113(3):476–483
Wood TK (2017) Strategies for combating persister cell and biofilm infections. Microb Biotechnol 10(5):1054–1056
Acknowledgements
This investigation is a part of the Ph.D. thesis of Reyhaneh Shams Khozani, approved by Faculty of Sciences, Karaj Branch, Islamic Azad University, Karaj, Iran and Pasteur Institute of Iran, Tehran-Iran.
Author information
Authors and Affiliations
Contributions
RSK performed all experiments and also contributed in writing the manuscript. DS, NH, and MMF contributed as advisor. KPB contributed in experimental design, writing and redaction of the manuscript and also supervised the project. The idea for application of melittin in removing the P. aeruginosa associated biofilm and killing the embedded bacteria belongs to the corresponding author, KPB.
Corresponding author
Ethics declarations
Conflict of interest
Reyhaneh Shams Khozani, Delavar Shahbazzadeh, Naser Harzandi, Mohammad Mehdi Feizabadi, and Kamran Pooshang Bagheri declare that they have no conflict of interest.
Research involving Human and Animal Participants
This article does not contain studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Shams Khozani, R., Shahbazzadeh, D., Harzandi, N. et al. Kinetics Study of Antimicrobial Peptide, Melittin, in Simultaneous Biofilm Degradation and Eradication of Potent Biofilm Producing MDR Pseudomonas aeruginosa Isolates. Int J Pept Res Ther 25, 329–338 (2019). https://doi.org/10.1007/s10989-018-9675-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-018-9675-z