Abstract
Algal endosymbionts of the genus Symbiodinium play a key role in the nutrition of reef building corals and strongly affect the thermal tolerance and growth rate of the animal host. This study reports that 14C photosynthate incorporation into juvenile coral tissues was doubled in Acropora millepora harbouring Symbiodinium C1 compared with juveniles from common parentage harbouring Symbiodinium D in a laboratory experiment. Rapid light curves performed on the same corals revealed that the relative electron transport rate of photosystem II (rETRMAX) was 87% greater in Symbiodinium C1 than in Symbiodinium D in hospite. The greater relative electron transport through photosystem II of Symbiodinium C1 is positively correlated with increased carbon delivery to the host under the applied experimental conditions (r 2 = 0.91). This may translate into a competitive advantage for juveniles harbouring Symbiodinium C1 under certain field conditions, since rapid early growth typically limits mortality. Both symbiont types exhibited severe reductions in 14C incorporation during a 10-h exposure to the electron transport blocking herbicide diuron (DCMU), confirming the link between electron transport through PSII and photosynthate incorporation within the host tissue. These findings advance the current understanding of symbiotic relationships between corals and their symbionts, providing evidence that enhanced growth rates of juvenile corals may result from greater translocation of photosynthates from Symbiodinium C1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Zooxanthellae (symbiotic dinoflagellates of the genus Symbiodinium) are critical to the survival of reef-building corals, providing a major source of energy from photosynthesis for cell maintenance, growth and reproduction of their coral hosts (Crossland et al. 1980; Muscatine et al. 1984). Molecular techniques using nuclear 28S, ITS1, ITS2, and organellar 23S and cox1 markers have uncovered a large diversity describing eight major groupings or clades within the genus Symbiodinium (van Oppen et al. 2001; LaJeunesse 2002; Santos et al. 2002; Baker 2003; Pochon et al. 2004; Rowan 2004; Takabayashi et al. 2004; Coffroth and Santos 2005; Apprill and Gates 2007). The term ‘clade’ refers to one of the eight major groupings of Symbiodinium named A-H. Sub-cladal groups are referred to as ‘types’ within this paper. Differences in photobiology are known to exist between in hospite Symbiodinium types, especially under conditions of thermal or irradiance stress (Rowan 2004; Goulet et al. 2005; Berkelmans and van Oppen 2006). As ocean warming associated with climate change is predicted to cause an increase in the frequency and intensity of coral bleaching events, assessing the performance of distinct coral-zooxanthella associations under conditions of photosystem stress will enhance the understanding of the future health of coral reefs (Little et al. 2004; Abrego et al. 2008).
Physiological traits of the coral host are at least partly shaped by the dominant symbiont type present within its tissues. For instance, the genetic type of symbiont within juveniles of Acropora millepora and Acropora tenuis has been linked to a 2- to 3-fold increase in growth rate within the first 6 months of development on the reef (Little et al. 2004). Furthermore, adult A. millepora corals on the Great Barrier Reef (GBR) have been shown to acquire a 1–1.5°C increase in thermal tolerance by shuffling the dominant symbiont type present within coral tissues (Berkelmans and van Oppen 2006). Likewise, in Guam, colonies of Pocillopora spp. associating with Symbiodinium D exhibited greater tolerance to thermal stress compared to corals associating with Symbiodinium C (Rowan 2004). Capacity for photoacclimation and tolerance to high irradiance stress has also been linked to the genetic type of Symbiodinium spp., both in culture and within multiple coral host species (Robison and Warner 2006; Warner et al. 2006). Recent studies have shown that symbiont type can affect the incorporation of algal-derived photosynthetic carbon (14C) into host tissues of an anemone (Loram et al. 2007) and the total amount of photosynthetically fixed carbon released from the symbiont (Stat et al. 2008), further supporting the notion that symbiont type can affect growth and resilience of invertebrate hosts to stress. Anemones hosting Symbiodinium clade A obtained a greater proportion of photosynthetically fixed carbon into animal lipids and amino acid pools than those hosting clade B. Total fixation increased at high temperatures for clade A anemones and was depressed for clade B anemones, demonstrating that these two distinct Symbiodinium clades are not functionally equivalent (Loram et al. 2007). The ability of some corals to associate with a diverse range of symbiont types (van Oppen et al. 2001; Baker 2003; Rowan 2004) may provide ecological advantages to the host colony, enabling it to colonize a variety of reef habitats and survive a changing global climate.
Acropora millepora is typical of most broadcast spawning corals, acquiring symbionts from the environment just prior to or following larval metamorphosis. It associates with Symbiodinium D on reefs surrounding Magnetic Island, a nearshore island in the central GBR. However, it commonly harbours Symbiodinium C1 and C2 and occasionally with C3 on inner-, mid- and outer-shelf reefs (van Oppen et al. 2001, 2005). On the GBR, Symbiodinium D has a predominantly inshore distribution and hence experiences high temperatures, turbidity and pollution events relatively frequently. Its rarity on mid- or outer-shelf reefs is possibly due to higher light intensities common at these sites (van Oppen et al. 2005).
This study compared the effect of hosting Symbiodinium C1 and D on the translocation of carbon-based energy (measured as tissue 14C incorporation) to the coral host. The relative electron transport rates of photosystem II (rETRMAX) of these symbiont types were then measured in hospite as a secondary measure of photosynthetic performance. Photosynthetic performance of the Symbiodinium types were also compared following exposure to the photosystem II (PSII) blocker DCMU (diuron), which is also an environmentally relevant contaminant (Jones et al. 2003).
Materials and methods
The experiment was conducted under controlled light and temperature conditions using 9-month-old A. millepora juveniles. In this set-up, juveniles experimentally infected with Symbiodinium C1 or D originated from crosses involving the same parent corals, thereby minimizing potential host genetic differences that may influence the physiology of the holobiont (host–symbiont partnership). The experimental design therefore controlled for both environmental and host influences on symbiont physiology (Coles and Brown 2003; Rowan 2004).
Juvenile inoculation and treatments
Gametes were collected following spawning from eight colonies of A. millepora and mixed for fertilization. Larvae were raised in filtered seawater (1 μm). On day 4 following spawning, when larvae were first observed to exhibit settlement behaviour, preconditioned (for 16 weeks on the reef at Magnetic Island), autoclaved (to kill any Symbiodinium spp. present on the tiles) terracotta tiles were placed on the bottom of two 500 l tanks as settlement surfaces. Symbiodinium type C1 (GenBank Accession No. AF380555) and D (GenBank Accession No. EU024793) based on ITS1 were selected for experimental infection of juveniles because both associate with A. millepora on inshore reefs of the GBR (van Oppen et al. 2005). Symbiodinium C1 and D were obtained from adult colonies of A. tenuis and A. millepora at Magnetic Island, respectively, by airbrushing the coral tissue and isolating the Symbiodinium cells from the coral-algal slurry through centrifugation (5 min at 350 × g). These isolated symbionts were offered to larvae and newly settled juveniles at 108 cells/tank after 3 and 5 days following spawning. Infection of the coral juveniles was confirmed by microscopic observation of squash preparations indicating the presence of symbionts within the tentacles of each juvenile. Symbiodinium genotypes were confirmed following infection prior to field deployment by single-stranded conformation polymorphism (SSCP, following methods described in van Oppen et al. 2001) of the ITS1 regions and potential presence of unexpected background strains was tested using quantitative PCR at the end of the experiment (see below). The juvenile corals were allowed to develop for a further 2 weeks in the laboratory, after which the tiles were attached vertically to racks on a fringing reef (Nelly Bay, Magnetic Island) in a zone where A. millepora is common, and were randomly arranged to minimize any effects of partial shading during this grow-out period. The juveniles were collected 9 months later and acclimated horizontally under identical natural illumination (75% shading, approximately 200 μmol photons m−2 s−1) in an outdoor flow-through aquarium for 2 days prior to experimental testing.
The juvenile corals were exposed to the herbicide DCMU (diuron or 3(3,4-dichlorophenyl)-1,1-dimethylurea, photosystem II inhibitor), an environmentally relevant contaminant that acts by inhibiting photosynthesis by blocking electron transport and causes photoinactivation of photosystem II (PSII) (Jones et al. 2003). Unlike high temperature and irradiation stresses, DCMU exposure influences the symbiont’s performance without directly affecting the coral host (Schreiber et al. 1997; Negri et al. 2005; Cantin et al. 2007), therefore enabling the effects of photoinhibition to be distinguished between symbiont types. Nine-month-old juveniles were randomly distributed within three glass tanks (4 l filtered sea water, 0.25 μm) per DCMU treatment [0 (control), 1 and 10 μg l−1; n = 9 juveniles per DCMU treatment and symbiont type; 10 h DCMU exposure]. Tanks were placed under metal halide lamps exposing the corals to a constant illumination of 180–200 μmol photons m−2 s−1.
Pulse amplitude modulation (PAM) fluorometry
All fluorescence measurements were taken with a Diving-PAM (Walz, Germany) by holding a 2-mm fibre-optic probe at a consistent distance of 2 mm directly above each juvenile coral using the manufacturer-supplied leaf clip. Rapid light curves (RLCs) can be used to assess the current photosynthetic capacity of PSII as a function of irradiance under increasing levels of light intensity (PAR) (Schreiber et al. 1997; Ralph and Gademann 2005). RLCs are constructed by plotting the effective quantum yield as measured with a PAM fluorometer against PAR. Cardinal points calculated from a RLC, such as the maximum relative electron transport rate of PSII (rETRMAX), reflect the present state of photosynthesis (of PSII) and are strongly dependent on the immediate light pre-history of the sample (Schreiber 2004). These measurements differ from traditional photosynthesis-irradiance (P-E) curves derived from gas exchange measurements, which effectively describe how the entire photosynthetic apparatus acclimates to different light intensities and are less dependent on light pre-history. RLC measurements calculated within this study were used comparatively among the different symbiont and DCMU treatments to reflect the current photosynthetic performance under the experimental light conditions.
After 2 h of light exposure, the corals were placed in total darkness for a short time (2-min dark adaptation) to allow substantial re-oxidation of the primary electron acceptor (QA) (Schreiber 2004) and minimize the total time required for RLC measurements. RLC measurements were performed in the dark on three juveniles for each symbiont type and DCMU treatment (n = 3), taking a total of 90 min. Each tank was immediately returned to the light regime following each RLC. The RLCs were measured using a pre-installed software routine, where the actinic measuring light was incremented over eight light steps (0, 44, 72, 116, 147, 222, 283, 428 and 653 μmol photons m−2 s−1), each with a duration of 10 s. The relative electron transport rate (rETR) (Eq. 1) obtained from RLCs provides a reliable approximation of relative electron flow through PSII when absorbance between samples is identical (Genty et al. 1989). In the present experiments, this was assumed since all juveniles (i) had similar colony heights (2–4 mm), (ii) received the same irradiance, (iii) contained the same quantity of symbionts (see below) and pigment concentrations in control treatments (see Results section) and (iv) were tested using the same host coral species; therefore, the corallite shapes were identical, thus reducing the influence of light scattering based on coral skeleton morphologies. Correction factors for absorbance were not used in calculations of rETR.
Minimum fluorescence (F in illuminated samples or Fo in dark-adapted samples) was determined by applying a weak pulse-modulated red measuring light (0.15 μmol photons m−2 s−1). The maximum fluorescence (\({F_{\text{m}^{\prime}}}\) in illuminated samples or F m in dark-adapted samples) was then measured following the application of a saturating pulse of actinic light (>3,000 μmol photons m−2 s−1). The maximum quantum yield (F v/F m, Eq. 2) is the proportion of light absorbed by chlorophyll (in PSII) used for photochemistry in the dark-adapted organisms, when all reaction centres are open (Genty et al. 1989). Maximum quantum yield (F v/F m) values were obtained from dark-adapted symbionts (n = 3 juvenile corals) following each RLC and an additional 10-min dark adaptation.
Radio-labelled 14C incorporation
Following the fluorescence measurements, the volume of filtered seawater was reduced to 1 l and 1 ml of NaH14CO3 (specific activity 74 MBq ml−1, Amersham Biosciences, USA) was added to each tank. The water level was subsequently raised to 2 l to ensure equal distribution of radiolabel throughout the tank. 14C incubation was carried out for 6 h under constant illumination (180–200 μmol photons m−2 s−1). At the end of the incubation, each tile was removed from the experimental light exposure and rinsed twice with fresh filtered sea water for 5 min to remove unincorporated 14C from the surface of coral tissues. Each juvenile (n = 9) was removed from the terracotta tile with a scalpel, snap frozen in liquid nitrogen and stored at −80°C until analysis. The time frame used to rinse each juvenile coral at lower light intensity (approx 15–30 μmol photons m−2 s−1 for 10–15 min) prior to freezing may have influenced the xanthophyll pigment ratios [conversion of diatoxanthin back to diadinoxanthin in the absence of light stress (Brown et al. 1999)]; however, this was not likely to influence the total xanthophyll pool. Tissue from each juvenile A. millepora (n = 9) was removed by airbrushing and the host tissue was separated from the symbionts by centrifugation (490 × g). Host tissue samples (100 μl) were acidified with 0.1 M HCl (100 μl) prior to scintillation counting to remove unincorporated 14C. Host tissue samples were counted on a 1450 Microbeta Plus scintillation counter (Perkin Elmer) for 2 min to determine disintegrations per minute (dpm) and the amount of 14C incorporation. Symbiodinium cells were resuspended in 10% formalin and cell densities were determined with a haemocytometer. Mean cell densities were not different between symbiont types: Symbiodinium C1 = 5.5 ± 0.4 (SE) × 105 cells cm−2 and Symbiodinium D = 4.6 ± 0.8 × 105 cells cm−2, P = 0.20]. Digital images of the juveniles were taken from a standardized height on a tripod and the perimeter of each juvenile coral was then traced using image analysis software (Optimas, Media Cybernetics, Silver Spring, MD, USA) and surface area of each juvenile coral was then calculated using Optimas (Negri et al. 2005). The radiolabelled photosynthate incorporation was expressed as radioactivity per unit area of juvenile per dinoflagellate cell (dpm zoox−1 cm−2) from the calculated surface area measurements to standardize for the variations in the size of individual juveniles and the Symbiodinium densities they host.
Extraction, amplification and sequencing of Symbiodinium chloroplast 23S ribosomal DNA (cp23S-rDNA)
In order to develop a clade-specific quantitative real-time PCR assay based on chloroplast DNA (cpDNA), small branches were collected from colonies of A. tenuis harbouring Symbiodinium C1 (from Magnetic Island, Great Barrier Reef (GBR)) and A. millepora harbouring Symbiodinium D (collected from Magnetic Island), Symbiodinium C2 (from Davies Reef) or Symbiodinium C2 or D (from Keppel Islands, GBR) and fixed in absolute ethanol. DNA was extracted following a previously published DNA isolation method (Wilson et al. 2002). An approximately 0.7 kb region of Symbiodinium cp23S-rDNA was PCR amplified using the primer pair 23S1 (5′-GGCTGTAACTATAACGGTCC-3′) and 23S2 (5′-CCATCGTATTGAACCCAGC-3′) (Santos et al. 2002). PCRs were performed in 25 μl volumes containing 2 mM MgCl, 200 μM dNTPs, 1 × DNA Polymerase PCR buffer, 0.02 U Taq polymerase (Fisher Biotec, Australia), 40 pmol of each primer and 50–70 ng of template DNA. Reactions were carried out in a Corbett Research PC-960G Gradient Thermal Cycler (Corbett Research, NSW Australia) under the following conditions: initial denaturing period of 1 min at 95°C, 35 cycles consisting of 95°C for 45 s, 55°C for 45 s and 72°C for 1 min, followed by a final extension period at 72°C for 7 min. PCR products were precipitated by adding 100% ethanol, 0.3 M ammonium acetate and then centrifuged at 1,530 × g in a bench top centrifuge at 4°C for 20 min. The products were cloned using the TOPO TA Cloning kit (Invitrogen, Victoria Australia) according to the manufacturer’s directions. Colony PCR was conducted using the universal M13 plasmid primers M13F (5′-GTAAAACGACGGCCAG-3′) and M13R (5′-CAGGAAACAGCTATGAC-3′). The products were then purified and sequenced at the Macrogen Sequencing Service (Macrogen Inc., South Korea).
Symbiodinium cp23S-rDNA quantitative real-time PCR assay
In order to determine the relative abundance of symbiont types within A. millepora juveniles used in this 14C study, local GBR cp23S-rDNA Symbiodinium sequences (one C1 from Magnetic Island, GenBank Accession No. EF140804; two C2 from Davies Reef, GenBank Accession No. EF140806 and Keppel Islands, GenBank Accession No. EF140805; and one D from Magnetic Island, GenBank Accession No. EF140808) were added to an existing alignment (Santos et al. 2002) to design Symbiodinium clade C and D specific primers for the quantitative real-time PCR (qPCR) assay. Relative symbiont abundance within the A. millepora juveniles was estimated from qPCR of the cp23S-rDNA region. The primer pairs 23S C FP2 (5′-GGGATAAAACTTGGGTAACATTC-3′) and 23S C RP2 (5′-CCAATTAAACAGTGGTCTTAGGAG-3′) were used to amplify Symbiodinium C and 23S D FP1 (5′-AACCCCCGATTGGCCTAG-3′) and 23S D RP1 (5′-CTTGATTGGGCCATTAAGCA-3′) were used to amplify Symbiodinium D. Clade specificity of each primer pair was confirmed by the absence of D amplification with the C primers and vice versa in qPCR assays with cloned Symbiodinium C1 and D 23S DNA. Each DNA sample was analyzed in duplicate with a Symbiodinium C specific reaction, a Symbiodinium D specific reaction and a quantified standard with DNA isolated from 100,000 cells of each Symbiodinium type, along with no-template controls. qPCR reactions were performed in 20 μl volumes containing 10.0 μl of Platinum SYBR Green qPCR Supermix UDG (Invitrogen, Victoria Australia), 1.0 μl of 4 μM C/D specific FP, 1.0 μl of 4 μM C/D specific RP, 6.0 μl of MilliQ water and 2.0 μl of DNA template. Reactions were carried out in the Rotor-Gene RG-3000A thermal cycler (Corbett Research, NSW Australia) under the following conditions: an initial denaturing period of 2 min at 50°C and 2 min at 95°C, 40 cycles of 15 s at 95°C and 30 s at 60°C. In order to check for the formation of primer dimers and to verify Symbiodinium type primer specificity, a melt curve was generated at the end of the run and the melting point (T m) for each symbiont specific reaction was determined by starting at 60°C for 30 s and raising the temperature by 1°C every 5 s until 95°C was reached. Fluorescent data collection took place after each 60°C step during cycling and after each temperature step of the melt curve. Serial dilutions over a range of 5 logs were run to ensure both the Symbiodinium C and D reactions had similar efficiencies (Symbiodinium C reaction efficiency = 0.92 and Symbiodinium D reaction efficiency = 0.95). Relative abundances (as a percentage of total copies per reaction) were determined from the calculated concentration (copies per reaction) within Rotor-Gene Analysis Software v. 6.0 (Corbett Research, NSW Australia) for each Symbiodinium type per reaction compared to the 100,000 copy standard after importing the standard curve from the serial dilution series. Although copy numbers may vary over the symbionts growth curve (Koumandou and Howe 2007), it is assumed that growth in hospite was not likely to be synchronous and that the application of duplicate 100,000 cell extracts is likely to have averaged out any differences. Quantification using multi-copy genes may also be affected by potential differences in copy numbers between symbiont types. A nuclear rDNA qPCR assay had previously been compared with the cpDNA assay used here and the quantification results were very similar (Mieog et al. 2007).
Although the cp23S-rDNA assay used here is Symbiodinium clade and not type specific, it is highly unlikely the C-juveniles would harbour C-types other than C1. First, the juveniles were experimentally infected with C1 and it was verified using SSCP of the rDNA ITS1 region that a symbiosis had been established with C1 prior to deployment of the juveniles in the field. Second, juveniles from the same batch that were used in other experiments only harboured C1 and D after the grow-out period in the field as verified by SSCP analysis (J.C. Mieog et al., pers. comm.). Third, C-types other than C1 are extremely rare at Magnetic Island; of 52 cnidarian species (136 specimens) surveyed at Magnetic Island (D. Abrego et al., pers. comm.), the vast majority harboured C1 or D, two Montipora species harboured C• (which has so far only been found in Porites and Montipora species and is not taken up by Acropora spp. in infection experiments), and a single colony harboured a mix of C1 and C2-like. Other previous reports on zooxanthellae types at Magnetic Island (van Oppen et al. 2001; van Oppen 2004; Ulstrup and van Oppen 2003) have shown only C1, C• and D with a single exception of an A. tenuis colony where C2 was present in addition to C1.
Pigment analysis by HPLC
Chlorophylls and carotenoids were extracted sequentially by sonication (Cole Parmer Ultrasonic Processor, Extech Equipment, Victoria, Australia) in 100% acetone from Symbiodinium cells that were separated from the host tissue (n = 8 juvenile corals). High performance liquid chromatography (HPLC) was used to analyse the extracts on a Waters 600 HPLC, combined with a Waters PDA 996 photodiodearray detector, on a 3 μm, 50 × 4.6 mm Phenomonex C-18 Gemini 110Å column (Phenomonex, NSW, Australia). A two solvent gradient with a flow rate of 1 ml min−1 was used to separate the pigments with a run time of 18 min. Percentages of the solvents A and B, respectively, were as follows: 0 min: 75, 25%; 0 to 5 min linear gradient to: 0, 100%; 5 to 10 min hold at: 0, 100%; 10–11 min linear gradient to: 75, 25%; 11–18 min hold at 75, 25%. Solvent A was 70:30 v/v methanol:28 mM tetrabutyl ammonium acetate (TBAA, 1.0 M aq. Sigma-Aldrich, Australia) and solvent B was 50:50 v/v methanol:acetone. Chlorophyll a, c 2 , peridinin and diadinoxanthin standards were obtained from the International Agency for 14C Determination (DHI, Denmark). The peaks reported were identified by comparison of retention times and absorption spectra with standards and published data (Wright and Jeffrey 1997).
Data analysis
Two way, Model I ANOVA (α = 0.05) was used to test the effect of Symbiodinium type and DCMU concentration on rETRMAX, F v/F m, 14C incorporation and total pigment concentrations. Fishers LSD post hoc test (P < 0.05) was used to identify statistical differences between treatments. Data were tested for assumptions of normality and homogeneity of variances and transformations were not required. Rapid light curves were fitted according to the functions of Platt et al. (1980) as outlined by Ralph et al. (2002). All figures and curve-fitting to determine the characteristic parameters of the rapid light curves (Ralph et al. 2002) were created using Sigmaplot 2001 for Windows (v. 7.1, SPSS Inc.). Statistica v. 6.0 (StatSoft, Inc. Oklahoma, USA) was used for all statistical analyses. Rotor-Gene Analysis Software v. 6.0 (Corbett Research, NSW Australia) was used for all real-time qPCR analysis.
Results
Quantitative real-time PCR assays indicated that the juvenile A. millepora colonies were dominated by the Symbiodinium types that they were exposed to during experimental infections after 9 months of growth on the reef at Magnetic Island in GBR. Juveniles exposed to Symbiodinium C1 were estimated to contain 98–100% relative abundance C (n = 27), while juveniles exposed to Symbiodinium D contained 94–100% relative abundance D (n = 27).
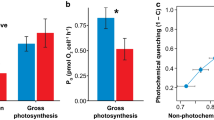
Incorporation of radiolabelled photosynthate (14C, energy) into the host tissue was 121% greater within juvenile A. millepora corals hosting Symbiodinium C1 than the colonies associated with Symbiodinium D in control treatments at 200 μmol photons m−2 s−1 (0 μg l−1, Fig. 1a, Two-way ANOVA, symbiont type: F 1,48 = 6.69, P = 0.02, DCMU: F 2,48 = 4.11, P = 0.01, symbiont*DCMU interaction: F 2,48 = 0.84, P = 0.4). Exposure to the electron transport inhibitor DCMU for 10 h reduced the incorporation of photosynthate into the tissues of corals hosting both Symbiodinium C1 and D (Fig. 1a). Corals hosting Symbiodinium C1 exhibited a 58% drop in photosynthate accumulation when exposed to 10 μg l−1 DCMU, while photosynthate level was reduced by 42% in corals hosting Symbiodinium D (Fig. 1a).
Photosynthate incorporation into juvenile corals and photosynthetic capacity of symbionts exposed to 3 DCMU (diuron) treatments (0, 1 and 10 μg l−1). a Photosynthate incorporation into juvenile A. millepora colonies hosting Symbiodinium C1 and Symbiodinium D as measured by uptake of 14C into host tissue n = 9 juveniles, mean ± SE. b Photosynthetic capacity of PSII in Symbiodinium C1 and D hosted by Acropora millepora juveniles as measured by relative electron transport (rETRMAX) derived from rapid light curve. n = 3 juveniles (mean ± SE). Asterisks (*) indicate significant differences (P < 0.05) between DCMU treatments compared to the control (0 μg l−1) and inequalities (≠) indicate significant differences between Symbiodinium C1 and D
Comparison of rapid light curves (RLCs, Fig. 2a, b) indicated that Symbiodinium C1 had an 87% greater relative electron transport rate through PSII (rETRMAX, Fig. 1b) than Symbiodinium D when associated with the same coral species in the absence of DCMU (Two-way ANOVA, symbiont type: F 1,12 = 6.85, P = 0.02, DCMU: F 2,12 = 14.77, P < 0.001, symbiont*DCMU interaction: F 2,12 = 2.07, P = 0.2). A strong, positive correlation was observed between rETRMAX and incorporation of 14C labelled photosynthates (r 2 = 0.91, P = 0.01, n = 6). Exposure of the juvenile colonies to DCMU significantly reduced the rETRMAX in both Symbiodinium C1 and D (Fig. 1b). At 10 μg l−1 DCMU, the rETRMAX was reduced by 86% in Symbiodinium C1 and by 71% in Symbiodinium D under the same experimental conditions (Fig. 1b). There was no difference between the maximum quantum yields (F v/F m) in dark-adapted corals for each symbiont type in the absence of DCMU (Fig. 3, Two-way ANOVA, symbiont type: F 1,102 = 0.05, P = 0.8, DCMU: F 2,102 = 103.76, P < 0.001, symbiont*DCMU interaction: F 2,102 = 0.61, P = 0.5), indicating similar efficiencies of excitation energy capture by PSII for each symbiont (Genty et al. 1989). DCMU exposure caused similar reductions in F v/F m in both symbionts (Fig. 3), indicating equivalent photoinactivation of PSII in both symbiont types (Schreiber 2004).
Rapid light curves from juvenile colonies of Acropora millepora. Relative electron transport rate (rETR) as a function of photosynthetically active radiation (PAR, μmol photons m−2 s−1) derived from colonies hosting a Symbiodinium C1 and b Symbiodinium D exposed to 3 DCMU (diuron) treatments (0, 1 and 10 μg l−1). n = 3 juveniles (mean ± SE)
Quantum yields of Symbiodinium C1 and D. Maximum quantum yields (F v/F m, mean ± SE, n = 3 juveniles) for dark-adapted Acropora millepora juveniles hosting Symbiodinium C1 and Symbiodinium D. Asterisks (*) indicate significant differences (P < 0.05) between DCMU treatments compared to the control (0 μg l−1) and inequalities (≠) indicate differences between Symbiodinium C1 and D
The pigments, chlorophyll a, c 2 and peridinin, which constitute the major light harvesting complex (LHC) within dinoflagellates, were detected with a molar ratio of 1:0.3:0.5 within the control juveniles. Diadinoxanthin was the major xanthophyll carotenoid within coral symbionts, along with low concentrations of diatoxanthin. No differences in light harvesting and xanthophyll pigments were evident between Symbiodinium types in the absence of DCMU (Fig. 4a and b) (Two-way ANOVA LHC symbiont type: F 1,42 = 0.25, P = 0.6, DCMU: F 2,42 = 3.14 P = 0.05, symbiont*DCMU interaction: F 2,42 = 2.54, P = 0.09, Fishers LSD, P = 0.04. Two-way ANOVA xanthophylls, symbiont type: F 1,42 = 0.52, P = 0.5, DCMU: F 2,42 = 2.76, P = 0.07, symbiont*DCMU interaction: F 2,42 = 2.91 P = 0.07, Fishers LSD, P = 0.02). Severe inhibition of PSII electron transport (10 μg l−1 DCMU treatment) for 10 h resulted in significant increases in both the total light harvesting and total xanthophyll pigments (by 108 and 114% respectively) in Symbiodinium C1, whereas pigment concentrations did not change in type D symbionts (Fig. 4). While the total light harvesting and xanthophyll pools increased for Symbiodinium C1, no changes in pigment ratios were observed for both symbiont types at any of the DCMU concentrations.
Pigment concentrations of Symbiodinium C1 and D. Pigments of Symbiodinium C1 and D within juveniles of Acropora millepora exposed to three different DCMU (diuron) treatments (0, 1 and 10 μg l−1). a Concentrations of total light harvesting pigments (LH), comprising chlorophyll a and c2 and peridinin (pg cell−1). b Concentrations of xanthophyll pigments, diadinoxanthin and diatoxanthin (pg cell−1). n = 8 juveniles (mean ± SE). Asterisks (*) indicate significant differences (P < 0.05) between DCMU treatment compared to the control (0 μg l−1) and inequalities (≠) indicate differences between Symbiodinium C1 and D
Discussion
Distinct patterns in rates of photosynthate (14C) incorporation and RLCs revealed physiological differences between Symbiodinium C1 and D when associated with the same host species. Symbiodinium C1 exhibited a 121% greater capacity for translocation of photosynthate to A. millepora juveniles along with 87% greater relative electron transport through photosystem II under identical environmental conditions. A. tenuis and A. millepora juveniles in a previous study exhibited 2 to 3 times faster growth rates when associated with Symbiodinium C1 compared to those associated with Symbiodinium D (Little et al. 2004) at the same field site where juveniles were reared in the present study. The higher growth rate previously found within C1 juveniles (Little et al. 2004) may result from greater translocation of photosynthate by colonies hosting Symbiodinium C1, as demonstrated here. The differences in carbon-based energy transfer between symbiont types may provide a competitive advantage to corals associating with Symbiodinium C1, particularly during their early life histories, when greater energy investment into rapid tissue and skeletal growth can prevent overgrowth of juveniles by competitors and mortality from grazers (Hughes and Jackson 1985).
The large differences in photosynthate incorporation into host tissue and photosynthetic performance observed between symbiont types in coral hosts of the same parentage highlights the functional influence of symbionts on the nutritional physiology of corals. Symbiodinium clade has also been shown to affect photosynthate transfer within the sea anemone, Condylactis gigantea (Loram et al. 2007). Photosynthetically fixed 14C incorporation into lipids and low molecular weight amino acids within the host tissue of anemones was significantly greater for those hosting clade A symbionts than B symbionts (Loram et al. 2007). Freshly isolated Symbiodinium C was also recently shown to fix higher amounts of carbon in the presence of synthetic host factor compared to Symbiodinium A (Stat et al. 2008). The efficiency of photosynthate transfer to the host and specific molecular allocation of fixed carbon have not been compared between symbiont types in corals, but are likely to contribute to the large (121%) differences in total photosynthate incorporation observed in the present study. Differences in the performance of Symbiodinium types are likely to be widespread in anthozoan endosymbioses and an important feature in the nutritional economy of reef corals.
Although both 14C uptake and rETRMAX were significantly higher for Symbiodinium C1 corals, the actual relationship between carbon fixation and symbiont performance is not likely to be simple due to the occurrence of both assimilatory and non-assimilatory electron flow (Jones et al. 1998). Hoogenboom et al. (2006) demonstrated that the saturation of O2 evolution in corals can occur at lower PAR than rETR saturation, indicating non-assimilatory electron flow through PSII. Further, 14C uptake in the present study was measured in the host tissue and not in the symbiont and translocation to the host tissue rather than total fixation of 14C may differ between the symbiont types. Additional respirometry and 14C fixation (and translocation) experiments over a wider range of PAR, in conjunction with detailed quenching analysis, are required to fully appreciate the complex relationship between the photosynthetic performance of different symbiont types and energetic benefit to the coral host.
The reduction of rETRMAX and 14C in both symbiont types by 10 μg l−1 DCMU to indistinguishable levels suggests that, under these conditions of severe electron transport inhibition and at this level of PAR, C1 colonies would receive the same 14C (energy) allocation from their symbionts as D-colonies. However, Symbiodinium C1 suffered a greater proportional drop since, in the absence of DCMU, its rETRMAX was 85% greater than that observed for Symbiodinium D (Fig. 1b). The relative reduction in 14C incorporation into host tissues in the presence of DCMU was also greater for C1 colonies (Fig. 1a). Therefore, C1 colonies may lose their potential for more rapid growth and any competitive advantage over D-colonies at the juvenile stage under stressful conditions that limit electron transport. While future experiments under a full range of irradiance conditions are required to confirm differences in physiology between Symbiodinium C1 and D, the present results reveal how PSII herbicide exposures might affect corals differently, depending on the symbiont types they harbour.
The identical maximum quantum yields (F v/F m) in dark-adapted samples for each symbiont type in the absence of DCMU (Fig. 3) indicates similar efficiencies of excitation energy capture by PSII for each symbiont type (Genty et al. 1989). This result is consistent with other reports, which show similar F v/F m for Symbiodinium within different coral species at the same depth when measured in the absence of stress (Rowan 2004; Berkelmans and van Oppen 2006; Robison and Warner 2006; Warner et al. 2006). Reductions in F v/F m at 10 μg l−1 DCMU were similar for both symbiont types, indicating equivalent level PSII photoinactivation (Genty et al. 1989). It is plausible that longer exposure to DCMU and exposures at higher irradiances might reveal differences in photoinactivation to PSII between symbiont types, analogous to that observed during longer exposure experiments to thermal stress. For example, far greater reductions in F v/F m were observed in C2 symbionts in adult A. millepora exposed to elevated seawater temperatures than for the more thermally tolerant D symbionts in the same species, indicating greater PSII photoinactivation of C2 symbionts (Berkelmans and van Oppen 2006). Pocillopora spp. hosting Symbiodinium C exhibited identical F v/F m values and higher levels of photosynthesis (measured as oxygen flux) at 28°C than Symbiodinium D, but suffered a greater decline in photosynthetic performance and more photoinactivation (damage) to PSII at elevated temperatures (Rowan 2004).
No differences in light harvesting and xanthophyll pigments were evident between Symbiodinium types in the absence of DCMU (Fig. 4). Thus, the greater incorporation of 14C labelled photosynthates and the observed higher rETRMAX of Symbiodinium C1 in control treatments did not result from higher concentrations of light harvesting pigments. In order to fully explain the functional mechanism leading to the differences observed between the two symbiont types, Symbiodinium C1 and D, future studies need to be conducted to determine which photosynthetic processes limit the photosynthetic performance of Symbiodinium D. Severe inhibition of PSII electron transport (10 μg l−1 DCMU treatment) for 10 h resulted in significant increases in both the light harvesting and xanthophyll pigments (by 108 and 114%, respectively) in Symbiodinium C1, whereas pigment concentrations did not change in type D symbionts (Fig. 4). Under these exposures, a large proportion of the PSII reaction centres are inactive due to DCMU inhibiting reduction of the plastoquinone pool, therefore mimicking the physiological transition to low light (Escoubas et al. 1995). It is likely that rapid pigment biosynthesis was stimulated in Symbiodinium C1 in response to the reduced electron transport and the effects of DCMU on the redox state of the plastoquinone pool. This type of rapid pigment biosynthesis was reported for high light acclimated green alga Dunaliella salina following a 12-h transition to low illumination (Masuda et al. 2002) and following 12-h exposure of Dunaliella tertiolecta to DCMU (Escoubas et al. 1995). It is unclear why the light harvesting pigments of Symbiodinium D did not increase following photoinactivation by DCMU. Further experiments that subject Symbiodinium spp. to DCMU under both low and high irradiances are needed to confirm this photoacclimatory response mechanism in corals.
This study identifies the potential energetic consequences to the coral host of association with genetically distinct types of the algal endosymbiont, Symbiodinium, that differ intrinsically in their photophysiology. It demonstrated that photosynthetic performance, as measured by photosynthate incorporation (carbon-based energy) and PSII relative electron transport, was significantly greater within Symbiodinium C1 compared to Symbiodinium D, which might explain the influence that symbiont type has previously been shown to have on juvenile coral growth (Little et al. 2004). The results suggest that a physiological trade-off between stress (thermal, irradiance or contamination) tolerance and photosynthetic performance underlies the growth advantage gained by corals when associated with Symbiodinium C1 in early life history (Little et al. 2004). As the community structure of coral reefs shift in response to global climate change and water quality impacts (Hughes et al. 2003), opportunistic corals harbouring symbionts that enable maximum rates of growth may similarly gain a competitive advantage. A shift in an A. millepora population from dominance by Symbiodinium C to D, a symbiont commonly associated with high-temperature, was identified in the southern GBR following a severe bleaching events (Jones et al. 2008). Further, a similar shift was observed in Pocillopora spp. from Pacific Panama, and was implied for a range of coral species in the Persian (Arabian) Gulf and Kenya following episodes of severe, high temperature bleaching, a response which may increase the resistance of coral reefs to future bleaching (Baker et al. 2004). While such shifts may increase the survival of corals under warming conditions (Berkelmans and van Oppen 2006), non-elevated sea temperatures between bleaching events may favour corals that harbour more photosynthetically active Symbiodinium types such as C1 (ITS1, used in this study) and enhance the growth rate of coral colonies that maintain stable symbiotic relationships observed following bleaching events (Thornhill et al. 2006). These findings reveal underlying photophysiological differences between genetically distinct algal endosymbionts that advance the understanding of the dynamic relationship between the coral host and its symbiotic partner.
References
Abrego D, Ulstrup KE, Willis BL, van Oppen MJH (2008) Species-specific interactions between algal endosymbionts and coral hosts define their bleaching response to heat and light stress. Proc R Soc B Biol Sci 275:2273–2282
Apprill AM, Gates RD (2007) Recognizing diversity in coral symbiotic dinoflagellate communities. Mol Ecol 16:1127–1134
Baker AC (2003) Flexibility and specificity in coral-algal symbiosis: diversity, ecology, and biogeography of Symbiodinium. Annu Rev Ecol Syst 34:661–689
Baker AC, Starger CJ, McClanahan TR, Glynn PW (2004) Corals’ adaptive response to climate. Nature 430:661–689
Berkelmans R, van Oppen MJH (2006) The role of zooxanthellae in the thermal tolerance of corals: a ‘nugget of hope’ for coral reefs in an era of climate change. Proc R Soc Lond B Biol Sci 273:2305–2312
Brown BE, Ambarasari I, Warner ME, Fitt WK, Dunne RP, Gibb SW, Cummings DG (1999) Diurnal changes in photochemical efficiency and xanthophyll concentrations in shallow water reef corals: evidence for photoinhibition and photoprotection. Coral Reefs 18:99–105
Cantin NE, Negri AP, Willis BL (2007) Photoinhibition from chronic herbicide exposure reduces reproductive output of reef-building corals. Mar Ecol Prog Ser 344:81–93
Coffroth MA, Santos SR (2005) Genetic diversity of symbiotic dinoflagellates in the genus Symbiodinium. Protist 156:19–34
Coles SL, Brown BE (2003) Coral bleaching—capacity for acclimatization and adaptation. Adv Mar Biol 46:183–244
Crossland CJ, Barnes DJ, Borowitzka MA (1980) Diurnal lipid and mucus production in the staghorn coral Acropora acuminata. Mar Biol 60:81–90
Escoubas J, Lomas M, LaRoche J, Falkowski PG (1995) Light intensity regulation of cab gene transcription is signalled by the redox state of the plastoquinone pool. Proc Natl Acad Sci USA 92:10237–10241
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Goulet TL, Cook CB, Goulet D (2005) Effect of short-term exposure to elevated temperatures and light levels on photosynthesis of different host-symbiont combinations in the Aiptasia pallida/Symbiodinium symbiosis. Limnol Oceanogr 50:1490–1498
Hoogenboom MO, Anthony KRN, Connolly SR (2006) Energetic cost of photoinhibition in corals. Mar Ecol Prog Ser 313:1–12
Hughes TP, Jackson JBC (1985) Population dynamics and life histories of foliaceous corals. Ecol Monogr 55:141–166
Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, Folke C, Grosberg R, Hoegh-Guldberg O, Jackson JBC, Kleypas J, Lough JM, Marshall P, Nystrom M, Palumbi SR, Pandolfi JM, Rosen B, Roughgarden J (2003) Climate change, human impacts, and the resilience of coral reefs. Science 301:929–933
Jones RJ, Hoegh-Guldberg O, Larkum AWD, Schreiber U (1998) Temperature-induced bleaching of corals begins with impairment of the CO2 fixation mechanism in zooxanthellae. Plant Cell Environ 21:1219–1230
Jones RJ, Müller J, Haynes D, Schreiber U (2003) Effects of herbicides diuron and atrazine on corals of the Great Barrier Reef, Australia. Mar Ecol Prog Ser 251:153–167
Jones AM, Berkelmans R, van Oppen MJH, Mieog JC, Sinclair W (2008) A community change in the algal endosymbionts of a scleractinian coral following a natural bleaching event: field evidence of acclimatization. Proc R Soc Lond B, Biol Sci 275:1359–1365
Koumandou VL, Howe CJ (2007) The copy number of chloroplast gene minicircles changes dramatically with growth phase in the dinoflagellate Amphidinium operculatum. Protist 158:89–103
LaJeunesse T (2002) Diversity and community structure of symbiotic dinoflagellates from Caribbean coral reefs. Mar Biol 141:387–400
Little AF, van Oppen MJH, Willis BL (2004) Flexibility in algal endosymbioses shapes growth in reef corals. Science 304:1492–1494
Loram JE, Trapido-Rosenthal HG, Douglas AE (2007) Functional significance of genetically different symbiotic algae Symbiodinium in a coral reef symbiosis. Mol Ecol 16:4849–4857
Masuda T, Jugen EWP, Melis A (2002) Biosynthesis and distribution of chlorophyll among the photosystems during recovery of the green alga Dunaliella salina from irradiance stress. Plant Physiol 128:603–614
Mieog JC, van Oppen MJH, Cantin NE, Stam WT, Olsen JL (2007) Real-time PCR reveals a high incidence of Symbiodinium clade D at low levels in four scleractinian corals across the Great Barrier Reef: implications for symbiont shuffling. Coral Reefs 26:449–457
Muscatine L, Falkowski PG, Porter JW, Dubinsky Z (1984) Fate of photosynthetic fixed carbon in light and shade-adapted colonies of the symbiotic coral Stylophora pistillata. Proc R Soc Lond B Biol Sci 222:181–202
Negri A, Vollhardt C, Humphrey C, Heyward A, Jones R, Eaglesham G, Fabricius K (2005) Effects of the herbicide diuron on the early life history stages of coral. Mar Pollut Bull 51:370–383
Platt T, Gallegos CL, Harrison WG (1980) Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J Mar Res 38:687–701
Pochon X, LaJeunesse TC, Pawlowski J (2004) Biogeographic partitioning and host specialization among foraminiferan dinoflagellate symbionts (Symbiodinium; Dinophyta). Mar Biol 146:17–27
Ralph PJ, Gademann R (2005) Rapid light curves: a powerful tool to assess photosynthetic activity. Aquat Ecol 82:222–237
Ralph PJ, Gademann R, Larkum AWD, Kuhl M (2002) Spatial heterogeneity in active fluorescence and PSII activity of coral tissues. Mar Biol 141:539–646
Robison JD, Warner ME (2006) Differential impacts of photoacclimation and thermal stress on the photobiology of four different phylotypes of Symbiodinium (Pyrrhophyta). J Phycol 42:568–579
Rowan R (2004) Thermal adaptation in reef coral symbionts. Nature 430:742
Santos SR, Taylor DJ, Kinzie RAIII, Hidaka M, Sakai K, Coffroth MA (2002) Molecular phylogeny of symbiotic dinoflagellates inferred from partial chloroplast large subunit (23S)-rDNA sequences. Mol Phylogenet Evol 23:97–111
Schreiber U (2004) Pulse-amplitude-modulation (PAM) fluorometry and saturation pulse method: an overview. In: Papageorgiou G, Govindjee (eds) Chlorophyll fluorescence: a signature of photosynthesis. Kluwer Academic Publishers, Dordrecht, pp 279–319
Schreiber U, Gademann R, Ralph PJ, Larkum AWD (1997) Assessment of photosynthetic performance of prochloron in Lissoclinum patella in hospite by chlorophyll fluorescence measurements. Plant Cell Physiol 38:945–951
Stat M, Morris E, Gates RD (2008) Functional diversity in coral-dinoflagellate symbiosis. Proc Natl Acad Sci USA 105:9256–9261
Takabayashi M, Santos SR, Cook CB (2004) Mitochondrial DNA phylogeny of the symbiotic dinoflagellates (Symbiodinium, Dinophyta). J Phycol 40:160–164
Thornhill DJ, Lajeunesse TC, Kemp DW, Fitt WK, Schmidt GW (2006) Multi-year, seasonal genotypic surveys of coral-algal symbioses reveal prevalent stability or post-bleaching reversion. Mar Biol 148:711–722
Ulstrup KE, van Oppen MJH (2003) Geographic and habitat partitioning of genetically distinct zooxanthellae (Symbiodinium) in Acropora corals on the Great Barrier Reef. Mol Ecol 12:3477–3484
van Oppen MJH (2004) Mode of zooxanthella transmission does not affect zooxanthella diversity in acroporid corals. Mar Biol 144:1–7
van Oppen MJH, Palstra FP, Piquet AMT, Miller D (2001) Patterns of coral-dinoflagellate associations in Acropora: significance of local availability and physiology of Symbiodinium strains and host-symbiont selectivity. Proc R Soc Lond B Biol Sci 268:1759–1767
van Oppen MJH, Mahiny AJ, Done TD (2005) Geographic distribution of zooxanthella types in three coral species on the Great Barrier Reef sampled after the 2002 bleaching event. Coral Reefs 24:482–487
Warner ME, Lajeunesse TC, Robison JD, Thur RM (2006) The ecological distribution and comparative photobiology of symbiotic dinoflagellates from reef corals in Belize: potential implications for coral bleaching. Limnol Oceanogr 51:1887–1897
Wilson K, Yutao L, Whan K, Lehnert S, Byrne K, Moore S, Pongsomboon S, Tassanakajon A, Rosenberg G, Ballment E, Fayazi Z, Swan J, Kenway M, Benzie J (2002) Genetic mapping of the black tiger shrimp Penaeus monodon with amplified fragment length polymorphism. Aquaculture 204:297–309
Wright SW, Jeffrey SW (1997) Phytoplankton pigments in oceanography: guidelines to modern methods. UNESCO, Paris
Acknowledgements
We thank J. Doyle and L. Peplow for technical advice, A. Baird for comments on the experimental design and P. Ralph for critical reading of the manuscript. This work was supported by a grant from AIMS@JCU.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Biology Editor Dr. Ruth Gates
Rights and permissions
About this article
Cite this article
Cantin, N.E., van Oppen, M.J.H., Willis, B.L. et al. Juvenile corals can acquire more carbon from high-performance algal symbionts. Coral Reefs 28, 405–414 (2009). https://doi.org/10.1007/s00338-009-0478-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-009-0478-8