Abstract
Ten new pentangular polyphenols, namely amexanthomycins A–J (1–10) were isolated from the strain Amycolatopsis mediterranei S699∆rifA constructed by deleting the polyketide synthase genes responsible for the biosynthesis of rifamycins. Their structures were elucidated on the basis of 1D and 2D NMR spectroscopic data and high-resolution ESIMS. Amexanthomycins A–C (1–3) showed inhibitory activity against human DNA topoisomerases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microorganisms, particularly actinomycetes, serve as a prolific source of structurally diverse bioactive metabolites for the pharmaceutical industry (Berdy 2005; Cragg and Newman 2013; Demain 2014). However, most of the strains belong to Streptomyces, with small portions from other genera called rare actinomycetes, such as Saccharopolyspora, Amycolatopsis, Micromonospora, Actinoplanes, and Amycolatopsis (Tiwari and Gupta 2012). Although Amycolatopsis is not a large group within the phylum Actinobacteria, this genus is well-known for the production of the antimicrobial agents currently available in the market such as vancomycin (glycopeptides) (Nagarajan 1991) and rifamycins (ansamycins) (August et al. 1998).
Amycolatopsis mediterranei S699 is a well-known rifamycin producer (August et al. 1998; Stratmann et al. 1999; Xu et al. 2005). Up to date, no other types of secondary metabolites were isolated from A. mediterranei S699. Bioinformatic analysis of the genome sequence of A. mediterranei S699 revealed diverse gene clusters (Verma et al. 2011). In order to exploit minor natural products with bioactivities or novel skeleton, the mutant strain A. mediterranei S699∆rifA was constructed by deleting the polyketide synthase genes responsible for the biosynthesis of rifamycins, the main components of the wild-type strain. In this study, ten new pentangular polyphenols, namely amexanthomycins A–J (1–10), were isolated from the fermentation products of A. mediterranei S699∆rifA on YMG agar media. Herein, we report the isolation, structure elucidation, bioactivity, and proposed biosynthetic pathway for compounds 1–10 (Fig. 1).
Materials and methods
General experimental procedures
The NMR spectra were recorded on Bruker DRX-600 MHz NMR spectrometer (Bruker Daltonics Inc., Billerica, Massachusetts) with tetramethylsilane (TMS) as an internal standard. The HRESIMS were measured on an LTQ-Orbitrap XL. Sephadex LH-20 was obtained from GE Amersham Biosciences (Piscataway, New Jersey). Reversed-phase (RP) C18 silica gel for column chromatography (CC) was obtained from Merck (Darmstadt, Germany). Silica gel GF254 for thin-layer chromatography (TLC) was purchased from Qingdao Marine Chemical Ltd. (Qingdao, China). Semipreparative HPLC was performed on Waters 1525 Binary HPLC Pump and Waters 996 Photodiode Array Detector, equipped with an Agilent Eclipse XDB-C18 column (5 μm, 9.4 × 250 mm). Compounds were visualized under UV light and sprayed with H2SO4/EtOH (1:9, v/v) and vanillic aldehyde, followed by heating.
Microbial materials
Amycolatopsis mediterranei S699 strain was provided by Prof. Linquan Bai at Shanghai Jiao Tong University. It was a derivative of the original type strain (ATCC 13685) isolated in 1957 at St. Raphael, France (Kim et al. 1992; Tang et al. 2012). A. mediterranei S699∆rifA strain was constructed by deleting the polyketide synthase genes responsible for the biosynthesis of rifamycins (Fig. S83).
Fermentation and isolation of compounds 1–10
Amycolatopsis mediterranei S699∆rifA strain was cultured for 12 days on YMG (yeast extract 4 g, malt extract 10 g, glucose 4 g, ddH2O 1000 mL, pH 7.2, agar 20 g) agar plates with a total volume of 20 L at 28 °C. The culture was diced and extracted three times overnight with EtOAc-MeOH (80:20, v/v) at room temperature to obtain the crude extract, which was partitioned between H2O and EtOAc (1:1, v/v) until the organic layer was colorless. The EtOAc extract was partitioned between 95% aqueous MeOH and petroleum ether (PE) until the PE layer was colorless. The 95% MeOH solution was concentrated under vacuum at 37 °C to afford the defatted MeOH extract (4.1 g). The MeOH extract was subjected to column chromatography over Sephadex LH-20 eluted with MeOH to give Fr. A–E.
Fr. A (357 mg) was purified by medium pressure liquid chromatography (MPLC) over RP-18 silica gel (30 g) eluted with gradient aqueous acetonitrile (30, 40, 50, 60, 70, 80, 90, and 100% CH3CN, 200 mL each) to afford three fractions Fr. A1–A3. Fr. A2 (22 mg) was purified by HPLC (Agilent Eclipse XDB-C18, 5 μm, 9.4 × 250 mm; 4 mL/min; UV 274 nm) eluted with gradient acetonitrile (0 min: 45% CH3CN; 10 min: 57% CH3CN; 15 min: 71% CH3CN; 16 min: 100% CH3CN; 18 min: 100% CH3CN; 19 min:45% CH3CN; 21 min:45% CH3CN) to afford 10 (t R 9 min, 4.5 mg), 8 (t R 12.5 min, 1.5 mg), 9 (t R 13.3 min, 4.9 mg), and 7 (t R 13.8 min, 4 mg) (Fig. S82).
Fr. B (1.62 g) was subjected to MPLC over RP-18 silica gel (60 g) eluted with gradient aqueous acetonitrile (30, 50, 70, and 100% CH3CN, 500 mL each) to afford five fractions Fr. B1–B5. Fr. B3 (475 mg) was then subjected to column chromatography over Sephadex LH-20 eluted with MeOH to afford Fr. B3a and Fr. B3b. Then they were purified by HPLC (Agilent Eclipse XDB-C18, 5 μm, 9.4 × 250 mm, 4 mL/min, UV 274 nm) eluted with gradient aqueous acetonitrile (0 min: 48% CH3CN; 10 min: 54% CH3CN; 15 min: 68% CH3CN; 16 min: 100% CH3CN; 18 min: 100% CH3CN; 19 min: 48% CH3CN; 21 min: 48% CH3CN) and 43% acetonitrile to obtain 6 (t R 11.3 min, 1.5 mg), 8 (t R 12 min, 4.5 mg), 9 (t R 13.2 min, 10 mg), 7 (t R 14 min, 8.2 mg), 4 (t R 12 min, 1.8 mg), 5 (t R 14 min, 6.9 mg), and 3 (t R 16 min, 4.2 mg), respectively (Fig. S82).
Fr. C (1.23 g) was subjected to MPLC over RP-18 silica gel (60 g) eluted with gradient aqueous acetonitrile (30, 40, 50, 70, and 100% CH3CN, 500 mL each) to afford five fractions Fr. C1–C5. Fr. C2 (100 mg) and Fr. C3 (26 mg) were purified by HPLC (Agilent Eclipse XDB-C18, 5 μm, 9.4 × 250 mm, 4 mL/min, UV 274 nm) eluted with 32 and 35% acetonitrile to afford 1 (t R 5.7 min, 4.8 mg) and 2 (t R 9.7 min, 4.5 mg), respectively (Fig. S82).
Results
Compound 1 was obtained as yellowish powder. Its molecular formula was determined to be C37H40O17 on the basis of high-resolution ESIMS (Fig. S72) at m/z 757.2336 [M + H]+ (calcd. for C37H41O17 +, 757.2338), 779.2144 [M + Na]+ (calcd. for C37H40O17Na+, 779.2158), and NMR data (Table S1, Figs. S1–5). Interpretation of the 1H, 13C, and HSQC NMR spectrum showed the presence of a ketone (δ C 182.1), a acyl carbon (δ C 172.3), 18 olefinic carbons, two sugar anomeric carbons (δ C 102.2, 102.4), eight oxymethine, two methoxyl, two methylene, and three methyl groups. The 1H-1H COSY correlations between H-10 (δ H 8.02)/H-11 (δ H 7.36)/H-12 (δ H 7.85), the HMBC correlations from H-10 to C-8 (δ C 182.1) and C-14 (δ C 146.5), H-11 to C-9 (δ C 121.9) and C-13 (δ C 146.9), and H-12 to C-10 (δ C 118.4) and C-14 indicated the presence of the benzene ring (ring E) with substitution of oxygen at C-13 and C-14, and a ketone linked to C-9, which confirmed the presence of substructure A (Fig. 2). The 1H-1H COSY correlations between H2-19 (δ H 2.72, 4.10) and H-20 (δ H 5.77), along with the HMBC correlations from H2-19 to C-5 (δ C 115.8), C-17 (δ C 139.1), and C-21 (δ C 136.3); H-20 to C-4 (δ C 118.6), C-18 (δ C 140.7), and C-22 (δ C 145.6); H3-24 (δ H 2.85) to C-2 (δ C 126.1), C-22, and C-23 (δ C 130.6); and 17-OMe (δ H 4.11) to C-17 revealed the presence of substructure B including ring B (Fig. 2). The presence of rings A and C was deduced from the analysis of unsaturation degrees and the five quaternary carbons including C-1 (δ C 172.3), C-3 (δ C 152.8), C-6 (δ C 152.4), C-7 (δ C 108.5), and C-16 (δ C 148.8). Ring C was connected to ring E via an ester bond between C-16 and C-14 and a ketone bond between C-7 and C-9, forming the ring D. The carbons C-3 and C-6 were attached to a hydroxyl group, respectively, and C-2 was attached to a carboxyl group, which was in accordance with the chemical shift and the molecular formula, and thus confirmed the aglycone moiety (Fig. 2). Furthermore, the NMR comparison with that of literature readily revealed that the aglycone moiety (rings A–E) of 1 was similar to arixanthomycin B, except for the presence of an oxazolidine ring at C-1 and C-24 and the absence of a hydroxyl group substitution at C-20 in arixanthomycin B (Kang and Brady 2014a).
The deoxysugar moiety E1 of 1 was determined by the 1H-1H COSY and HMBC correlations (Table S1). The 1H-1H COSY correlations of H-1’ (δ H 5.61)/H-2’ (δ H 3.92)/H-3’ (δ H 4.25)/H-4’ (δ H 3.87)/H-5’ (δ H 4.01)/H-6’ (δ H 1.66), and the NOE correlation between H-1’ and H-5’, along with the HMBC correlation from 2’-OMe (δ H 4.18) to C-2’ (δ C 85.0) indicated the sugar moiety E1 is 2-O-methyl β-quinovose. The large constant couplings between H-1’ and H-2’ (7.7 Hz), H-2’ and H-3’ (9.0 Hz), H-3’ and H-4’ (9.1 Hz), and H-4’ and H-5’ (9.1 Hz) revealed all the protons of this deoxysugar were in the axial position, which was further confirmed by the NOE correlations between H-1’ and H-3’, H-1’ and H-5’ (Fig. 2). Similarly, the deoxysugar moiety W1 of 1 was determined to be β-olivose. The linkages between C-1’ of E1 and C-13, and C-1’ of W1 and C-22 through O-glycosidic bridge were revealed by the HMBC correlations from H-1’ of E1 to C13, H-1’ of W1 to C22, respectively. Thus, the structure of compound 1 was determined as amexanthomycin A.
Compound 2 has the molecular formula of C44H52O20 based on its HRESIMS (Fig. S73) and NMR data (Table S2, Figs. S9–14). The NMR spectra of 2 were similar to that of 1, indicating that both have the same aglycone moiety, 2-O-methyl β-quinovose (E1), and β-olivose (W1) moiety (Fig. 1). However, one deoxysugar moiety was demonstrated in 2 by the NMR spectra and the molecular formula (Tables 1 and 3). The long-range couplings from H-1’ (δ H 5.02) to H-6’ (δ H 1.55) and the HMBC correlations from H-6’ to C-4’ (δ C 66.5), H-5’ (δ H 3.67) to C-1’ (δ H 102.3), and 3’-OMe (δ H 3.42) to C-3’ (δ C 78.8) in E2, along with the large constant couplings between H-1’ and H-2’a (8.8 Hz) and the NOE correlations between H-1’ and H-3’ (δ H 3.47) and H-1’ and H-5’ revealed the sugar moiety E2 is a β-oleandrose. The deoxysugar E2 connected to E1 through O-glycosidic bridge between C-1’ of E2 and C-3’ (δ C 86.6) of E1 was indicated by the HMBC correlation from H-1’ in E2 to C-3’ in E1 and the NOE correlation between H-1’ in E2 to H-3’ (δ H 4.07) in E1. Thus, the structure of compound 2 was elucidated as amexanthomycin B.
Compound 3 was determined to have the molecular formula of C57H74O25 (Fig. S74). Five anomeric carbons were observed (δ C 102.3, 102.0, 101.9, 101.6, and 101.0) in the 13C spectra, revealing the presence of five sugar residues, two more than those of compound 2 (Table 3). Analysis of the NMR spectra and the molecular formula in the same way as that of 2 showed that 2 and 3 possess the same aglycone moiety, 2-O-methyl β-quinovose (E1), β-oleandrose (E2), and β-olivose (W1) moieties, but two other deoxysugars named β-amicetose (W2) and β-oleandrose (W3) (Tables 1 and 3). The HMBC correlations from H-1’ (δ H 4.74) in W2 to C-3’ (δ C 80.1) in W1 and H-1’ (δ H 4.76) in W3 to C-4’ (δ C 80.0) in W2, along with the NOE correlations between H-1’ in W2 to H-3’ (δ H 4.00) in W1 and H-1’ in W3 to H-4’ (δ H 3.39) in W2 revealed that the attachment of the oligosaccharide chain in 3 was β-ole (1→4)-β-ami (1→3)-β-oli to C-22 (Table S3). Thus, the structure of compound 3 was elucidated as amexanthomycin C (Table 2).
The molecular formula of compound 4 was elucidated as C58H76O26 (Fig. S75). A close NMR comparison (Tables 1 and 3) with that of 3 revealed that the only evident difference was the 3’-OMe (δ H 3.53) in W2, indicating that the sugar moiety W2 β-amicetose was replaced by β-oleandrose, which was confirmed by the HMBC correlations from 3’-OMe to C-3’ (δ C 79.0) in 4 (Table S4). Thus, the structure of compound 4 was elucidated as amexanthomycin D.
Compound 5 has the molecular formula of C59H78O27 (Fig. S76). The NMR data (Tables 1 and 3) were closely similar to those of 4 except for the presence of the 2’-OMe (δ H 3.72) in W2, revealing that the sugar moiety W2 β-oleandrose was replaced by 2,3-O-dimethyl β-quinovose, which was confirmed by the HMBC correlations from 2’-OMe to C-2’ (δ C 84.2) in 5 (Table S5). Thus, the structure of compound 5 was elucidated as amexanthomycin E.
Compounds 6 and 7 were determined to have the molecular formula C63H84O27 (Fig. S77) and C69H94O29 (Fig. S78), respectively. Six anomeric carbons (δ C 102.3, 102.2, 102.0, 101.9, 101.6, and 101.0) in 6 and seven ones (δ C 103.5, 102.3, 102.1, 102.0, 101.9, 101.6, and 101.0) in 7 were observed in their respective 13C spectrum, revealing the presence of six sugar residues in 6 and seven ones in 7. (Tables 2 and 4) Analysis of the NMR spectrum and the molecular formula in the same way as those of 3 showed that 3, 6, and 7 share the same aglycone moiety, 2-O-methyl β-quinovose (E1), β-oleandrose (E2), β-olivose (W1), β-amicetose (W2), and β-oleandrose (W3) moieties, but one more deoxysugar named β-amicetose (E3) in 6 and two more ones in 7. The HMBC correlations of 6 from H-1’ (δ H 5.04) in E3 to C-4’ (δ C 71.8) in E2 and the NOE correlation between H-1’ in E3 and H-4’ (δ H 4.06) in E2 revealed that the attachment of the oligosaccharide chain in 6 was β-ami (1→4)-β-ole (1→3)-2-O-methyl β-qui to C-13 (Table S6). The HMBC correlations of 7 from H-1’ (δ H 4.70) in E4 to C-4’ (δ C 80.0) in E3 and the NOE correlation between H-1’ in E4 to H-4’ (δ H 3.40) in E3 revealed that the attachment of the oligosaccharide chain in 7 was β-ami (1→4)-β-ami (1→4)-β-ole (1→3)-2-O-methyl β-qui to C-13 (Table S7). Thus, the structures of compounds 6 and 7 were determined as amexanthomycins F and G, respectively.
The molecular formula of compounds 8 and 9 were determined to be C70H96O30 (Fig. S79) and C71H98O31 (Fig. S80), respectively. Their NMR data (Tables 1 and 4) were closely similar to those of 7, except that the 3’-OMe (δ H 3.53) in W2 in 8 and 2’-OMe (δ H 3.72) and 3’-OMe (δ H 3.79) in W2 in 9 were present, revealing that the sugar moiety W2 β-amicetose was replaced by β-oleandrose in 8 and 2,3-O-dimethyl β-quinovose in 9, which was confirmed by the HMBC correlations from 3’-OMe to C-3’ (δ C 79.1) in W2 in 8, from 2’-OMe to C-2’ (δ C 84.2), and from 3’-OMe to C-3 (δ C 84.9) in W2 in 9 (Tables S8 and S9). Thus, the structures of compounds 8 and 9 were determined as amexanthomycins H and I, respectively.
Compound 10 was elucidated as C77H108O35 (Fig. S81). Eight anomeric carbons were observed (δ C 106.6, 103.3, 102.3, 102.1, 101.9, 101.8, 101.7, and 100.6) in the 13C spectrum, revealing the presence of eight sugar residues, one more than those of compound 9 (Table 4). Analysis of the NMR spectrum and the molecular formula in the same way as that of 7 showed that 9 and 10 possess the same aglycone moiety, 2-O-methyl β-quinovose (E1), β-oleandrose (E2), β-amicetose (E3), β-amicetose (E4), β-olivose (W1), 2,3-O-dimethyl β-quinovose (W2), and β-oleandrose (W3) moieties, but one more deoxysugar named β-quinovose (E5) in 10 than in 9 (Tables 2 and 4). The HMBC correlations from H-1’ (δ H 4.76) in E5 to C-4’ (δ C 80.7) in E4 and the NOE correlation between H-1’ in E5 to H-4’ (δ H 3.51) in E4 revealed that the attachment of the oligosaccharide chain in 10 was β-qui (1→4)-β-ami (1→4)-β-ami (1→4)-β-ole (1→3)-2-O-methyl β-qui to C-13 (Table S10). Thus, the structure of compound 10 was determined as amexanthomycin J.
The structures of amexanthomycins A–J (1–10) feature a xanthone-containing pentangular polyphenol core. The xanthone derivatives have been reported to function as DNA topoisomerase II inhibitors (Woo et al. 2007; Woo et al. 2010). Thus, we assessed the topoisomerases IIα (Topo IIα) inhibitory activities of 1–10 using Topo-mediated supercoiled DNA relaxation assay (Zhang et al. 2017). Compounds 1–3 exhibited Topo IIα inhibitory activity at 500 μM, while 4–10 showed no activities (Fig. 3a). The dose-dependent assays showed that 1 and 2 had stronger Topo IIα inhibitory activity than 3 (Fig. 3b). These results indicated that the number and type of deoxysugar substitutions in amexanthomycins had impact on the topoisomerase inhibitory activities, which provided valuable information regarding structure–activity relationships for the development of new topoisomerase inhibitors.
Topoisomerase IIα inhibitory activities of amexanthomycins A–J (1–10) isolated from Amycolatopsis mediterranei S699 ∆rifA. a The effects of amexanthomycins A–J (1–10) on the DNA relaxation activity by Topo IIα. Supercoiled pBR322 DNA (SC) was relaxed by Topo IIα alone or with 500 μM of compounds 1–10. Relaxation reaction products containing nicked open circular or relaxed DNA (NOC/R) were indicated. Etoposide (VP16) was known inhibitors of topoisomerase II and used as positive controls. b Compounds 1–3 inhibited Topo IIα mediated DNA relaxation in a dose-dependent manner
Discussion
It has been more than half a century since rifamycins were isolated from Amycolatopsis mediterranei S699 (Sensi et al. 1959). The semisynthetic derivatives of rifamycin B have been used against Mycobacterium tuberculosis (Woodley and Kilburn 1982). Thus, A. mediterranei S699 has been the focus for researchers to explore the biosynthesis of rifamycins, making it one of the most thoroughly studied strains (August et al. 1998; Tang et al. 2012; Verma et al. 2011; Xu et al. 2005; Yu et al. 2001). However, as of today, no other types of secondary metabolites isolated from A. mediterranei S699 were reported. In our continuing studies on A. mediterranei S699, ten new pentangular polyphenols, namely amexanthomycins A–J (1–10) were obtained from the mutant strain S699∆rifA constructed by deleting the polyketide synthase genes responsible for the biosynthesis of rifamycins (Fig. S83). It is possible that the production of amexanthomycins was inhibited by the main products rifamycins in the wild strain S699; nevertheless, the gene cluster of amexanthomycins was activated after inactivation of rifamycins in the mutant strain S699∆rifA. Another possibility is that the minor components of amexanthomycins were difficult to be detected with the background of rifamycins in S699, while in S699∆rifA, amexanthomycins turned to be the main products and were easier to be detected without the influence of rifamycins. It is most possible that S699∆rifA was cultured on agar plates, while S699 was usually studied in shake flask culture (Ma et al. 2008; Xu et al. 2003), and the change of cultivation condition led to the production of amexanthomycins. It needs to be further studied how amexanthomycins were activated or detected in S699∆rifA.
The amexanthomycins shared the similar aglycone moiety of xanthone-containing pentangular polyphenol with arixanthomycin A (Kang and Brady 2014a) or calixanthomycin A (Kang and Brady 2014b), except for the absence of an oxazolidine or lactone ring at C-1 and C-24. And the substituted positions of hydroxyl or methoxyl group in amexanthomycins were different from the two reported compounds. Furthermore, the oligosaccharide chains were linked to C-13 and C-22, respectively, and there were eight deoxysugar substitutions in amexanthomycin J, which were first reported in this literature.
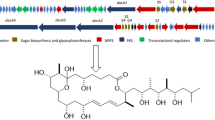
In silico genome analyses with the antibiotics and Secondary Metabolite Analysis Shell (antiSMASH) algorithm revealed that the gene cluster of GC22 in Amycolatopsis mediterranei S699 was involved in the biosynthesis of amexanthomycins (Table S13) (Verma et al. 2011), which was further confirmed by the deletion of the minimal polyketide synthase (min-PKS) of GC22 to obtain the mutant strain S699∆rifA∆PKS without the production of amexanthomycins (Fig. 4). Thus, the gene cluster responsible for amexanthomycins was identified, which provides strong support for exploring the biosynthesis of amexanthomycins. The min-PKS consists of two ketosynthase units (KS α and KS β , or chain length factor (CLF)) and an acyl carrier protein (ACP), which catalyzes the iterative condensation of malonyl-CoA into polyketide chains ranging from 16 to 30 carbons to form structurally diverse aromatic polyketides of secondary metabolites (Hertweck 2009; Hertweck et al. 2007). Accordingly, we proposed the biosynthetic pathway of amexanthomycins (Fig. 5). The pentacyclic xanthone core was predicted to be generated by min-PKS synthase, cyclase, and oxidoreductase using an acetyl-CoA starter unit and 11 malonyl-CoA extender units, which was followed by an oxidative rearrangement catalyzed by the predicted Bayer-Villiger oxidase (BVO). Finally, this aglycone was glycosylated by the glycosyl transferases, completing the biosynthesis of compounds 1–10.
Disruption of PKS genes. a Scheme of the gene inactivation of PKS genes. b PCR amplification using check primers confirmed the genotype of double crossover mutants. c Comparison of the HPLC profiles between the extracts of Amycolatopsis mediterranei S699∆rifA and Amycolatopsis mediterranei S699∆rifA∆PKS
a Amexanthomycin gene cluster (MIBiG BGC-ID: BGC0000200_c1). b Proposed biosynthetic pathway for amexanthomycins
References
August PR, Tang L, Yoon YJ, Ning S, Muller R, Yu TW, Taylor M, Hoffmann D, Kim CG, Zhang X, Hutchinson CR, Floss HG (1998) Biosynthesis of the ansamycin antibiotic rifamycin: deductions from the molecular analysis of the rif biosynthetic gene cluster of Amycolatopsis mediterranei S699. Chem Biol 5(2):69–79. https://doi.org/10.1016/S1074-5521(98)90141-7
Berdy J (2005) Bioactive microbial metabolites. J Antibiot (Tokyo) 58(1):1–26. https://doi.org/10.1038/ja.2005.1
Cragg GM, Newman DJ (2013) Natural products: a continuing source of novel drug leads. Biochim Biophys Acta 1830(6):3670–3695. https://doi.org/10.1016/j.bbagen.2013.02.008
Demain AL (2014) Importance of microbial natural products and the need to revitalize their discovery. J Ind Microbiol Biotechnol 41(2):185–201. https://doi.org/10.1007/s10295-013-1325-z
Hertweck C (2009) The biosynthetic logic of polyketide diversity. Angew Chem Int Ed Engl 48(26):4688–4716. https://doi.org/10.1002/anie.200806121
Hertweck C, Luzhetskyy A, Rebets Y, Bechthold A (2007) Type II polyketide synthases: gaining a deeper insight into enzymatic teamwork. Nat Prod Rep 24(1):162–190. https://doi.org/10.1039/b507395m
Kang HS, Brady SF (2014a) Arixanthomycins A-C: phylogeny-guided discovery of biologically active eDNA-derived pentangular polyphenols. ACS Chem Biol 9(6):1267–1272. https://doi.org/10.1021/cb500141b
Kang HS, Brady SF (2014b) Mining soil metagenomes to better understand the evolution of natural product structural diversity: pentangular polyphenols as a case study. J Am Chem Soc 136(52):18111–18119. https://doi.org/10.1021/ja510606j
Kim CG, Kirschning A, Bergon P, Ahn Y, Wang JJ, Shibuya M, Floss HG (1992) Formation of 3-amino-5-hydroxybenzoic acid, the precursor of Mc(7)N units in ansamycin antibiotics, by a new variant of the shikimate pathway. J Am Chem Soc 114:4941-4943 doi: Doi https://doi.org/10.1021/Ja00038a090
Ma FX, Kim JH, Kim SB, Seo YG, Chang YK, Hong SK, Kim CJ (2008) Medium optimization for enhanced production of rifamycin B by Amycolatopsis mediterranei S699: combining a full factorial design and a statistical approach. Process Biochem 43(9):954–960. https://doi.org/10.1016/j.procbio.2008.04.021
Nagarajan R (1991) Antibacterial activities and modes of action of vancomycin and related glycopeptides. Antimicrob Agents Chemother 35(4):605–609. https://doi.org/10.1128/AAC.35.4.605
Sensi P, Margalith P, Timbal MT (1959) Rifomycin, a new antibiotic; preliminary report. Farmaco Sci 14(2):146–147
Stratmann A, Toupet C, Schilling W, Traber R, Oberer L, Schupp T (1999) Intermediates of rifamycin polyketide synthase produced by an Amycolatopsis mediterranei mutant with inactivated rifF gene. Microbiology 145(12):3365–3375. https://doi.org/10.1099/00221287-145-12-3365
Tang B, Zhao W, Zheng H, Zhuo Y, Zhang L, Zhao GP (2012) Complete genome sequence of Amycolatopsis mediterranei S699 based on de novo assembly via a combinatorial sequencing strategy. J Bacteriol 194(20):5699–5700. https://doi.org/10.1128/JB.01295-12
Tiwari K, Gupta RK (2012) Rare actinomycetes: a potential storehouse for novel antibiotics. Crit Rev Biotechnol 32(2):108–132. https://doi.org/10.3109/07388551.2011.562482
Verma M, Kaur J, Kumar M, Kumari K, Saxena A, Anand S, Nigam A, Ravi V, Raghuvanshi S, Khurana P, Tyagi AK, Khurana JP, Lal R (2011) Whole genome sequence of the rifamycin B-producing strain Amycolatopsis mediterranei S699. J Bacteriol 193(19):5562–5563. https://doi.org/10.1128/JB.05819-11
Woo S, Jung J, Lee C, Kwon Y, Na Y (2007) Synthesis of new xanthone analogues and their biological activity test—cytotoxicity, topoisomerase II inhibition, and DNA cross-linking study. Bioorg Med Chem Lett 17(5):1163–1166. https://doi.org/10.1016/j.bmcl.2006.12.030
Woo S, Kang DH, Nam JM, Lee CS, Ha EM, Lee ES, Kwon Y, Na Y (2010) Synthesis and pharmacological evaluation of new methyloxiranylmethoxyxanthone analogues. Eur J Med Chem 45(9):4221–4228. https://doi.org/10.1016/j.ejmech.2010.06.017
Woodley CL, Kilburn JO (1982) In vitro susceptibility of Mycobacterium avium complex and Mycobacterium tuberculosis strains to a spiro-piperidyl rifamycin. Am Rev Respir Dis 126(3):586–587. https://doi.org/10.1164/arrd.1982.126.3.586
Xu J, Mahmud T, Floss HG (2003) Isolation and characterization of 27-O-demethylrifamycin SV methyltransferase provides new insights into the post-PKS modification steps during the biosynthesis of the antitubercular drug rifamycin B by Amycolatopsis mediterranei S699. Arch Biochem Biophys 411(2):277–288. https://doi.org/10.1016/S0003-9861(03)00004-3
Xu J, Wan E, Kim CJ, Floss HG, Mahmud T (2005) Identification of tailoring genes involved in the modification of the polyketide backbone of rifamycin B by Amycolatopsis mediterranei S699. Microbiology 151(8):2515–2528. https://doi.org/10.1099/mic.0.28138-0
Yu TW, Muller R, Muller M, Zhang X, Draeger G, Kim CG, Leistner E, Floss HG (2001) Mutational analysis and reconstituted expression of the biosynthetic genes involved in the formation of 3-amino-5-hydroxybenzoic acid, the starter unit of rifamycin biosynthesis in Amycolatopsis Mediterranei S699. J Biol Chem 276(16):12546–12555. https://doi.org/10.1074/jbc.M009667200
Zhang ZQ, Wu XK, Song RT, Zhang JL, Wang HX, Zhu J, Lu CH, Shen YM (2017) Ansavaricins F-I, new DNA topoisomerase inhibitors produced by Streptomyces sp S012. RSC Adv 7(24):14857–14867. https://doi.org/10.1039/c7ra00961e
Funding
This work was supported by the National Natural Science Foundation of China (81373304, 81673317), Science Foundation of Two Sides of Strait (U1405223), and Program for Changjiang Scholars and Innovative Research Team in University (IRT_17R68).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Electronic supplementary material (ESI) available: The NMR spectra and HRESIMS of all the compounds and disruption of PKS genes.
Electronic supplementary material
ESM 1
(PDF 14512 kb)
Rights and permissions
About this article
Cite this article
Li, X., Wu, X., Zhu, J. et al. Amexanthomycins A–J, pentangular polyphenols produced by Amycolatopsis mediterranei S699∆rifA . Appl Microbiol Biotechnol 102, 689–702 (2018). https://doi.org/10.1007/s00253-017-8648-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-017-8648-z