Abstract
Coral reef ecosystems have great economic, social, and ecological value. The ecological success of coral reef ecosystems critically depends on coral-algal symbiosis and coral-prokaryotic partnership. However, seasonal changes underlying these relationships in subtropical hard corals of Hong Kong are poorly studied. Here, we compared the community changes of algal symbionts and prokaryotic partners in Platygyra carnosa and Galaxea fascicularis from Hong Kong collected at two seasonal time points of winter and summer via massively parallel sequencing of genetic markers and multivariate analysis. The results indicated that algal symbionts showed no significant changes between the two seasonal time points but prokaryotic partners changed substantially. Prokaryotic partners putatively involved in photosynthesis, nitrogen fixation, and sulfur oxidation increased significantly from winter to summer, while prokaryotic partners potentially associated with chemosynthesis, ammonia oxidation, and nitrite oxidation decreased significantly from winter to summer. Dissolved oxygen and pH served as the main contributors influencing prokaryotic partners in winter, while temperature, turbidity, and salinity played a dominant role in shaping prokaryotic partners in summer. The findings of the present study suggest that season structures prokaryotic partners but not algal symbionts in subtropical hard corals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coral reefs, one of the most biologically complex and diverse ecosystems, not only house the highest diversity of marine species but also provide essential ecological goods and services such as carbon sequestration and food production (Moberg and Folke 1999). However, the fitness of coral reefs critically relies on the holobiont complex associations among algal symbionts, prokaryotic partners (i.e., collective bacteria and archaea in corals), and scleractinian corals (Rohwer et al. 2002; Thompson et al. 2014). Algal symbionts of the genus Symbiodinium can form endosymbioses with scleractinian corals and provide the majority of coral energy budget through photosynthesis (Gordon and Leggat 2010). Nine clades (i.e., A–I) of Symbiodinium have been identified until now, among which six of them (i.e., A–D, F, and G) have been reported to be tightly associated with scleractinian corals (Arif et al. 2014). Previous studies have shown that algal symbionts in Galaxea fascicularis can change from clade C to clade D in a latitudinal gradient from subtropical to tropical regions (Tong et al. 2017) and in a temperature gradient along the tropical Hainan Island (Zhou et al. 2017), but it remains unknown whether they would change in subtropical hard corals along a seasonal gradient. Highly diverse prokaryotic partners can colonize coral mucus, tissue, and skeleton, which can benefit corals through carbon, nitrogen, and sulfur metabolism and antimicrobial defense (Krediet et al. 2013; Rädecker et al. 2015; Rosenberg et al. 2007). The communities of prokaryotic partners in corals are highly complex and dynamic, usually changing with environmental gradients (Peixoto et al. 2017). Thus, there is an increasing interest in studying the species richness, evenness, variations, and functions of prokaryotic partners in corals and their associations with the hosts under various environmental conditions.
Hong Kong sits on the northern border of the South China Sea with a subtropical climate, providing a marginal habitat for coral development. The sea surface temperature of Hong Kong usually ranges from 14 °C in winters to 31 °C in summers (Goodkin et al. 2011). Hard corals are unable to form real reefs in Hong Kong largely because sea surface temperature in winters is too cold to sustain coral growth and reef building. Nevertheless, compared to other regions with similar latitudes, Hong Kong’s coral diversity is high, with 84 species, about 10% of the world’s 845 species (Polidoro and Carpenter 2013). Hard corals develop on the shallow-water bedrock of many small islands, but there are substantial differences in coral cover and diversity along a water quality gradient created by freshwater runoff from the Pearl River (Morton 1994). Western Hong Kong is estuarine with a very low coral cover and diversity, while Eastern Hong Kong is oceanic and thus, the coral cover and diversity is very high in some protected bays (Xie et al. 2016). Hong Kong usually has two types of climates all year around, that is, cold and dry winters and hot and wet summers. The sea surface temperature usually increases or decreases sharply during the winter and summer alternations; thus, springs and falls are not typical and last for a very short time. According to the seawater temperature monitoring, we have collected coral samples at two extreme conditions after a long exposure of cold and heat, including a winter time point (i.e., the near end of winter in March) and a summer time point (i.e., the near end of summer in October).
According to the Coral Field Guide established by the Agriculture, Fisheries and Conservation Department of Hong Kong SAR Government, a dominant species Platygyra carnosa and a rare species Galaxea fascicularis were selected as the target corals for the present study, which were collected from the West and the East of Hong Kong at two seasonal time points of winter and summer, respectively. The communities of algal symbionts and prokaryotic partners in these corals were compared comprehensively between the two seasonal time points through massively parallel sequencing of genetic markers and multivariate statistical analyses. The objective of the present study was to explore potential community changes of algal symbionts and prokaryotic partners in subtropical hard corals between the two seasonal time points of winter and summer. We believe the present study will provide a new understanding of seasonal community changes of algal symbionts and prokaryotic partners in subtropical hard corals.
Materials and methods
Sample collection and fixation
In the present study, we examined a dominant species P. carnosa and a rare species G. fascicularis in Hong Kong to study seasonal community changes of algal symbionts and prokaryotic partners. P. carnosa and G. fascicularis were collected at Lamma Island (E 114.135°, N 22.187°) in western Hong Kong and Crescent Bay (E 114.314°, N 22.531°) in eastern Hong Kong, respectively. Coral collection outside marine parks or marine reserve for scientific or educational study does not require a local license. Coral colonies for each site were sampled within about 10 m around and at a water depth of 3–5 m. Based on local seawater temperature monitoring, winter and summer samplings were conducted in March and October of 2014, respectively. Six colonies of each coral species (n = 6) and two nearby seawater samples (n = 2) of each site were collected at the two seasonal time points of winter and summer, which generated 24 coral samples and 8 seawater samples in total. During the sampling, small pieces of coral fragments (~ 1 cm × 1 cm) were picked from apparently healthy colonies, washed using sterilized seawater to remove those loosely attached microbes, and fixed in 70% ethanol immediately. Microbes in ~ 1 L seawater were concentrated through filtration using a 0.22-μm polycarbonate membrane and then fixed in 70% ethanol. All the fixed samples were stored in a − 30 °C refrigerator prior to DNA extraction.
DNA extraction, PCR, and sequencing
The fixed coral specimens were rinsed using 1× PBSE (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4·7H2O, 1.4 mM KH2PO4, and 10 mM EDTA) and further ground into slurry using a mortar and pestle. The generated slurry was centrifuged to collect pellets for DNA extraction. FastDNA® Spin Kit for Soil (MP Biomedicals, USA) was used to extract the total DNA following the instructions recommended by the manufacturer. The quality of DNA extracts was determined using 1% agarose gel electrophoresis before PCR. The internal transcribed spacer 2 region (ITS2) of algal rRNA genes and the hypervariable V3V4 region of prokaryotic 16S rRNA genes (16S) were used as the fingerprints to explore seasonal community changes of algal symbionts and prokaryotic partners, respectively. Two primer sets, i.e., ITSintfor2: 5′-GAATTGCAGAACTCCGTG-3′ and ITS2CLAMP: 5′-GGGATCCATATGCTTAAGTTCAGCGGGT-3′ (Arif et al. 2014; LaJeunesse and Trench 2000), 341F: 5′-CCTAYGGGRBGCASCAG-3′ and 802R: 5′-TACNVGGGTATCTAATCC-3′ (Behrendt et al. 2011; Cai et al. 2013), were used to amplify the regions of ITS2 and 16S V3V4, respectively. Nucleotide barcodes were fused to the 5′ terminus of the forward primers to conduct multiplexed sequencing of ITS2 and 16S V3V4 amplicons (Bartram et al. 2011). The PCR for ITS2 was conducted with 50 ng of DNA template, 200 nM of each primer, 25 μL of 2× Taq Platinum PCR Master (Tiangen, China), and ddH2O up to 50 μL. The amplification was performed under an initial denaturation at 94 °C for 5 min, 35 cycles of 94 °C for 30 s, 51 °C for 30 s, 72 °C for 30 s, and a final extension at 72 °C for 5 min. The PCR for 16S V3V4 was conducted with 50 ng of DNA template, 200 nM of each primer, 25 μL of 2× Premix Ex Taq solution (TaKaRa, China), and ddH2O up to 50 μL. The amplification was performed under an initial denaturation at 94 °C for 5 min, 30 cycles of 94 °C for 30 s, 50 °C for 30 s, and 72 °C for 60 s, and a final extension at 72 °C for 5 min. The PCR products were purified using PureLin® PCR Purification Kit (Invitrogen, USA). The purified PCR products were measured with a Thermo NanoDrop 2000 UV-Vis Spectrophotometer and pooled with equal mass for multiplexed sequencing of ITS2 and 16S V3V4 amplicons. The final DNA samples were submitted to Novogene Corporation (Beijing, China) for library construction and sequencing on an Illumina MiSeq sequencer with a paired-end mode of 300 bp × 2. The raw ITS2 and 16S V3V4 sequencing datasets have been deposited in the NCBI Sequence Read Archive under accession numbers SRP066283 and SRP066229, respectively.
Data processing and bioinformatic analysis
The sequencing adaptors and low-quality reads (accounting for ~ 5%) were first removed from the raw data. The PEAR (paired-end read merger) tool (Zhang et al. 2014) was used to merge the paired-end reads into the full-length sequences of ITS2 and 16S V3V4. The QIIME platform (Caporaso et al. 2010) was employed to demultiplex ITS2 and 16S V3V4 sequences into each sample through barcode identification. Potential chimeras generated by the PCR were detected by ChimeraSlayer (Haas et al. 2011) and removed under the QIIME platform. After quality filtering of low-quality and chimeric sequences, there were 1,591–45,053 clean ITS2 sequences and 1,715–41,943 clean 16S V3V4 sequences for each sample, which were recovered and normalized using the lowest number to obtain equal sequencing depth for further bioinformatic analysis. Six ITS2 datasets (Tong et al. 2017) and six 16S datasets (Cai et al. 2018) derived from the winter G. fascicularis samples have been previously used for algal and microbial community analyses across different regions in the South China Sea, respectively. BLAST analysis for ITS2 sequences and annotation at subclade level for algal symbionts followed a published pipeline, i.e., search against the constructed non-redundant ITS2 database and annotation using a cutoff of 97% sequence similarity (Tong et al. 2017). For prokaryotic partners, operational taxonomic units (OTUs) were clustered at 97% similarity and taxonomically annotated under the QIIME platform using default settings. Taxonomic compositions including phylum, genus, and 97% OTU were generated for plotting and multivariate statistical analyses.
The t test, permutational multivariate analysis of variance (PERMANOVA), principal coordinates analysis (PCoA), and canonical correspondence analysis (CCA) were conducted in PAST 3.14 (Hammer et al. 2001). Stacked bar charts and box charts were all plotted in OriginPro 9.0. The R Project for Statistical Computing (https://www.r-project.org/) was employed for heat map plotting to visualize seasonal changes of the 100 most abundant genera of prokaryotic partners. Functional prokaryotic partners were searched taxonomically to identify potential photoautotrophs, chemoautotrophs, nitrogen-fixing bacteria (NFB), ammonia-oxidizing archaea (AOA), ammonia-oxidizing bacteria (AOB), nitrite-oxidizing bacteria (NOB), nitrate-reducing bacteria (NRB), sulfur-oxidizing bacteria (SOB), sulfate-reducing bacteria (SRB), dimethylsulfoniopropionate-degrading bacteria (DMSP-DB), and dimethylsulfide-degrading bacteria (DMS-DB), respectively. PICRUSt software (Langille et al. 2013) using 16S sequences was further employed to validate the functional prokaryotic partners identified taxonomically. Environmental data for CCA ordination were downloaded from the database of Environmental Protection Department of the Hong Kong SAR Government (http://epic.epd.gov.hk/EPICRIVER/marine/) including temperature, salinity, dissolved oxygen, turbidity, pH, suspended solids, volatile suspended solids, 5-day biochemical oxygen demand, ammonia nitrogen, nitrite nitrogen, nitrate nitrogen, total inorganic nitrogen, total Kjeldahl nitrogen, total nitrogen, orthophosphate phosphorus, and chlorophyll-a. The seawater parameters of the monitoring stations SM3, SM5, SM6, SM18, and SM19 around the sampling site Lamma Island and the monitoring stations MM3, MM4, MM5, MM7, and MM17 near the sampling site Crescent Bay were downloaded and averaged for each sampling location and each sampling month, respectively.
Results
No seasonal community changes of algal symbionts
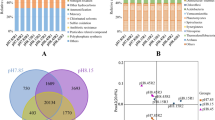
The compositions of algal symbionts in subtropical hard corals of Hong Kong were shown in Fig. 1a. C2r was the most dominant subclade for P. carnosa and G. fascicularis at the two seasonal time points of winter and summer, with a mean relative abundance of 91.54%. C161, C3d/C21, and C116 were the minor subclades, with a mean relative abundance of 2.50, 1.09, and 0.78%, respectively. Subclade D17 was abundant in only one winter colony of P. carnosa. Subclades C163b, C1p/C1.8, C1c/C45, and C1ca/C1b/C1e became more abundant from the winter time point to the summer time point, which were observed in four summer colonies of G. fascicularis. Even though one P. carnosa colony and four G. fascicularis colonies showed some minor differences in relative abundance for several subclades, their main subclades in algal community structure remained rather stable. The profiles of the other 19 colonies of both coral species from both seasonal time points were very similar (Fig. 1a). Symbiotic algal community structures for the samples studied were rather stable, showing no significant changes between the two coral species and between the two seasonal time points (PERMANOVA, P > 0.05).
Seasonal community changes of prokaryotic partners
The compositions of coral prokaryotic partners exhibited significant seasonal changes between the two seasonal time points of winter and summer at phylum level (PERMANOVA, P < 0.05). As shown in Fig. 1b, Proteobacteria was the most dominant phylum for both coral species at both seasonal time points, which is consistent with related studies (Lee et al. 2012; Lema et al. 2014; Li et al. 2014; Pantos et al. 2015). However, a P. carnosa colony dominated by Chlorobi and a G. fascicularis colony dominated by Chloroflexi were found, both of which were collected at the summer time point. As shown in Fig. 2, the relative abundance of the phyla Chlorobi and Actinobacteria increased significantly from the winter time point to the summer time point (t test, P < 0.05). While the relative abundance of the following phyla Bacteroidetes, Thaumarchaeota, Nitrospirae, Gemmatimonadetes, Planctomycetes, Verrucomicrobia, Fusobacteria, and Deferribacteres decreased significantly from the winter time point to the summer time point (t test, P < 0.05). The relative abundance of the phyla Proteobacteria, Cyanobacteria, Chloroflexi, Firmicutes, and Acidobacteria showed no significant changes between the two seasonal time points (t test, P > 0.05). Coral prokaryotic partners also exhibited seasonal community changes at species level, as revealed by the PCoA ordination using 97% OTU data (Fig. 3). There were four clumped dispersion patterns for prokaryotic communities including winter coral samples, winter seawater samples, summer coral samples, and summer seawater samples. However, prokaryotic communities of the two coral species at the two seasonal time points were not dispersed. In summary, significant community changes in prokaryotic partners were observed at both phylum and species levels between the two seasonal time points of winter and summer.
A box and whisker plot illustrating seasonal community changes in coral prokaryotic partners at phylum level. Blue and red boxes represent coral samples of the winter time point and the summer time point, respectively. Standard error is used for plotting the box and whisker range. Filled squares located in the boxes represent the mean value. Stars indicate significant increase or decrease (t test, P < 0.05)
Distribution patterns of coral prokaryotic partners revealed by principal coordinates analysis (PCoA) using the 97% OTU data. Clumped dispersion patterns were observed, which were dispersed by season, coral, and seawater. PC1 mainly explains seasonal community changes and PC2 mainly explains changes between coral and seawater. No dispersion patterns are shown between P. carnosa and G. fascicularis at the each time point of winter and summer. Shading indicates the four clumped dispersion patterns
Seasonal community changes of functional prokaryotic partners
Seasonal changes of the 100 most abundant prokaryotic partners are shown at genus level in a heat map (Fig. 4). Relative abundance of some genera decreased from the winter time point to the summer time point, especially for those genera located in the upper part of the heat map, while the others increased from the winter time point to the summer time point, for example, those genera distributed in the lower part of the heat map. The overall patterns for the two coral species were highly similar at each seasonal time point, which was consistent with the PCoA result. As marked in Fig. 4, a total of 39 functional prokaryotic partners were identified, including 14 photoautotrophs, 5 chemoautotrophs, 7 NFB, 1 AOA, 2 AOB, 2 NOB, 15 NRB, 1 SOB, 2 SRB, 8 DMSP-DB, and 5 DMS-DB, respectively. However, some of them might have multiple functions such as Prosthecochloris, capable of performing nitrogen fixation, sulfur oxidation, and photosynthesis (Cai et al. 2017). These functional prokaryotic partners were summarized into ten ecological functions including photosynthesis, chemosynthesis, nitrogen fixation, ammonia oxidation, nitrite oxidation, nitrate reduction, sulfur oxidation, sulfate reduction, DMSP degradation, and DMS degradation (Fig. 5). For both coral species, the relative abundance of the prokaryotic partners putatively involved in photosynthesis, nitrogen fixation, and sulfur oxidation increased significantly from the winter time point to the summer time point (t test, P < 0.05), while the relative abundance of the prokaryotic partners potentially associated with chemosynthesis, ammonia oxidation, and nitrite oxidation decreased significantly (t test, P < 0.05). The relative abundance of the prokaryotic partners possibly related to nitrate reduction increased significantly for G. fascicularis (t test, P < 0.05) but not for P. carnosa (t test, P > 0.05). The relative abundance for the prokaryotic partners with other putative ecological functions including DMSP degradation, DMS degradation, and sulfate reduction showed no significant changes between the two seasonal time points of winter and summer for both coral species (t test, P > 0.05).
A heat map illustrating seasonal community changes in coral prokaryotic partners at genus level. The 100 most abundant genera were analyzed and the lowest taxa were annotated for potential unclassified genera. Flammeovirgaceae_f, Rhizobiales_o, and Deltaproteobacteria_c indicate unclassified genera that are affiliated with the family Flammeovirgaceae, the order Rhizobiales, and the class Deltaproteobacteria, respectively. Abbreviations NFB, AOA, AOB, NOB, NRB, SOB, SRB, DMSP-DB, and DMS-DB represent nitrogen-fixing bacteria, ammonia-oxidizing archaea, ammonia-oxidizing bacteria, nitrite-oxidizing bacteria, nitrate-reducing bacteria, sulfur-oxidizing bacteria, sulfate-reducing bacteria, dimethylsulfoniopropionate-degrading bacteria, and dimethylsulfide-degrading bacteria, respectively. Potentially functional prokaryotic partners participating carbon, nitrogen, and sulfur metabolism are shown in green, blue, and red fonts, respectively. The color scale ND (not detected), 0.1, 1, and 10% indicate the relative abundance
Community changes in putative functional prokaryotic partners between the two seasonal time points of winter and summer. Ten important ecological functions are summarized, including photosynthesis (photoautotroph), chemosynthesis (chemoautotroph), nitrogen fixation (NFB), ammonia oxidation (AOA and AOB), nitrite oxidation (NOB), nitrate reduction (NRB), sulfur oxidation (SOB), sulfate reduction (SRB), DMSP degradation (DMSP-DB), and DMS degradation (DMS-DB). Blue and red boxes represent coral samples of the winter time point and the summer time point, respectively. Standard error is used for plotting the box and whisker range. Filled squares located in the boxes represent the mean value. Stars indicate significant increase or decrease (t test, P < 0.05)
Seasonal community changes of prokaryotic partners explained by environmental changes
The seasonal community changes of coral prokaryotic partners were readily observed, as presented above. The relationships between these changes and seasonal environmental gradients were further revealed by CCA ordination. As shown in Fig. 6, CCA1 axis (69.0% variation explained) interpreted much more variation than CCA2 axis (20.1% variation explained) and the other CCA axes (10.9% variation explained), which seemed to exhibit a seasonal changing pattern along the two sides of the CCA1 axis from the origin. Among all environmental factors analyzed, dissolved oxygen, pH, temperature, turbidity, and salinity exhibited a longer length and a smaller angle with the CCA1 axis (the most percent of variation explained) at the same time; thus, they served as the main contributors shaping the community changes of prokaryotic partners between the two seasonal time points of winter and summer. The communities of prokaryotic partners at the winter time point were positively related with dissolved oxygen and pH, while negatively related with temperature, turbidity, and salinity. In contrast, the communities of prokaryotic partners at the summer time point were positively related with temperature, turbidity, and salinity, while negatively related with dissolved oxygen and pH. The other environmental factors showed some minor impacts on the seasonal community changes of prokaryotic partners with different levels. In summary, dissolved oxygen and pH served as the main environmental factors influencing the communities of prokaryotic partners at the winter time point, while temperature, turbidity, and salinity played a dominant role in shaping the communities of prokaryotic partners at the summer time point.
Discussion
Types and stability of algal symbionts in subtropical hard corals
In the present study, it is understandable that the communities of algal symbionts in the two studied coral species were dominated by clade C Symbiodinium. Corals hosting symbionts of clade C Symbiodinium can grow faster but lack thermal tolerance (Stat and Gates 2011). Certain algal symbionts from clade D Symbiodinium are considered to strengthen the heat tolerance of the coral hosts (D'Angelo et al. 2015), but they can decrease the rate of carbon dioxide fixation and even limit the translocation of photosynthates to the host for calcification (Cantin et al. 2009; Pettay et al. 2015). Thus, scleractinian corals in subtropical regions are more likely to form symbiosis with clade C Symbiodinium, which is consistent with our results and previous findings (Tong et al. 2017; Wong et al. 2016). However, it can be assumed that there would be certain coral species from subtropical regions mainly hosting clade D Symbiodinium if they require a higher heat tolerance and rely on clade D Symbiodinium for survival. For example, Oulastrea crispata is the only species mainly hosting clade D Symbiodinium within the 14 coral species surveyed in Hong Kong (Wong et al. 2016). Although clade C Symbiodinium hosted by G. fascicularis was reported to shuffle to clade D from a subtropical region to a tropical region (Tong et al. 2017), the present study demonstrates the stability of clade C Symbiodinium between the two seasonal time points of winter and summer in the subtropical region. Taken together, it can be concluded that clade C Symbiodinium is generally hosted by subtropical hard corals of Hong Kong and forms a relatively stable symbiosis with corals across seasons.
Comparisons for compositions of prokaryotic partners in corals and seawater
It is commonly found that the community structures of coral prokaryotic partners were quite different from the seawater and changed from a time point of a season to another, as demonstrated in the present and previous studies (Chen et al. 2011; Lee et al. 2012; Lema et al. 2014; Li et al. 2014; Sharp et al. 2017). However, it is believed that coral prokaryotic partners are derived from the seawater and form specific associations with the hosts. The community structures of prokaryotic partners at the same seasonal time points were found to be similar between the two coral species collected from two different sites but they were different between the two seasonal time points, as shown in Fig. 3. This is understandable because both coral species might host certain similar prokaryotic partners and change simultaneously from a seasonal time point to another. For example, the relative abundance of Prosthecochloris in both coral species was extremely low at the winter time point but was very high at the summer time point (Fig. 4). These types of prokaryotic partners might play a main role in driving the PCoA ordination.
Changes of prokaryotic partners linked to seasonal environmental variables
Compared to the stable compositions of algal symbionts, prokaryotic partners varied significantly between the two seasonal time points of winter and summer, suggesting a seasonally changeable coral-prokaryotic partnership. This is not strange because more and more studies have demonstrated the seasonal/temporal variations of the communities of prokaryotic partners in different corals from different regions (Chen et al. 2011; Lema et al. 2014; Li et al. 2014; Sharp et al. 2017; Yang et al. 2017; Zhang et al. 2016). Our findings revealed that the changes of certain prokaryotic partners also conformed to the seasonal features. For example, the photosynthetic prokaryotic partners increased significantly in relative abundance at the summer time point possibly due to the increased light intensity and temperature, while the increased dissolved oxygen at the winter time point might drive the significant increase in relative abundance of the chemosynthetic prokaryotic partners because of dependency on oxygen for ammonia and nitrite oxidation. Both types of these autotrophic prokaryotic partners might be beneficial to coral hosts because they might provide fixed carbon compounds to the hosts like algal symbionts. Interestingly, the relative abundance of the photoautotrophic and chemoautotrophic prokaryotic partners changed reversely between the two seasonal time points of winter and summer. Thus, further investigations are needed to reveal their relatedness and associations with the coral hosts in different seasons. It has been reported that scleractinian corals can survive in oligotrophic ocean environments with limited nutrients, especially for nitrogen sources (Rädecker et al. 2015). We found that the relative abundance of prokaryotic partners involved in nitrogen fixation increased significantly from the winter time point to the summer time point. This might be important for the coral hosts because they can provide biologically available nitrogenous nutrients to fulfill the large holobiont requirements in summer.
It is a limitation that the present study only collected coral samples at two time points of winter and summer within a year. More time point sampling in the following years would provide more data to support our findings and unveil how these changes of prokaryotic partners happen in subtropical hard corals. In the present study, we employed massively parallel sequencing of ITS2 and 16S V3V4 amplicons to study seasonal community changes of algal symbionts and prokaryotic partners in subtropical scleractinian corals of Hong Kong. The algal symbionts were stable between the two time points of winter and summer, while the prokaryotic partners changed significantly. Prokaryotic partners putatively involved in photosynthesis, nitrogen fixation, and sulfur oxidation increased significantly from the winter time point to the summer time point, while prokaryotic partners potentially related to chemosynthesis, ammonia oxidation, and nitrite oxidation decreased significantly from the winter time point to the summer time point. Dissolved oxygen and pH served as the main environmental factors influencing the communities of prokaryotic partners at the winter time point, while temperature, turbidity, and salinity played a dominant role in shaping the communities of prokaryotic partners at the summer time point. In short, seasonal environmental gradients can structure coral prokaryotic partners but not algal symbionts. The present study gives a basic understanding of how algal symbionts and prokaryotic partners would change between the two seasonal time points of winter and summer in subtropical hard corals and how environmental variables would shape such changes.
References
Arif C, Daniels C, Bayer T, Banguera-Hinestroza E, Barbrook A, Howe CJ, LaJeunesse TC, Voolstra CR (2014) Assessing Symbiodinium diversity in scleractinian corals via next-generation sequencing-based genotyping of the ITS2 rDNA region. Mol Ecol 23:4418–4433. https://doi.org/10.1111/mec.12869
Bartram AK, Lynch MD, Stearns JC, Moreno-Hagelsieb G, Neufeld JD (2011) Generation of multimillion-sequence 16S rRNA gene libraries from complex microbial communities by assembling paired-end illumina reads. Appl Environ Microbiol 77:3846–3852. https://doi.org/10.1128/AEM.02772-10
Behrendt L, Larkum AW, Norman A, Qvortrup K, Chen M, Ralph P, Sorensen SJ, Trampe E, Kuhl M (2011) Endolithic chlorophyll d-containing phototrophs. ISME J 5(6):1072–1076. https://doi.org/10.1038/ismej.2010.195
Cai L, Tian RM, Zhou G, Tong H, Wong YH, Zhang W, Chui APY, Xie JY, Qiu JW, Ang PO, Liu S, Huang H, Qian PY (2018) Exploring coral microbiome assemblages in the South China Sea. Sci Rep 8:2428. https://doi.org/10.1038/s41598-018-20515-w
Cai L, Ye L, Tong AH, Lok S, Zhang T (2013) Biased diversity metrics revealed by bacterial 16S pyrotags derived from different primer sets. PLoS One 8:53649. https://doi.org/10.1371/journal.pone.0053649
Cai L, Zhou G, Tian RM, Tong H, Zhang W, Sun J, Ding W, Wong YH, Xie JY, Qiu JW, Liu S, Huang H, Qian PY (2017) Metagenomic analysis reveals a green sulfur bacterium as a potential coral symbiont. Sci Rep 7:9320. https://doi.org/10.1038/s41598-017-09032-4
Cantin NE, van Oppen MJH, Willis BL, Mieog JC, Negri AP (2009) Juvenile corals can acquire more carbon from high-performance algal symbionts. Coral Reefs 28:405–414. https://doi.org/10.1007/s00338-009-0478-8
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. https://doi.org/10.1038/nmeth.f.303
Chen CP, Tseng CH, Chen CA, Tang SL (2011) The dynamics of microbial partnerships in the coral Isopora palifera. ISME J 5:728–740. https://doi.org/10.1038/ismej.2010.151
D'Angelo C, Hume BC, Burt J, Smith EG, Achterberg EP, Wiedenmann J (2015) Local adaptation constrains the distribution potential of heat-tolerant Symbiodinium from the Persian/Arabian Gulf. ISME J 9:2551–2560. https://doi.org/10.1038/ismej.2015.80
Goodkin NF, Switzer AD, McCorry D, DeVantier L, True JD, Hughen KA, Angeline N, Yang TT (2011) Coral communities of Hong Kong: long-lived corals in a marginal reef environment. Mar Ecol Prog Ser 426:185–196. https://doi.org/10.3354/meps09019
Gordon BR, Leggat W (2010) Symbiodinium-invertebrate symbioses and the role of metabolomics. Mar Drugs 8:2546–2568. https://doi.org/10.3390/md8102546
Haas BJ, Gevers D, Earl AM, Feldgarden M, Ward DV, Giannoukos G, Ciulla D, Tabbaa D, Highlander SK, Sodergren E, Methe B, DeSantis TZ, Human Microbiome C, Petrosino JF, Knight R, Birren BW (2011) Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res 21:494–504. https://doi.org/10.1101/gr.112730.110
Hammer Ø, Harper DAT, Ryan DR (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:9pp
Krediet CJ, Ritchie KB, Paul VJ, Teplitski M (2013) Coral-associated micro-organisms and their roles in promoting coral health and thwarting diseases. P Roy Soc B-Biol Sci 280:20122328. https://doi.org/10.1098/rspb.2012.2328
LaJeunesse TC, Trench RK (2000) Biogeography of two species of Symbiodinium (Freudenthal) inhabiting the intertidal sea anemone Anthopleura elegantissima (Brandt). Biol Bull 199:126–134. https://doi.org/10.2307/1542872
Langille MGI, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Thurber RLV, Knight R, Beiko RG, Huttenhower C (2013) Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 31:814–821. https://doi.org/10.1038/nbt.2676
Lee OO, Yang JK, Bougouffa S, Wang Y, Batang Z, Tian RM, Al-Suwailem A, Qian PY (2012) Spatial and species variations in bacterial communities associated with corals from the Red Sea as revealed by pyrosequencing. Appl Environ Microbiol 78:7173–7184. https://doi.org/10.1128/AEM.01111-12
Lema KA, Willis BL, Bourne DG (2014) Amplicon pyrosequencing reveals spatial and temporal consistency in diazotroph assemblages of the Acropora millepora microbiome. Environ Microbiol 16:3345–3359. https://doi.org/10.1111/1462-2920.12366
Li J, Chen Q, Long LJ, Dong JD, Yang J, Zhang S (2014) Bacterial dynamics within the mucus, tissue and skeleton of the coral Porites lutea during different seasons. Sci Rep 4:7320. https://doi.org/10.1038/srep07320
Moberg F, Folke C (1999) Ecological goods and services of coral reef ecosystems. Ecol Econ 29:215–233. https://doi.org/10.1016/S0921-8009(99)00009-9
Morton B (1994) Hong Kong’s coral communities: status, threats and management plans. Mar Pollut Bull 29:74–83. https://doi.org/10.1016/0025-326X(94)90429-4
Pantos O, Bongaerts P, Dennis PG, Tyson GW, Hoegh-Guldberg O (2015) Habitat-specific environmental conditions primarily control the microbiomes of the coral Seriatopora hystrix. ISME J 9:1916–1927. https://doi.org/10.1038/ismej.2015.3
Peixoto RS, Rosado PM, Leite DC, Rosado AS, Bourne DG (2017) Beneficial microorganisms for corals (BMC): proposed mechanisms for coral health and resilience. Front Microbiol 8:341. https://doi.org/10.3389/fmicb.2017.00341
Pettay DT, Wham DC, Smith RT, Iglesias-Prieto R, LaJeunesse TC (2015) Microbial invasion of the Caribbean by an Indo-Pacific coral zooxanthella. Proc Natl Acad Sci U S A 112:7513–7518. https://doi.org/10.1073/pnas.1502283112
Polidoro B, Carpenter K (2013) Dynamics of coral reef recovery. Science 340:34–35. https://doi.org/10.1126/science.1236833
Rädecker N, Pogoreutz C, Voolstra CR, Wiedenmann J, Wild C (2015) Nitrogen cycling in corals: the key to understanding holobiont functioning? Trends Microbiol 23:490–497. https://doi.org/10.1016/j.tim.2015.03.008
Rohwer F, Seguritan V, Azam F, Knowlton N (2002) Diversity and distribution of coral-associated bacteria. Mar Ecol Prog Ser 243:1–10. https://doi.org/10.3354/meps243001
Rosenberg E, Koren O, Reshef L, Efrony R, Zilber-Rosenberg I (2007) The role of microorganisms in coral health, disease and evolution. Nat Rev Microbiol 5:355–362. https://doi.org/10.1038/nrmicro1635
Sharp KH, Pratte ZA, Kerwin AH, Rotjan RD, Stewart FJ (2017) Season, but not symbiont state, drives microbiome structure in the temperate coral Astrangia poculata. Microbiome 5:120. https://doi.org/10.1186/s40168-017-0329-8
Stat M, Gates RD (2011) Clade D Symbiodinium in scleractinian corals: a “nugget” of hope, a selfish opportunist, an ominous sign, or all of the above? J Mar Bio 2011:730715. https://doi.org/10.1155/2011/730715
Thompson JR, Rivera HE, Closek CJ, Medina M (2014) Microbes in the coral holobiont: partners through evolution, development, and ecological interactions. Front Cell Infect Microbiol 4:176. https://doi.org/10.3389/fcimb.2014.00176
Tong H, Cai L, Zhou G, Yuan T, Zhang W, Tian R, Huang H, Qian PY (2017) Temperature shapes coral-algal symbiosis in the South China Sea. Sci Rep 7:40118. https://doi.org/10.1038/srep40118
Wong JCY, Thompson P, Xie JY, Qiu JW, Baker DM (2016) Symbiodinium clade C generality among common scleractinian corals in subtropical Hong Kong. Reg Stud Mar Sci 8:439–444. https://doi.org/10.1016/j.rsma.2016.02.005
Xie JY, Wong JC, Dumont CP, Goodkin N, Qiu JW (2016) Borehole density on the surface of living Porites corals as an indicator of sedimentation in Hong Kong. Mar Pollut Bull 108:87–93. https://doi.org/10.1016/j.marpolbul.2016.04.055
Yang SH, Tseng CH, Huang CR, Chen CP, Tandon K, Lee STM, Chiang PW, Shiu JH, Chen CA, Tang SL (2017) Long-term survey is necessary to reveal various shifts of microbial composition in corals. Front Microbiol 8:1094. https://doi.org/10.3389/fmicb.2017.01094
Zhang J, Kobert K, Flouri T, Stamatakis A (2014) PEAR: a fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 30:614–620. https://doi.org/10.1093/bioinformatics/btt593
Zhang Y, Yang Q, Ling J, Van Nostrand JD, Shi Z, Zhou J, Dong J (2016) The shifts of diazotrophic communities in spring and summer associated with coral Galaxea astreata, Pavona decussata, and Porites lutea. Front Microbiol 7:1870. https://doi.org/10.3389/fmicb.2016.01870
Zhou G, Cai L, Li Y, Tong H, Jiang L, Zhang Y, Lei X, Guo M, Liu S, Qian PY, Huang H (2017) Temperature-driven local acclimatization of Symbiodinium hosted by the coral Galaxea fascicularis at Hainan Island, China. Front Microbiol 8:2487. https://doi.org/10.3389/fmicb.2017.02487
Acknowledgements
The authors would like to thank Drs. Yue Him Wong, Apple Pui Yi Chui, and James Y. Xie for their kind help in experimental design and field sample collection.
Funding
This study was supported by the NSFC-Guangdong Joint Fund (U1301232).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Corals are marine invertebrates. The authors declare that this article follows the guidelines established by the Agriculture, Fisheries and Conservation Department of Hong Kong SAR Government.
Rights and permissions
About this article
Cite this article
Cai, L., Zhou, G., Tong, H. et al. Season structures prokaryotic partners but not algal symbionts in subtropical hard corals. Appl Microbiol Biotechnol 102, 4963–4973 (2018). https://doi.org/10.1007/s00253-018-8909-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-8909-5