Abstract
Understanding the drivers of algal endosymbiont communities hosted by reef corals is a requisite for predicting coral resilience. For the biodiverse reefs of Southeast Asia, few studies have characterised the spatial variability of Symbiodiniaceae communities amongst reefs and investigated species and environmental effects on community structure and diversity. To profile the endosymbionts associated with reef corals inhabiting Southeast Asia, three common species, Pachyseris speciosa, Pocillopora acuta and Diploastrea heliopora, were sampled from 10 reef sites along the coasts of the Malay Peninsula. The nuclear internal transcribed spacer 2 region of Symbiodiniaceae was targeted for high-throughput sequencing, and the SymPortal framework was used to establish the identities of endosymbiont genera and types. Effects of environmental variables on endosymbiont community structure and diversity were then tested. Analyses revealed that Symbiodiniaceae diversity in this region is higher than previously known. Endosymbiont communities are structured significantly by host species and are relatively invariant in D. heliopora, with P. speciosa associating strongly with both Cladocopium and Durusdinium while P. acuta and D. heliopora are dominated by Durusdinium. Environmental parameters influence Symbiodiniaceae communities and diversity distinctly between host species. In particular, higher sea surface temperature (SST) affects endosymbiont diversity positively for P. acuta while higher SST range affects diversity negatively for P. speciosa and D. heliopora. Overall, this study has uncovered the hidden diversity of Symbiodiniaceae types previously unrecorded in the region and established a baseline for future comparative studies on how Southeast Asian reef corals acclimatise and adapt to changing environments through the natural variation of endosymbiont communities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coral reefs are most diverse in the Central Indo-Pacific region, with species diversity of corals and reef fishes reaching a global maximum in the Coral Triangle (Roberts et al. 2002; Hoeksema 2007; Veron et al. 2015)—an area extending from Indonesia and Philippines to the Solomon Islands (Veron et al. 2011). Southeast Asia accounts for a large part of this biodiversity, harbouring over 600 of the world’s 800 reef-building coral species (Burke et al. 2002; Huang et al. 2015). For example, 480 and 255 species have been recorded on the reefs of Peninsular Malaysia and Singapore, respectively (Huang et al. 2009; Affendi and Rosman 2011). As is the case in many other tropical and subtropical regions, coral reefs form a major marine ecosystem in Southeast Asia and its persistence is highly reliant on the endosymbiotic relationship between reef corals and Symbiodiniaceae dinoflagellates. In terms of carbon sequestration alone, the combined carbon storage value of reefs on the west coast of Peninsular Malaysia and Singapore has been estimated at U$288,700 annually (Chou 2000), though this value would be considerably higher today (Wagner 2021).
Despite their high ecological, cultural and economic values, corals are under threat from various anthropogenic activities and climate change. Indeed, one-third of reef-building corals are deemed to be at an elevated risk of extinction (Carpenter et al. 2008). Due to rapid ocean warming, the greatest threat to corals is coral bleaching, in which the relationship between reef corals and the Symbiodiniaceae dinoflagellates breaks down (Brown 1997). Mass mortality events due to bleaching are occurring at increasing frequency, scale and severity on a global level and can be exacerbated by other anthropogenic stressors (Glynn 1993; Hughes et al. 2017, 2018a, b; Oliver et al. 2018). For instance, high levels of maritime traffic, land reclamation and coastal development can increase sediment smothering of corals, resulting in bleaching (Weber et al. 2006; Bessell-Browne et al. 2017). The ability to recover from coral bleaching can also be impeded by previous or ongoing anthropogenic disturbances such as contaminant retention and land-sea biological exchange from coastal development and urban land use (Carlson et al. 2019).
Studies have observed that dominant Symbiodiniaceae taxa present in corals are associated with bleaching tolerance (Hume et al. 2015; Silverstein et al. 2015) and contribute to the stability and resilience of reef corals during chronic and acute disturbances (Sampayo et al. 2008). Broadly, each genus can be distinct in ecological breadth and function (LaJeunesse et al. 2018): Symbiodinium (formally clade A) is adapted to living in high irradiance or variable light conditions (Shoguchi et al. 2018); Breviolum (formerly clade B) contains species that can be found in coral hosts with a wide depth range and other species in shallow waters in the Caribbean (e.g. Diekmann et al. 2003; Finney et al. 2010); Cladocopium (formally clade C) comprises many host specialists which are physiologically diverse, with many members adapted to various light and temperature conditions (Fabina et al. 2013); and Durusdinium (formally clade D) includes species that are tolerant of large fluctuations in temperature (Baker et al. 2013; LaJeunesse et al. 2018). The biogeographic distributions of Symbiodiniaceae genera also differ: Symbiodinium and Breviolum are more frequently found in higher latitudes while Cladocopium and Durusdinium are more common in tropical latitudes (see Baker, 2003). Furthermore, different host species can be associated with multiple Symbiodiniaceae types (e.g. Chankong, et al. 2020). The identification of Symbiodiniaceae taxa hosted by corals is now relatively routine in studies involving coral endosymbiosis and commonly relies on high-throughput sequencing (HTS) of high-resolution amplicon markers, of which the most widely used is the internal transcribed spacer 2 (ITS2) rRNA region (e.g. Hume, et al. 2018a, b; Terraneo et al. 2019). Recent development of the analytical SymPortal framework specifically for processing ITS2 data has further enhanced the speed and resolution of Symbiodiniaceae community and diversity analyses (Hume et al. 2019; Howells et al. 2020).
The Malay Peninsula is amongst many areas in Southeast Asia currently facing declining coral diversity and abundance (Chou 2000, 2006; Guest et al. 2016a). Singapore supports one of the world’s busiest harbours which yield high levels of maritime traffic, land reclamation and seabed dredging (Chou 2006; Chou et al. 2019). These activities lead to increased sediment load and turbidity in the water column (Dikou and van Woesik 2005; Chow et al. 2019), changes in hydrodynamic patterns and tidal speeds, as well as threats of grounding and accidental spillage of hazardous materials (Chan et al. 2006; Sin et al. 2016). These degraded marine conditions severely threaten coral survival (Wong et al. 2018; Poquita-Du et al. 2019; Bollati et al. 2022; Ng et al. 2021). Along the coasts of Peninsular Malaysia, live coral cover has been variable over the years but is generally declining at many sites (Reef Check Malaysia, 2018) as a result of destructive fishing (e.g. blast fishing and use of cyanide), sedimentation and pollution (Chou 2000; Praveena et al. 2012). Throughout the region, global-scale thermal stress has led to extensive coral bleaching events, such as in 2010 (Guest et al. 2012, 2016b; Chan and Sukarno 2016) and 2016 (Kimura et al. 2018; Toh et al. 2018; Ng et al. 2020).
Few studies have characterised the Symbiodiniaceae communities present in reef corals of the Malay Peninsula. Using denaturing gel gradient electrophoresis, Tanzil et al. (2016) documented the dominance of Cladocopium and Durusdinium in common corals in Singapore, the high prevalence of Durusdinium in three of seven coral species examined, as well as variable symbiosis in Pachyseris speciosa which associated with both Cladocopium and Durusdinium. In another study, however, high-throughput amplicon sequencing of endosymbionts in five coral species established Cladocopium as the dominant clade, with low community diversity which was attributed to the high turbidity of Singapore’s coastal environment (Smith et al. 2020). Across the Malay Peninsula, Tan et al. (2020) found Cladocopium to be the most dominant genus hosted by Porites lutea as expected (see Gong et al. 2018, 2019; Pootakham et al. 2018; Terraneo et al. 2019), with community diversity driven by a combination of mean monthly cloud cover, variance in monthly sea surface temperature and the interaction between both factors. These findings and studies in other regions also suggest that endosymbiont communities in various host species differ in how they are structured spatially and environmentally (e.g. Chen et al. 2019; Leveque et al. 2019; Qin et al. 2019; Hume et al. 2020; Tong et al. 2020).
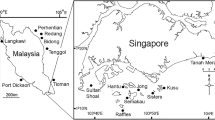
The present study expands upon the sampling of Symbiodiniaceae communities in Southeast Asia, focusing on three common host species—Pachyseris speciosa (Agariciidae; plating; Jain et al. 2020, 2021), Pocillopora acuta (Pocilloporidae; branching; Poquita-Du et al. 2017) and Diploastrea heliopora (Diploastraeidae; mounding; Huang et al. 2014) (Fig. 1). While samples were collected with those of Tan et al. (2020), this study goes beyond that work in two ways. First, it examines and compares three different host species of distinct lineages and colony forms. Colony form is a very important factor to consider because it has been observed to be associated with bleaching vulnerability; branching Pocillopora is highly vulnerable to thermal bleaching but massive and encrusting corals tend to be more resistant (Loya et al. 2001; Darling et al. 2012; Ng et al. 2020; but see Swain et al. 2018a). There is also evidence showing that skeletal structures of different host species scatter light distinctly and modulate light availability to the algal endosymbionts (Swain et al. 2018b). Second, this study analyses a larger suite of environmental variables with greater model complexity. These parameters include those related to thermal conditions (e.g. temperature and cloud cover), productivity and nutrient levels (e.g. photosynthetically available radiation, phosphate and nitrate concentrations), which are known to affect Symbiodiniaceae communities (Baker et al. 2013; Chow et al. 2019). Our primary objectives are to profile the diversity and distribution patterns of Symbiodiniaceae in the three coral species along the Malay Peninsula (Fig. 1) and to assess the influence of relevant satellite-derived environmental parameters on endosymbiont community structure and diversity. We show that corals here are dominated by Cladocopium and Durusdinium, whose communities are structured by host species and site. Various spatio-environmental factors influence Symbiodiniaceae community structure and diversity, but the effects differ between host species. By identifying the coral species and populations hosting particular Symbiodiniaceae taxa and endosymbiont communities that are sensitive or resistant to stress, our findings contribute to the growing literature on coral resilience and establish a baseline for reef rehabilitation studies.
Materials and methods
Coral sampling
Pachyseris speciosa, Pocillopora acuta and D. heliopora were sampled between November 2017 and July 2018 from seven sites around Peninsular Malaysia (PM) and three sites in Singapore (Raffles Lighthouse, Sisters’ Islands and Kusu Island) (Fig. 1), alongside the sampling for Tan et al. (2020). Of the seven sites in PM, two are from the western coast (West PM; Langkawi with two collection points, Pulau Payar and Datai Beach, as well as Port Dickson) and five are from the eastern coast (East PM; Pulau Perhentian, Pulau Redang, Pulau Bidong, Pulau Tenggol and Pulau Tioman). At least 20 samples of Pachyseris speciosa and Pocillopora acuta were collected from each site, except for Pachyseris speciosa at Port Dickson and Tenggol (six and 17 samples, respectively). Collection of D. heliopora was more variable—Langkawi, Pulau Tioman and Pulau Tenggol yielded 20 samples each while 9–16 samples were collected from Port Dickson, Raffles Lighthouse, Kusu Island, Pulau Perhentian and Pulau Redang (Electronic Supplementary Material, ESM Table S1). Sampling protocol followed Tan et al. (2020). Briefly, tissue samples were collected from coral colonies visually determined to be healthy at depths of 2–6 m in Singapore and 4–25 m in PM, spanning the full depth ranges of reefs at the respective sites. The vast majority of samples from PM were collected between 4 and 11 m depth. Samples were immersed in water in separate sealed containers and kept in the shade until processing. Samples were preserved in 100% molecular-grade ethanol within 6 h and thereafter stored at −80 °C upon return to the laboratory.
DNA library preparation and sequencing
Symbiodiniaceae DNA was extracted from coral samples using the DNeasy Blood and Tissue Kit (Qiagen, Singapore) according to the manufacturer’s recommended protocol. We followed the 16S Metagenomic Sequencing Library Preparation guide for the Illumina MiSeq System (Illumina 2013) using the SYM_VAR primer pair for Symbiodiniaceae ITS2 amplification (ESM Table S2) (Hume et al. 2018b). Polymerase chain reaction (PCR) was performed using 2.5 µL of sample DNA, 5 µL of forward and reverse primers each and 12.5 µL of 2 × KAPA HiFi HotStart ReadyMix to make a total volume of 25 µL per reaction mix. The PCR conditions were initial denaturation at 95 °C for 3 min, followed by 25 cycles of 95 °C for 30 s, 55 °C for 30 s and 72 °C for 30 s, and a final extension step at 72 °C for 5 min. Amplicon purification was carried out using AMPure XP magnetic beads (Agencourt).
Adaptor-index PCR was then performed using 2.5 µL of sample DNA, 2.5 µL each of Index 1 (i5F) and Index 2 (i7R) primers (Integrated DNA Technologies, Coralville, IA, USA), 12.5 µL of 2 × KAPA HiFi HotStart ReadyMix and 5 µL of PCR-grade water to make a total volume of 25 µL per reaction mix. The PCR conditions were initial denaturation at 95 °C for 3 min, followed by 8 cycles of 95 °C for 30 s, 55 °C for 30 s and 72 °C for 30 s, and a final extension step at 72 °C for 5 min. PCR purification was then carried out again using the AMPure XP magnetic beads (Agencourt).
Successful adaptor-index PCR was checked by running 1 µL of each PCR product on a 1% agarose gel. Samples were also quantified on a Qubit 3 Fluorometer using the dsDNA HS Assay Kit (Invitrogen, Carlsbad, CA, USA) and 1 µL of PCR product. Sample normalisation was performed using the SequelPrep Normalization Plate Kit (Invitrogen, Carlsbad, CA, USA) to achieve a concentration of 1–2 ng/μL. Samples for each library were then pooled for a minimum concentration of 5 nM. Libraries were sequenced on the Illumina MiSeq platform (V3 chemistry, 300-bp paired-end reads) at Macrogen (Seoul, South Korea).
Bioinformatics
Sequence data were processed locally using the SymPortal framework (Hume et al. 2019). Demultiplexed and paired forward and reverse FASTQ files were submitted to SymPortal, where sequence filtering and quality control were carried out using Mothur 1.43.0 (Schloss et al. 2009), BLAST + suite of executables (Camacho et al. 2009) and minimum entropy decomposition (MED) (Eren et al. 2015).
Mothur-processing generated contiguous sequences and sequences were screened with maximum ambiguities allowed set to 0 and maximum homopolymer set to 5 in order to discard sequences putatively generated from sequencing errors. Distinct sequences were identified, and singletons and doubletons were removed. Non-Symbiodiniaceae sequences were then filtered out using BLASTn searches against the SymPortal reference database (accessed on 3 October 2019) on a sample-by-sample basis. Sequences that had an identity > 80% and coverage > 95% to any sequence in the reference database were assigned to be of Symbiodiniaceae origin. Size screening was performed with minimum and maximum cut-offs of 184 bp and 310 bp, respectively (~ 50 bp ± average lengths for the shortest and longest fragments across Symbiodiniaceae genera). Finally, MED nodes within a given set of sequence were identified by the nucleotide positions most informative in differentiating between these sequences to yield relative abundance data for Symbiodiniaceae types. Samples with fewer than 10,000 sequencing reads were removed, and rarefaction curves were plotted for the retained samples to verify sufficient sequencing depth had been achieved (ESM Fig. S1).
Environmental data
Environmental data associated with the coral reef environment were extracted from Bio-ORACLE v2.1 (Tyberghein et al. 2012; Assis et al. 2018) (ESM Table S3). All data layers were at a resolution of 5 arcmin, or approximately 9.2 km at the equator, and the long-term averages for the selected variables used in this study at each site were extracted from their respective layers. All 14 parameters obtained were analysed as mean values to characterise baseline environmental conditions at each study site (ESM Table S4). Environmental conditions within each region (West PM, Singapore and East PM) were more similar to one another than between regions (ESM Fig. S2). Within each region, however, these parameters also varied considerably between sites (ESM Table S4), so data analyses were performed at the site level to take this variation into account.
Data analyses
All analyses were performed in R version 3.6.3 (R Core Team 2013). Symbiodiniaceae richness was calculated for each host species based on absolute abundance count tables of post-MED ITS2 sequences, and the majority (top 20 most abundant) ITS2 sequences for each species were identified.
A Bray–Curtis dissimilarity matrix was computed for the entire dataset using the vegan package (Oksanen et al. 2018). Permutational multivariate analysis of variance (PERMANOVA) was performed to test the significance of host species and collection site in structuring Symbiodiniaceae communities. Following results showing significant differences between coral species, data for each host species were analysed separately. Analysis of similarities (ANOSIM) was performed for each host species using the Bray–Curtis dissimilarity measure to test for differences in community structure between sites, and distance decay of similarities was performed with Mantel test on the shortest over-water distance between each pair of collection sites.
Variation of Symbiodiniaeceae communities was visualised via non-metric multidimensional scaling (NMDS; Bray–Curtis dissimilarity; vegan package; Oksanen et al. 2018) of the count tables of the entire dataset as well as separately for each host species. Using the envfit function from the vegan package, environmental data modelled below were fitted onto the NMDS for each host species to assess the correlation between these parameters and Symbiodiniaceae community structure. The betadisper function from the same package was used to test for differences in group dispersions.
Shannon diversity index was calculated for each host species. Generalised linear mixed models (GLMMs) were constructed using the lme4 package (Bates et al. 2015) to analyse the fixed effects of environmental variables on Symbiodiniaceae diversity, with monsoon period (October to March, and April to September) and site indicated as random factors. Multicollinearity tests were performed using the GGally package (Schloerke et al. 2021) and parameters with pairwise collinearity values greater that 0.7 were removed. The parameters retained were scaled using the scale function in R due to being of different orders of magnitude. The maximal GLMM was constructed and variables that remained collinear (variable inflation factor > 2.5) were identified using the vif function from the car package (Fox and Weisberg 2011) and sequentially removed. Models were assessed by the Akaike information criterion corrected for small sample sizes (AICc). Model averaging was carried out using the MuMIn package for models with ΔAICc ≤ 2. Coefficient estimates reported are from the “full” average which assumes that a variable is included in every model; where the model does not originally include the variable, the coefficient of that variable is set to zero.
Results
Sequencing resulted in average sample read counts of 143,079 for Pachyseris speciosa, 140,547 for Pocillopora acuta (excluding samples that returned with 0 reads) and 184,664 for D. heliopora. Samples with read counts less than 10,000 were removed for poor quality; of the 430 samples processed and sequenced, 100 were removed from the final analyses. Thereafter, average sample read counts increased to 145,488 for Pachyseris speciosa, 211,365 for Pocillopora acuta and 221,211 for D. heliopora. All sequencing data have been deposited at the National Center for Biotechnology Information (BioProject ID PRJNA785764).
Comparing Symbiodiniaceae communities between species, Pocillopora acuta (n = 89) hosted the highest Symbiodiniaceae richness with a total of 315 unique Symbiodiniaceae types and Pachyseris speciosa (n = 142) had an intermediate richness of 269 types, while D. heliopora (n = 99) hosted the lowest richness of 165 types (Table 1). Samples of D. heliopora and Pachyseris speciosa had mean richness of 13.4 (SD = 5.54 and 5.18, respectively) while Pocillopora acuta samples had a lower mean richness of 10.8 (SD = 5.84).
The top 20 most abundant ITS2 sequences comprised 97.7% of the total read abundance present in Pachyseris speciosa, while the proportion was 89.2% for Pocillopora acuta and 96.4% for D. heliopora, revealing that Pocillopora acuta had a greater representation of background types than the other two host species (ESM Fig. S3). These differences were statistically significant (Kruskal–Wallis test: Chi-squared = 33.5, df = 2, p < 0.05; pairwise Wilcoxon rank sum tests: p < 0.05). Pachyseris speciosa was found to be strongly associated with both Cladocopium and Durusdinium while Pocillopora acuta and D. heliopora were dominated by Durusdinium (Fig. 2a; ESM Fig. S3 and Table S5). Gerakladium was detected in the background in one Pocillopora acuta sample from Perhentian and one Pachyseris speciosa sample from Redang, both being the northernmost sites in East PM. PERMANOVA results revealed host species identity was a significant driver of Symbiodiniaceae community (df = 2, F = 172, p < 0.05) and the interaction between species and site was significant (df = 11, F = 17.6, p < 0.05).
Diversity of Symbiodiniaceae communities hosted by Pachyseris speciosa, Pocillopora acuta and Diploastrea heliopora at each sampling site. The top 20 most abundant ITS2 sequences composed 97.7% of the total read abundance present in Pachyseris speciosa, 89.2% in Pocillopora acuta and 96.4% in D. heliopora. a Stacked bar charts showing the relative abundances of the 20 most abundant ITS2 types. b Boxplots showing Shannon diversity index of Symbiodiniaceae communities. Colours represent regions in Peninsular Malaysia (PM) and Singapore
Symbiodiniaceae community composition was variable amongst sites for every host species (Fig. 2). ANOSIM showed that communities were significantly different between sites, with communities in Pachyseris speciosa being the most dissimilar amongst sites while those in D. heliopora were least dissimilar (Table 2). Results of the combined NMDS supported this pattern as D. heliopora had the smallest spread of data points (Fig. 3a), with significant differences in the community dispersion of Symbiodiniaceae between host species (betadisper: df = 2, F = 19.9, p < 0.05).
Non-metric multidimensional scaling (NMDS) of Symbiodiniaceae communities based on Bray–Curtis dissimilarity. Each point represents a coral sample; ellipses denote 95% confidence level. a NMDS plot of all three host species (stress = 0.0776). b NMDS plot for each species (stress < 0.2). In each plot, symbols represent regions in Peninsular Malaysia (PM) and Singapore, with fitted vectors of environmental variables denoted by arrows
NMDS performed separately for each host species showed evidence of Symbiodiniaceae communities clustering by region (Fig. 3b), except in D. heliopora for which the different regions overlapped substantially. Community dispersion of Symbiodiniaceae amongst the three regions according to betadisper was significantly heterogeneous in Pachyseris speciosa (df = 2, F = 5.20, p < 0.05) and Pocillopora acuta (df = 2, F = 11.5. p < 0.05) but not D. heliopora (df = 2, F = 0.0372, p = 0.964). These results are in line with the outcomes of the distance decay analysis based on Mantel tests, which showed isolation by distance of Symbiodiniaceae communities in Pachyseris speciosa and Pocillopora acuta, but no correlation between community dissimilarity and spatial separation in D. heliopora (Table 2). In other words, endosymbiont communities in Pachyseris speciosa and Pocillopora acuta were more similar between corals in closer proximity than those farther apart, but this was not evident for D. heliopora.
The environmental parameters that were significant in structuring Symbiodiniaceae communities varied amongst host species (Table 3). Of the main effects, calcite and phosphate concentrations and sea surface temperature (SST) were significant in structuring endosymbiont communities in Pachyseris speciosa. Cloud fraction, salinity and SST range were significant for Pocillopora acuta, while only primary productivity was significant for D. heliopora. Symbiodiniaceae communities were not structured by monsoon period of collection (ESM Fig. S4).
Shannon diversity of Symbiodiniaceae types was found to be significantly different amongst the three host species (df = 2, F = 29.7, p < 0.05) and between sites (df = 9, F = 11.7, p < 0.05) based on a two-way ANOVA. Overall, Shannon diversity was least variable in D. heliopora and the greatest variation occurred in Pocillopora acuta (Table 4, Fig. 2b). Across sites and regions, Symbiodiniaceae diversity was higher in Pocillopora acuta and D. heliopora compared to Pachyseris speciosa (Table 4). While sites in West PM had the highest Symbiodiniaceae diversity across all host species, sites in Singapore had the lowest diversity for Pachyseris speciosa and D. heliopora but not for Pocillopora acuta, indicating the presence of site by species interaction (df = 11, F = 3.79, p < 0.05).
For Shannon diversity data pooled across all three host species, the full GLMM model had high levels of multicollinearity. Since the ANOVA above showed that Shannon diversity differed significantly between host species, GLMMs for each coral species were used to analyse the environmental parameters. Assessment of all candidate models with strong support (ΔAICc ≤ 2) resulted in two models for Pachyseris speciosa and six models each for Pocillopora acuta and D. heliopora (ESM Table S6). Results showed that different sets of environmental variables affected Symbiodiniaceae diversity distinctly amongst species (Table 5). After model averaging, the only significant parameters were SST (p < 0.05) and calcite concentration × phosphate concentration (p < 0.01) for Pachyseris speciosa, although calcite and phosphate concentrations were also present in the best model. Cloud fraction, salinity and their interaction were associated with Symbiodiniaceae diversity in Pocillopora acuta. For D. heliopora, current velocity, primary productivity, SST range and current velocity × SST range were the most important predictors. Overall, parameters related to temperature (SST and SST range) influenced Symbiodiniaceae diversity in all three host species, though with varying effects—SST had a positive effect in P. speciosa while SST range had negative effects in Pocillopora acuta and D. heliopora. Primary productivity and nutrient availability also had different effects, shown by the positive relationship with phosphate concentration in Pachyseris speciosa but negative relationship with primary productivity in D. heliopora (Table 5).
Discussion
Building extensively upon the works of Tanzil et al. (2016), Smith et al. (2020) and Tan et al. (2020), we provide the most comprehensive profiling thus far of the community structure of Symbiodiniaceae along the Malay Peninsula and furthermore assess the influence of environmental parameters on endosymbiont community diversity. We focus on host species which are representative of reef corals in the region and of different lineages, colony forms and life histories. Importantly, the use of SymPortal for the identification of Symbiodiniaceae types reveals a high diversity of Symbiodiniaceae, higher than what might have been predicted from previous studies in this region (Leveque et al. 2019; Tan et al. 2020). Our analyses further highlight the dominance of Cladocopium and Durusdinium, with the presence of Gerakladium in the background. Cladocopium has been reported to be the most common genus in several Indo-Pacific host genera (e.g. Leveque et al. 2019; Tan et al. 2020). While Cladocopium dominate assemblages in the western Indian Ocean (LaJeunesse et al. 2010) and western Pacific Ocean (LaJeunesse et al. 2004), Durusdinium had been found to occur frequently in the Andaman Sea and the Gulf of Thailand (LaJeunesse et al. 2010; Chankong et al. 2020). This pattern has been attributed to higher water temperatures and turbidity (LaJeunesse et al. 2010), factors that can also be extended to the tropical coastal waters off Peninsular Malaysia and Singapore. Indeed, Tanzil et al. (2016) found high prevalence of Durusdinium in three of seven coral species examined, highlighting the importance of this Symbiodiniaceae genus for the persistence of urban coral reefs in Singapore (Poquita-Du et al. 2020). The 20 most abundant Symbiodiniaceae types make up more than 89% of total abundance of Symbiodiniaceae for each host species, with hundreds making up the low-abundance background types that may be transient or permanent. While Lee et al. (2016) has suggested that background Symbiodiniaceae are transient and have minimal ecological significance in temperate reef corals, emerging studies indicate that background Symbiodiniaceae may have a functional role in host coral stress response and resilience to perturbations (Ziegler et al. 2018).
Pachyseris speciosa is associated with two Symbiodiniaceae genera (Cladocopium and Durusdinium) while both Pocillopora acuta and D. heliopora are dominated by Durusdinium. Pocillopora acuta has the highest Symbiodiniaceae richness, consistent with findings of large genetic variation in the Durusdinium types present in this species (Tanzil et al. 2016). A recent study of samples from the same localities found that Porites lutea was dominated by Cladocopium, but it was also associated with Symbiodinium and Durusdinium (Tan et al. 2020). Comparisons of Symbiodiniaceae richness between host species and their life histories can be informative for characterising the relationship between the endosymbionts and the resilience of the holobiont against environmental stressors. Pocillopora acuta is considered a weedy, opportunistic coloniser of recently disturbed habitats, D. heliopora a stress-tolerant species, while Pachyseris speciosa is a generalist with traits overlapping between the weedy and stress-tolerant life histories (Darling et al. 2012, 2013). These life history classifications appear consistent with their Symbiodiniaceae richness, with Pocillopora acuta having the highest total richness, in line with its opportunistic life history, and D. heliopora having the lowest total richness (Fig. 2a), possibly as fewer endosymbiont types are required for a stress-tolerant coral species residing in stressful urban marine environments, especially Singapore (Smith et al. 2020). Pachyseris speciosa, with a life history that partially overlaps with Pocillopora acuta and D. heliopora, has an intermediate Symbiodiniaceae total richness. Another stress-tolerant coral, Porites lutea, also registered relatively low community richness in the Malay Peninsula (Tan et al. 2020), and this is consistent with the recent finding that urbanised environments tend to depress Symbiodiniaceae richness (Smith et al. 2020; Jain et al. 2021).
Symbiodiniaceae communities in Pachyseris speciosa are most dissimilar amongst sites while those in D. heliopora are least dissimilar (Table 2). The site-wise distinctiveness exhibited by Pachyseris speciosa is contributed by the different combinations of Cladocopium and Durusdinium. At the regional level, this is most evident where the West PM confidence ellipse in the NMDS is largely distinct from the East PM and Singapore ellipses (Fig. 3b), as supported by the ANOSIM results showing significantly distinct communities between sites. Analysis of the most abundant Symbiodiniaceae types demonstrates that Pachyseris speciosa in West PM has a greater proportion of Durusdinium (mean 0.838 ± SD 0.325; ESM Fig. S3), with Cladocopium present in the background in very low abundances in Langkawi (Fig. 2a). For Pocillopora acuta, 12 of the majority Symbiodiniaceae types are unique to one region only, with eight types unique to West PM, three to Singapore and one to East PM. These biogeographic patterns contribute to dissimilarities in Symbiodiniaceae communities.
Interestingly, even though D. heliopora has relatively high overall Symbiodiniaceae diversity (Table 4), its endosymbiont communities are largely similar amongst sites based on the low ANOSIM statistic (Table 2), overlapping NMDS confidence ellipses (Fig. 3b), and insignificant betadisper result. The relative stability of Symbiodiniaceae communities amongst D. heliopora colonies is an interesting parallel to findings of limited genetic and morphological variation amongst D. heliopora colonies (Todd et al. 2004; Lam et al. 2006; Huang et al. 2014), suggesting that host similarity may constrain Symbiodiniaceae types and community structure. More research on coral species of varying host characteristics would help clarify this relationship. Relatedly, the lack of distance decay of similarity in D. heliopora could suggest either high connectivity between sites or an endosymbiont community structure that is relatively unaffected by environmental variation. In view of the genetic invariance of D. heliopora and its stress-tolerant life history, the latter may be the more likely explanation. That Porites lutea, also a stress-tolerant species, exhibits similar Symbiodiniaceae composition throughout the Malay Peninsula (Tan et al. 2020) further lends support to this hypothesis.
Different environmental variables appear to structure Symbiodiniaceae communities distinctly amongst host species (Table 3), a finding that is in line with work elsewhere (e.g. Great Barrier Reef (Tonk et al. 2013) and Red Sea (Terraneo et al. 2019; Osman et al. 2020)) showing host-specific community responses to various environmental variables. In particular, our GLMM results reveal that across the three host species, different sets of environmental parameters have varying effects on Symbiodiniaceae diversity (Table 5). Common to all is sea surface temperature (mean SST or SST range), which also consistently features across GLMMs with high relative importance across host species. Higher SST seems to be associated with an increase in Symbiodiniaceae diversity in Pachyseris speciosa; at warmer sites, Symbiodiniaceae types that are more thermally tolerant may be recruited as Pachyseris speciosa “shuffles” its symbionts (Jain et al. 2020), potentially driving diversity higher. Meanwhile, smaller SST range is associated with higher Symbiodiniaceae diversity in Pocillopora acuta and D. heliopora. Inhabiting shallow reefs, temperature stability may be an important influencing factor for these two host species.
Primary productivity and nutrients also have varying effects on Symbiodiniaceae diversity (Table 5). In particular, primary productivity is negatively associated with endosymbiont diversity for D. heliopora while phosphate concentration has a positive influence for Pachyseris speciosa, underscoring the fact that different host species tend to have different water quality or nutrient requirements. With nutrients being limiting in reef waters, influx of phosphates can impact corals greatly (Duprey et al. 2016). The increase in Pachyseris speciosa’s Symbiodiniaceae diversity on reefs with higher phosphate concentration may be a sign that Pachyseris speciosa could adapt to poorer water quality by altering its Symbiodiniaceae community. Finally, surface current velocity has a moderately important positive effect on endosymbiont diversity in D. heliopora, possibly resulting in greater water movement surrounding the coral colonies. The enhanced flushing may moderate the effects of extremely high temperature or nutrient concentrations (West and Salm 2003; Fabricius 2005), preventing the dominance of Symbiodiniaceae types that are more well-adapted to stressful conditions (Smith et al. 2020).
While these findings reveal species-specific effects of different environmental parameters on Symbiodiniaceae diversity, the details of interactions amongst modelled predictors remain challenging to explain because the physiologies of most Symbiodiniaceae types are yet to be fully characterised (LaJeunesse et al. 2018). Other factors known to affect Symbiodiniaceae communities but are not tested here add to the complexity of variations in endosymbiont diversity. Such parameters can include turbidity, sedimentation rate, sediment type and nutrients (Cooper et al. 2011; Savage 2019; Zhou et al. 2021), which require concerted in situ measurements. The depth of host corals can also potentially drive Symbiodiniaceae community structure because temperature and light levels tend to be lower at deeper habitats (Chow et al. 2019; Eckert et al. 2020). Bacterial associates in corals are also important in maintaining the dynamic relationship between the coral host and its endosymbionts (Peixoto et al. 2017). For example, Epstein et al. (2019) found that Pocillopora acuta in the Great Barrier Reef had a relatively stable microbiome and Symbiodiniaceae community throughout a thermal stress event that likely contributed to the species’ thermal resilience. While the dynamics between the trio of coral host, Symbiodiniaceae and bacteria are not fully understood, it is possible that associated bacterial microbes may play a role in determining the composition of Symbiodiniaceae that is taken up by the coral host throughout its lifetime (Quigley et al. 2019; Claar et al. 2020; Matthews et al. 2020). Thus, the environment may not always affect Symbiodiniaceae community structure directly, but it may do so indirectly by influencing the composition of the coral microbiome (see Chen et al. 2021).
Critically, studies monitoring corals over seasonal to interannual timescales are needed to uncover fine-scale temporal variations in Symbiodiniaceae communities and physico-chemical parameters, preferably with comparable sampling intervals between the biotic and abiotic data. Studies suggest that corals under thermal stress may become increasingly reliant on Durusdinium (Baker et al. 2004; Kemp et al. 2014; Huang et al. 2020), and this may have long-term implications on coral nutrition and growth. Since Durusdinium is known to withhold more nutrition from the coral host under warm-water conditions (Baker et al. 2018), growth rates may be reduced for corals which host Durusdinium. This effect may also differ between corals dominated by Durusdinium, such as Pocillopora acuta and D. heliopora, and those which associate with both Cladocopium and Durusdinium, like Pachyseris speciosa. Endosymbiont communities and growth rates of corals at the sites examined here could be quantified over time and environmental gradients to assess if such a prediction—where corals become increasingly reliant on Durusdinium as SST rises (Heron et al. 2016; Hughes et al. 2018b)—holds true in this region and to predict future coral performance under climate change.
This study has shed light on the natural variation of Symbiodiniaceae communities hosted by different coral species inhabiting the reefs of Malay Peninsula, showing how spatio-environmental variables affect endosymbiont diversity. The high richness of Symbiodiniaceae present in the region is also uncovered here for the first time, filling a critical biodiversity knowledge gap. Our results have established a baseline and foundation for future studies aiming to better understanding how different Symbiodiniaceae assemblages confer adaptive advantages on corals amidst local impacts and climate change.
References
Affendi YA, Rosman FR (2011) Current knowledge on scleractinian coral diversity of Peninsular Malaysia. In: Kamarruddin I, Mohamed CAR, Rozaimi MJ, Kee Alfian AA, Fitra AZ, Lee JN (eds) Malaysia’s marine biodiversity: Inventory and current status. Department of Marine Park Malaysia, Putrajaya, pp 21–31
Assis J, Tyberghein L, Bosch S, Verbruggen H, Serrão EA, Clerck OD, Tittensor D (2018) Bio-ORACLE v2.0: extending marine data layers for bioclimatic modelling. Glob Ecol Biogeogr 27:277–284
Baker AC (2003) Flexibility and specificity in coral-algal symbiosis: diversity, ecology, and biogeography of Symbiodinium. Annu Rev Ecol Evol Syst 34:661–689
Baker AC, Starger CJ, Mcclanahan TR, Glynn PW (2004) Corals adaptive response to climate change. Nature 430:741
Baker DM, Andras JP, Jordán-Garza AG, Fogel ML (2013) Nitrate competition in a coral symbiosis varies with temperature among Symbiodinium clades. ISME J 7:1248–1251
Baker DM, Freeman CJ, Wong JCY, Fogel ML, Knowlton N (2018) Climate change promotes parasitism in a coral symbiosis. ISME J 12:921–930
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Bessell-Browne P, Negri AP, Fisher R, Clode PL, Duckworth A, Jones R (2017) Impacts of turbidity on corals: the relative importance of light limitation and suspended sediments. Mar Pollut Bull 117:161–170
Bollati E, Rosenberg Y, Simon-Blecher N, Tamir R, Levy O, Huang D (2022) Untangling the molecular basis of coral response to sedimentation. Mol Ecol 31:884–901
Brown B (1997) Coral bleaching: causes and consequences. Coral Reefs 16:S129–S138
Burke L, Selig E, Spalding M (2002) Reefs at risk in Southeast Asia. World Resources Institute, Washington, DC
Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL (2009) BLAST+: architecture and applications. BMC Bioinform 10:1–9
Carlson R, Foo S, Asner G (2019) Land use impacts on coral reef health: a ridge-to-reef perspective. Front Mar Sci 6:562
Carpenter KE, Abrar M, Aeby G, Aronson RB, Banks S, Bruckner A, Chiriboga A, Cortés J, Delbeek JC, DeVantier L, Edgar GJ, Edwards AJ, Fenner D, Guzmán HM, Hoeksema BW, Hodgson G, Johan O, Licuanan WL, Livingstone SR, Lovell ER, Moore JA, Obura DO, Ochavillo D, Polidoro BA, Precht WF, Quibilan MC, Reboton C, Richard ZT, Rogers AD, Sanciangco J, Sheppard A, Sheppard C, Smith J, Stuart S, Turak E, Veron JEN, Wallace C, Weil E, Wood E (2008) One-third of reef-building corals face elevated extinction risk from climate change and local impacts. Science 321:560–563
Chan ES, Tkalich P, Gin KY-H, Obbard JP (2006) The physical oceanography of Singapore coastal waters and its implications for oil spills. In: Wolanski E (ed) The environment in Asia Pacific harbours. Springer, Netherlands, pp 393–412
Chan AA, Sukarno W (2016) Coral reef rehabilitation and restoration: Experience of Malaysia. In Kawamura H, Iwata T, Theparoonrat Y, Manajit N, Sulit VT (Eds.), Consolidating the strategies for fishery resources enhancement in Southeast Asia. Proceedings of the Symposium on Strategy for Fisheries Resources Enhancement in the Southeast Asian Region, Pattaya, Thailand, 27–30 July 2015 (pp 113–116). Samutprakan, Thailand: Training Department, Southeast Asian Fisheries Development Center
Chankong A, Kongjandtre N, Senanan W, Manthachitra V (2020) Community composition of Symbiodiniaceae among four scleractinian corals in the eastern Gulf of Thailand. Reg Stud Mar Sci 33:100918
Chen B, Yu K, Liang J, Huang W, Wang G, Su H, Qin Z, Huang X, Pan Z, Luo W, Luo Y, Wang Y (2019) Latitudinal variation in the molecular diversity and community composition of Symbiodiniaceae in coral from the South China Sea. Front Microbiol 10:1278
Chen B, Yu K, Liao Z, Yu X, Qin Z, Liang J, Wang G, Wu Q, Jiang L (2021) Microbiome community and complexity indicate environmental gradient acclimatisation and potential microbial interaction of endemic coral holobionts in the South China Sea. Sci Total Environ 765:142690
Chou LM (2006) Marine habitats in one of the world’s busiest harbours. In: Wolanski E (ed) The environment in Asia Pacific harbours. Springer, Dordrecht, pp 377–391
Chou LM, Huang D, Tan KS, Toh TC, Goh BPL, Tun K (2019) Singapore. In: Sheppard CRC (ed) World seas: an environmental evaluation, vol II. The Indian Ocean to the Pacific Academic Press, London, pp 539–558
Chou LM (2000) Southeast Asian Reefs-Status update: Cambodia, Indonesia, Malaysia, Philippines, Singapore, Thailand and Viet Nam. In C. Wilkinson (Ed.), Status of Coral Reefs of the World: 2000, Australian Institute of Marine Science, pp 117–129
Chow GSE, Chan YKS, Jain SS, Huang D (2019) Light limitation selects for depth generalists in urbanised reef coral communities. Mar Environ Res 147:101–112
Claar DC, McDevitt-Irwin JM, Garren M, Vega Thurber R, Gates RD, Baum JK (2020) Increased diversity and concordant shifts in community structure of coral-associated Symbiodiniaceae and bacteria subjected to chronic human disturbance. Mol Ecol 29:2477–2491
Cooper TF, Berkelmans R, Ulstrup KE, Weeks S, Radford B, Jones AM, Doyle J, Canto M, O’Leary RA, van Oppen MJH (2011) Environmental factors controlling the distribution of Symbiodinium harboured by the coral Acropora millepora on the great barrier reef. PLoS ONE 6:e25536
Darling ES, Alvarez-Filip L, Oliver TA, McClanahan TR, Côté IM (2012) Evaluating life-history strategies of reef corals from species traits. Ecol Lett 15:1378–1386
Darling ES, McClanahan TR, Coté IM (2013) Life histories predict coral community disassembly under multiple stressors. Glob Chang Biol 19:1930–1940
Diekmann O, Olsen J, Stam W, Bak R (2003) Genetic variation within Symbiodinium clade B from the coral genus Madracis in the Caribbean (Netherlands Antilles). Coral Reefs 22:29–33
Dikou A, van Woesik R (2005) Survival under chronic stress from sediment load: spatial patterns of hard coral communities in the southern islands of Singapore. Mar Pollut Bull 52:7–21
Duprey NN, Yasuhara M, Baker DM (2016) Reefs of tomorrow: eutrophication reduces coral biodiversity in an urbanized seascape. Glob Chang Biol 22:3550–3565
Eckert RJ, Reaume AM, Sturm AB, Studivan MS, Voss JD (2020) Depth influences Symbiodiniaceae associations among Montastraea cavernosa corals on the Belize Barrier Reef. Front Microbiol 11:518
Epstein HE, Torda G, van Oppen MJH (2019) Relative stability of the Pocillopora acuta microbiome throughout a thermal stress event. Coral Reefs 38:373–386
Eren A, Morrison HG, Lescault PJ, Reveillaud J, Vineis JH, Sogin ML (2015) Minimum entropy decomposition: unsupervised oligotyping for sensitive partitioning of high-throughput marker gene sequences. ISME J 9:968–979
Fabricius KE (2005) Effects of terrestrial runoff on the ecology of corals and coral reefs: review and synthesis. Mar Pollut Bull 50:125–146
Finney JC, Pettay DT, Sampayo EM, Warner ME, Oxenford HA, LaJeunesse TC (2010) The relative significance of host–habitat, depth, and geography on the ecology, endemism, and speciation of coral endosymbionts in the genus Symbiodinium. Microb Ecol 60:250–263
Fox J, Weisberg S (2011) An R companion to applied regression. Sage, Thousand Oaks
Gong S, Chai G, Xiao Y, Xu L, Yu K, Li J, Liu F, Cheng H, Zhang F, Liao B, Li Z (2018) Flexible symbiotic associations of Symbiodinium with five typical coral species in tropical and subtropical reef regions of the northern South China Sea. Front Microbiol 9:2485
Gong S, Xu L, Yu K, Zhang F, Li Z (2019) Differences in Symbiodiniaceae communities and photosynthesis following thermal bleaching of massive corals in the northern part of the South China Sea. Mar Pollut Bull 144:196–204
Guest JR, Tun K, Low J, Vergés A, Marzinelli EM, Campbell AH, Bauman AG, Feary DA, Chou LM, Steinberg PD (2016a) 27 years of benthic and coral community dynamics on turbid, highly urbanised reefs off Singapore. Sci Rep 6:36260
Guest JR, Low J, Tun K, Wilson B, Ng C, Raingeard D, Ulstrup KE, Tanzil JT, Todd PA, Toh TC, McDougald D, Chou LM, Steinberg PD (2016b) Coral community response to bleaching on a highly disturbed reef. Sci Rep 6:20717
Guest JR, Baird AH, Maynard JA, Muttaqin E, Edwards AJ, Campbell SJ, Yewdall K, Affendi YA, Chou LM (2012) Contrasting patterns of coral bleaching susceptibility in 2010 suggest an adaptive response to thermal stress. PLoS ONE 7:e33353
Heron SF, Maynard JA, van Hooidonk R, Eakin CM (2016) Warming trends and bleaching stress of the world’s coral reefs 1985–2012. Sci Rep 6:38402
Hoeksema BW (2007) Delineation of the Indo-Malayan centre of maximum marine biodiversity: the Coral Triangle. In: Renema W (ed) Biogeography, time, and place: distributions, barriers, and islands. Topics in geobiology. Springer, Dordrecht, pp 117–178
Howells EJ, Bauman AG, Vaughan GO, Hume BC, Voolstra CR, Burt JA (2020) Corals in the hottest reefs in the world exhibit symbiont fidelity not flexibility. Mol Ecol 29:899–911
Huang D, Tun K, Chou L, Todd P (2009) An inventory of zooxanthellate scleractinian corals in Singapore, including 33 new records. Raffles Bull Zool S22:69–80
Huang D, Benzoni F, Fukami H, Knowlton N, Smith ND, Budd AF (2014) Taxonomic classification of the reef coral families Merulinidae, Montastraeidae, and Diploastraeidae (Cnidaria: Anthozoa: Scleractinia). Zool J Linn Soc 171:277–355
Huang D, Licuanan WY, Hoeksema BW, Chen CA, Ang PO, Huang H, Lane DJW, Vo ST, Waheed Z, Affendi YA, Yeemin T, Chou LM (2015) Extraordinary diversity of reef corals in the South China Sea. Mar Biodivers 45:157–168
Huang Y-Y, Carballo-Bolaños R, Kuo C-Y, Keshavmurthy S, Chen CA (2020) Leptoria phrygia in Southern Taiwan shuffles and switches symbionts to resist thermal-induced bleaching. Sci Rep 10:7808
Hughes TP, Kerry JT, Álvarez-Noriega M, Álvarez-Romero JG, Anderson KD, Baird AH, Babcock RC, Beger M, Bellwood DR, Berkelmans R, Bridge TC, Butler IR, Byrne M, Cantin NE, Comeau S, Connolly SR, Cumming GS, Dalton SJ, Diaz-Pulido G, Eakin CM, Figueira WF, Gilmour JP, Harrison HB, Heron SF, Hoey AS, Hobbs J-PA, Hoogenboom MO, Kennedy EV, Kuo C-Y, Lough JM, Lowe RJ, Liu G, McCulloch MT, Malcolm HA, McWilliam MJ, Pandolfi JM, Pears RJ, Pratchett MS, Schoepf V, Simpson T, Skirving WJ, Sommer B, Torda G, Wachenfeld DR, Willis BL, Wilson SK (2017) Global warming and recurrent mass bleaching of corals. Nature 543:373–377
Hughes TP, Kerry JT, Baird AH, Connolly SR, Dietzel A, Eakin CM, Heron SF, Hoey AS, Hoogenboom MO, Liu G, McWilliam MJ, Pears RJ, Pratchett MS, Skirving WJ, Stella JS, Torda G (2018a) Global warming transforms coral reef assemblages. Nature 556:492–496
Hughes TP, Anderson KD, Connolly SR, Heron SF, Kerry JT, Lough JM, Baird AH, Baum JK, Berumen ML, Bridge TC, Claar DC, Eakin CM, Gilmour JP, Graham NAJ, Harrison H, Hobbs J-PA, Hoey AS, Hoogenboom M, Lowe RJ, McCulloch MT, Pandolfi JM, Pratchett M, Schoepf V, Torda G, Wilson SK (2018b) Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359:80–83
Hume BCC, D’Angelo C, Burt JA, Wiedenmann J (2018a) Fine-scale biogeographical boundary delineation and sub-population resolution in the Symbiodinium thermophilum coral symbiont group from the Persian/Arabian gulf and gulf of Oman. Front Mar Sci 5:138
Hume BC, Smith EG, Ziegler M, Warrington HJ, Burt JA, LaJeunesse TC, Wiedenmann J, Voolstra CR (2019) SymPortal: a novel analytical framework and platform for coral algal symbiont next-generation sequencing ITS2 profiling. Mol Ecol Resour 19:1063–1080
Hume BCC, Mejia-Restrepo A, Voolstra CR, Berumen ML (2020) Fine-scale delineation of Symbiodiniaceae genotypes on a previously bleached central Red Sea reef system demonstrates a prevalence of coral host-specific associations. Coral Reefs 39:583–601
Hume BC, D’Angelo C, Smith EG, Stevens JR, Burt J, Wiedenmann J (2015) Symbiodinium thermophilum sp. nov., a thermotolerant symbiotic alga prevalent in corals of the world’s hottest sea, the Persian/Arabian gulf. Sci Rep 5:8562
Hume BC, Ziegler M, Poulain J, Pochon X, Romac S, Boissin E, De Vargas C, Planes S, Wincker P, Voolstra CR (2018b) An improved primer set and amplification protocol with increased specificity and sensitivity targeting the Symbiodinium ITS2 region. Peer J 6:e4816
Illumina (2013) 16S metagenomic sequencing library preparation. Accessed from https://support.illumina.com/documents/documentation/chemistry_documentation/16s/16s-metagenomic-library-prep-guide-15044223-b.pdf on 22 July 2020
Jain SS, Afiq-Rosli L, Feldman B, Levy O, Phua JW, Wainwright BJ, Huang D (2020) Homogenization of endosymbiont communities hosted by equatorial corals during the 2016 mass bleaching event. Microorganisms 8:1370
Jain SS, Afiq-Rosli L, Feldman B, Kunning I, Levy O, Mana RR, Wainwright BJ, Huang D (2021) Endosymbiont communities in Pachyseris speciosa highlight geographical and methodological variations. Front Mar Sci 8:759744
Kemp DW, Hernandez-Pech X, Iglesias-Prieto R, Fitt WK, Schmidt GW (2014) Community dynamics and physiology of Symbiodinium spp. before, during, and after a coral bleaching event. Limnol Oceanogr 59:788–797
Kimura T, Tun K, Chou LM (2018) Status of coral reefs in East Asian Seas region: 2018. In: Kimura T, Tun K, Chou LM (eds). Ministry of the Environment of Japan and Japan Wildlife Research Center, Tokyo, pp 58
LaJeunesse TC, Bhagooli R, Hidaka M, DeVantier L, Done T, Schmidt GW, Fitt WK, Hoegh-Guldberg O (2004) Closely related Symbiodinium spp. differ in relative dominance in coral reef host communities across environmental, latitudinal and biogeographic gradients. Mar Ecol Prog Ser 284:147–161
LaJeunesse TC, Pettay DT, Sampayo EM, Phongsuwan N, Brown B, Obura DO, Hoegh-Guldberg O, Fitt WK (2010) Long-standing environmental conditions, geographic isolation and host–symbiont specificity influence the relative ecological dominance and genetic diversification of coral endosymbionts in the genus Symbiodinium. J Biogeogr 37:785–800
LaJeunesse TC, Parkinson JE, Gabrielson PW, Jeong HJ, Reimer JD, Voolstra CR, Santos SR (2018) Systematic revision of Symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Curr Biol 28:2570–2580
Lam KKY, Loo AHB, Todd PA, Chew FT, Chou LM (2006) Existence of intra-colonial paralogues of the ribosomal internal transcribed spacer (ITS) impedes studies of intra-colonial genetic variation in the scleractinian coral Diploastrea heliopora (Lamark 1816). Raffles Bull Zool 54:485–489
Lee MJ, Jeong HJ, Jang SH, Lee SY, Kang NS, Lee KH, Kim HS, Wham DC, LaJeunesse TC (2016) Most low-abundance “background” Symbiodinium spp. are transitory and have minimal functional significance for symbiotic corals. Microb Ecol 71:771–783
Leveque S, Afiq-Rosli L, Ip YCA, Jain SS, Huang D (2019) Searching for phylogenetic patterns of Symbiodiniaceae community structure among Indo-Pacific Merulinidae corals. Peer J 7:e7669
Loya Y, Sakai K, Yamazato K, Nakano Y, Sambali H, van Woesik R (2001) Coral bleaching: the winners and the losers. Ecol Lett 4:122–131
Matthews JL, Raina JB, Kahlke T, Seymour JR, van Oppen MJ, Suggett DJ (2020) Symbiodiniaceae-bacteria interactions: rethinking metabolite exchange in reef-building corals as multi-partner metabolic networks. Environ Microbiol 22:1675–1687
Ng CSL, Huang D, Toh KB, Sam SQ, Kikuzawa YP, Toh TC, Taira D, Chan YKS, Hung LZT, Sim WT, Rashid AR, Afiq-Rosli L, Ng NK, Chou LM (2020) Responses of urban reef corals during the 2016 mass bleaching event. Mar Pollut Bull 154:111111
Ng CSL, Chan YKS, Nguyen NTH, Kikuzawa YP, Sam SQ, Toh TC, Mock AYJ, Chou LM, Huang D (2021) Coral community composition and carbonate production in an urbanized seascape. Mar Environ Res 168:105322
Oksanen J, Guillaume FB, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2018) Vegan: community ecology package. R package version 2. 4–6. https://CRAN.R-project.org/package=vegan
Oliver J, Berkelmans R, Eakin C (2018) Coral bleaching in space and time. In: van Oppen MJH, Lough JM (eds) Coral bleaching. Springer, Cham, pp 27–49
Osman EO, Suggett DJ, Voolstra CR, Pettay DT, Clark DR, Pogoreutz C, Sampayo EM, Warner ME, Smith DJ (2020) Coral microbiome composition along the northern Red Sea suggests high plasticity of bacterial and specificity of endosymbiotic dinoflagellate communities. Microbiome 8:8
Peixoto RS, Rosado PM, Leite DC, Rosado AS, Bourne DG (2017) Beneficial microorganisms for corals (BMC): proposed mechanisms for coral health and resilience. Front Microbiol 8:341
Pootakham W, Mhuantong W, Putchim L, Yoocha T, Sonthirod C, Kongkachana W, Sangsrakru D, Naktang C, Jomchai N, Thongtham N, Tangphatsornruang S (2018) Dynamics of coral-associated microbiomes during a thermal bleaching event. MicrobiologyOpen 7:e00604
Poquita-Du RC, Quek ZBR, Jain SS, Schmidt-Roach S, Tun K, Heery EC, Chou LM, Todd PA, Huang D (2019) Last species standing: loss of Pocilloporidae corals associated with coastal urbanization in a tropical city state. Mar Biodivers 49:1727–1741
Poquita-Du RC, Huang D, Chou LM, Todd PA (2020) The contribution of stress-tolerant endosymbiotic dinoflagellate Durusdinium to Pocillopora acuta survival in a highly urbanized reef system. Coral Reefs 39:745–755
Poquita-Du RC, Ng CSL, Loo JB, Afiq-Rosli L, Tay YC, Todd PA, Chou LM, Huang D (2017) New evidence shows that Pocillopora “damicornis-like” corals in Singapore are actually Pocillopora acuta (Scleractinia: Pocilloporidae). Biodivers Data J 5:e11407
Praveena SM, Siraj SS, Aris AZ (2012) Coral reefs studies and threats in Malaysia: a mini review. Rev Environ Sci Biotechnol 11:27–39
Qin Z, Yu K, Chen B, Wang Y, Liang J, Luo W, Xu L, Huang X (2019) Diversity of Symbiodiniaceae in 15 coral species from the southern south China Sea: potential relationship with coral thermal adaptability. Front Microbiol 10:2343
Quigley KM, Alvarez Roa C, Torda G, Bourne DG, Willis BL (2019) Co-dynamics of Symbiodiniaceae and bacterial populations during the first year of symbiosis with Acropora tenuis juveniles. MicrobiologyOpen 9:e959
R Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Reef Check Malaysia (2018) Status of coral reefs in Malaysia, 2018. Reef Check Malaysia, Kuala Lumpur
Roberts CM, McClean CJ, Veron JE, Hawkins JP, Allen GR, McAllister DE, Mittermeier CG, Schueler FW, Spalding M, Wells F, Vynne C, Werner TB (2002) Marine biodiversity hotspots and conservation priorities for tropical reefs. Science 295:1280–1284
Sampayo EM, Ridgway T, Bongaerts P, Hoegh-Guldberg O (2008) Bleaching susceptibility and mortality of corals are determined by fine-scale differences in symbiont type. Proc Natl Acad Sci USA 105:10444–10449
Savage C (2019) Seabird nutrients are assimilated by corals and enhance coral growth rates. Sci Rep 9:4284
Schloerke B, Cook D, Larmarange J, Francois B, Marbach M, Thoen E, Elberg A, Crowley J (2021) GGally: extension to “ggplot2”. R package version 2.1.2. https://CRAN.R-project.org/package=GGally
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541
Shoguchi E, Beedessee G, Tada I, Hisata K, Kawashima T, Takeuchi T, Arakaki N, Fujie M, Royanagi R, Roy MC, Kawachi M, Hidaka M, Satoh N, Shinzato C (2018) Two divergent Symbiodinium genomes reveal conservation of a gene cluster for sunscreen biosynthesis and recently lost genes. BMC Genom 19:458
Silverstein R, Cunning R, Baker A (2015) Change in algal symbiont communities after bleaching, not prior heat exposure, increases heat tolerance of reef corals. Glob Chang Biol 21:236–249
Sin TM, Ang HP, Buurman J, Lee AC, Leong YL, Ooi SK, Steinberg P, Teo SL-M (2016) The urban marine environment of Singapore. Reg Stud Mar Sci 8:331–339
Smith EG, Gurskaya A, Hume BC, Voolstra CR, Todd PA, Bauman AG, Burt JA (2020) Low Symbiodiniaceae diversity in a turbid marginal reef environment. Coral Reefs 39:545–553
Swain TD, Lax S, Lake N, Grooms H, Backman V, Marcelino LA (2018a) Relating coral skeletal structures at different length scales to growth, light availability to Symbiodinium, and thermal bleaching. Front Mar Sci 5:450
Swain TD, Bold EC, Osborn PC, Baird AH, Westneat MW, Backman V, Marcelino LA (2018b) Physiological integration of coral colonies is correlated with bleaching resistance. Mar Ecol Prog Ser 586:1–10
Tan YTR, Wainwright BJ, Afiq-Rosli L, Ip YCA, Lee JN, Nguyen NTH, Pointing SB, Huang D (2020) Endosymbiont diversity and community structure in Porites lutea from Southeast Asia are driven by a suite of environmental variables. Symbiosis 80:269–277
Tanzil JT, Ng AP, Tey YQ, Tan BH, Yun EY, Huang D (2016) A preliminary characterisation of Symbiodinium diversity in some common corals from Singapore. Cosmos 12:15–27
Terraneo TI, Fusi M, Hume BC, Arrigoni R, Voolstra CR, Benzoni F, Forsman ZH, Berumen ML (2019) Environmental latitudinal gradients and host-specificity shape Symbiodiniaceae distribution in Red Sea Porites corals. J Biogeogr 46:2323–2335
Todd PA, Ladle RJ, Lewin-Koh NJI, Chou LM (2004) Genotype × environment interactions in transplanted clones of the massive corals Favia speciosa and Diploastrea heliopora. Mar Ecol Prog Ser 271:167–182
Toh TC, Huang D, Tun K, Chou LM (2018) Summary of coral bleaching from 2014 to 2017 in Singapore. In: Kimura T, Tun K, Chou LM (eds) Status of coral reefs in East Asian Seas region: 2018. Ministry of the Environment of Japan and Japan Wildlife Research Center, Tokyo, pp 21–23
Tong H, Cai L, Zhou G, Zhang W, Huang H, Qian PY (2020) Correlations between prokaryotic microbes and stress-resistant algae in different corals subjected to environmental stress in Hong Kong. Front Microbiol 11:686
Tonk L, Sampayo EM, Weeks S, Magno-Canto M, Hoegh-Guldberg O (2013) Host-specific interactions with environmental factors shape the distribution of Symbiodinium across the Great Barrier Reef. PLoS ONE 8:e68533
Tyberghein L, Verbruggen H, Pauly K, Troupin C, Mineur F, De Clerck O (2012) Bio-ORACLE: a global environmental dataset for marine species distribution modelling. Glob Ecol Biogeogr 21:272–281
Veron JEN, DeVantie LM, Turak E, Green AL, Kininmonth S, Stafford-Smith M, Peterson N (2011) The Coral Triangle. In: Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer, Dordecht, pp 47–55
Veron JEN, Stafford-Smith M, DeVantier L, Turak E (2015) Overview of distribution patterns of zooxanthellate Scleractinia. Front Mar Sci 1:81
Wagner G (2021) Recalculate the social cost of carbon. Nat Clim Chang 11:293–294
Weber M, Lott C, Fabricius K (2006) Sedimentation stress in a scleractinian coral exposed to terrestrial and marine sediments with contrasting physical, organic and geochemical properties. J Exp Mar Biol Ecol 336:18–32
West JM, Salm RV (2003) Resistance and resilience to coral bleaching: implications for coral reef conservation and management. Conserv Biol 17:956–967
Wong JSY, Chan YKS, Ng CSL, Tun KPP, Darling ES, Huang D (2018) Comparing patterns of taxonomic, functional and phylogenetic diversity in reef coral communities. Coral Reefs 37:737–750
Zhou Z, Zhang K, Wang L, Su Y, Wang J, Song T, Yang X, Tang J, Lin S (2021) Nitrogen availability improves the physiological resilience of coral endosymbiont Cladocopium goreaui to high temperature. J Phycol 57:1187–1198
Ziegler M, Eguíluz VM, Duarte CM, Voolstra CR (2018) Rare symbionts may contribute to the resilience of coral–algal assemblages. ISME J 12:161–172
Acknowledgements
This study was funded by the National Research Foundation, Prime Minister’s Office, Singapore, under its Marine Science R&D Programme (MSRDP-P38) and by the Mind the Gap—Sustainable Earth Fund. Collections from Peninsular Malaysia and Singapore were made under permits JTLM 630-7Jld.9(9) and NP/RP16-156, respectively. We thank two anonymous reviewers for constructive comments that helped improve the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Topic Editor Steve Vollmer
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ong, J.H., Wainwright, B.J., Jain, S.S. et al. Species and spatio-environmental effects on coral endosymbiont communities in Southeast Asia. Coral Reefs 41, 1131–1145 (2022). https://doi.org/10.1007/s00338-022-02254-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-022-02254-7